Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills
Abstract
1. Introduction
- (a)
- To determine the major metabolites present in the fish lens and gill, including osmolytes, antioxidants, amino acids, organic acids etc., and to measure their concentrations;
- (b)
- To compare the metabolomic profiles of the fish lens and gill. The gill is a very blood-rich tissue, while the metabolomic composition of AH surrounding the lens is very similar to that of blood plasma [22]. Therefore, the comparison of metabolomic compositions of the lens and gill may help to determine which compounds enter the lens from blood via AH, and which ones are specifically synthesized inside the lens;
- (c)
- To compare the metabolomic composition of gills and lenses from herbivorous–omnivorous (R. rutilus lacustris) and predatory (S. lucioperca) fish;
- (d)
- To compare the lens metabolomic composition of fish caught at different times of year in order to estimate the influence of a seasonal factor on the lens metabolomic profile.
2. Results
2.1. Metabolite Identification
2.2. Metabolite Quantification
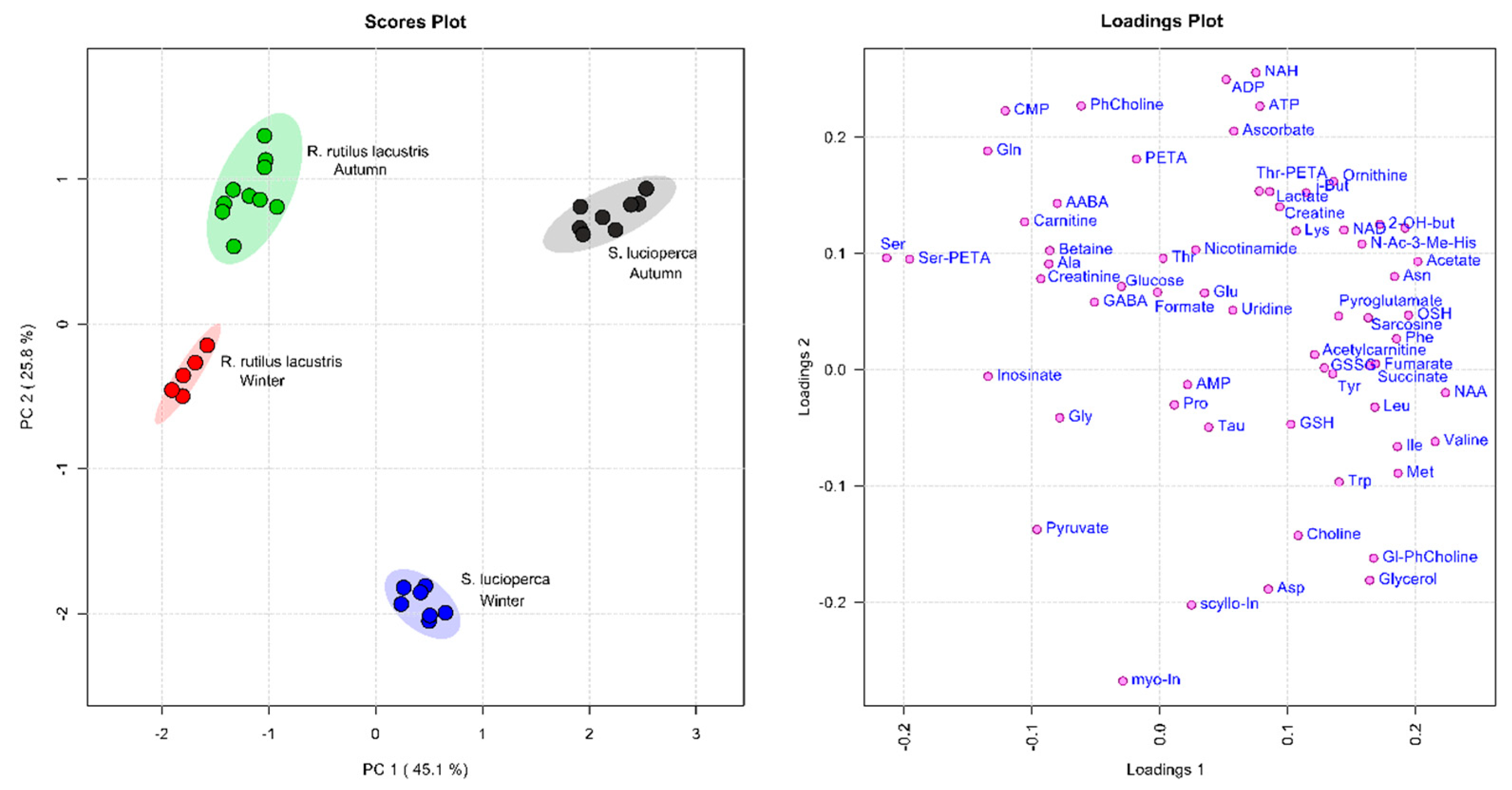
2.3. Quantitative Data Analysis
2.4. Metabolite Group Analysis
2.4.1. Amino Acids
2.4.2. Organic Acids
2.4.3. Alcohols, Amines, and Sugars
2.4.4. Osmolytes
2.4.5. Antioxidants
2.4.6. Nitrogenous Bases, Nucleotides, Nucleosides
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Fish Sample Collection
4.3. Fish Lens and Gill Preparation
4.4. NMR Measurements
4.5. LC-MS Measurements
4.6. LC Fraction Collection
4.7. Data Analysis
5. Conclusions
Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Mishur, R.J.; Rea, S.L. Applications of mass spectrometry to metabolomics and metabonomics: Detection of biomarkers of aging and of age-related diseases. Mass Spectrom. Rev. 2012, 31, 70–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Mass spectrometry-based metabolomics: Applications to biomarker and metabolic pathway research. Biomed. Chromatogr. 2016, 30, 7–12. [Google Scholar] [CrossRef]
- Viant, M.R.; Pincetich, C.A.; Tjeerdema, R.S. Metabolic effects of dinoseb, diazinon and esfenvalerate in eyed eggs and alevins of Chinook salmon (Oncorhynchus tshawytscha) determined by 1H NMR metabolomics. Aquat. Toxicol. 2006, 77, 359–371. [Google Scholar] [CrossRef]
- Li, M.-H.; Ruan, L.-Y.; Zhou, J.-W.; Fu, Y.-H.; Jiang, L.; Zhao, H.; Wang, J.-S. Metabolic profiling of goldfish (Carassius auratis) after long-term glyphosate-based herbicide exposure. Aquat. Toxicol. 2017, 188, 159–169. [Google Scholar] [CrossRef]
- Cappello, T.; Maisano, M.; Mauceri, A.; Fasulo, S. 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2017, 142, 417–422. [Google Scholar] [CrossRef]
- Cappello, T.; Pereira, P.; Maisano, M.; Mauceri, A.; Pacheco, M.; Fasulo, S. Advances in understanding the mechanisms of mercury toxicity in wild golden grey mullet (Liza aurata) by 1H NMR-based metabolomics. Environ. Pollut. 2016, 219, 139–148. [Google Scholar] [CrossRef]
- Allen, P.J.; Wise, D.; Greenway, T.; Khoo, L.; Griffin, M.J.; Jablonsky, M. Using 1-D 1H and 2-D 1H J-resolved NMR metabolomics to understand the effects of anemia in channel catfish (Ictalurus punctatus). Metabolomics 2015, 11, 1131–1143. [Google Scholar] [CrossRef]
- Remø, S.C.; Hevrøy, E.M.; Breck, O.; Olsvik, P.A.; Waagbø, R. Lens metabolomic profiling as a tool to understand cataractogenesis in Atlantic salmon and rainbow trout reared at optimum and high temperature. PLoS ONE 2017, 12, e0175491. [Google Scholar] [CrossRef] [PubMed]
- Sambraus, F.; Fjelldal, P.G.; Remø, S.C.; Hevrøy, E.M.; Nilsen, T.O.; Thorsen, A.; Hansen, T.J.; Waagbø, R. Water temperature and dietary histidine affect cataract formation in Atlantic salmon (Salmo salar L.) diploid and triploid yearling smolt. J. Fish Dis. 2017, 40, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Low, C.-F.; Rozaini, M.Z.H.; Musa, N.; Baharum, S.N. Current knowledge of metabolomic approach in infectious fish disease studies. J. Fish Dis. 2017, 40, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Bjerkas, E. The role of nutrition in cataract formation in farmed fish. CAB Rev. 2006, 1, 16. [Google Scholar] [CrossRef]
- Jonassen, T.; Hamadi, M.; Remø, S.C.; Waagbø, R. An epidemiological study of cataracts in wild and farmed lumpfish (Cyclopterus lumpus L.) and the relation to nutrition. J. Fish Dis. 2017, 40, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.E. Cataracts in farmed Atlantic salmon (Salmo salar) in Ireland, Norway and Scotland from 1995 to 1997. Vet. Rec. 1998, 142, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Breck, O.; Bjerkås, E.; Sanderson, J.; Waagbø, R.; Campbell, P. Dietary histidine affects lens protein turnover and synthesis of N-acetylhistidine in Atlantic salmon (Salmo salar L.) undergoing parr–smolt transformation. Aquac. Nutr. 2005, 11, 321–332. [Google Scholar] [CrossRef]
- Remø, S.C.; Hevrøy, E.M.; Olsvik, P.A.; Fontanillas, R.; Breck, O.; Waagbø, R. Dietary histidine requirement to reduce the risk and severity of cataracts is higher than the requirement for growth in Atlantic salmon smolts, independently of the dietary lipid source. Br. J. Nutr. 2014, 111, 1759–1772. [Google Scholar] [CrossRef]
- Waagbø, R.; Trösse, C.; Koppe, W.; Fontanillas, R.; Breck, O. Dietary histidine supplementation prevents cataract development in adult Atlantic salmon, Salmo salar L., in seawater. Br. J. Nutr. 2010, 104, 1460–1470. [Google Scholar] [CrossRef]
- Baslow, M.H. Function of the N-acetyl-L-histidine system in the vertebrate eye. Evidence in support of a role as a molecular water pump. J. Mol. Neurosci. 1998, 10, 193–208. [Google Scholar] [CrossRef]
- Rhodes, J.D.; Breck, O.; Waagbo, R.; Bjerkas, E.; Sanderson, J. N-acetylhistidine, a novel osmolyte in the lens of Atlantic salmon (Salmo salar L.). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1075–R1081. [Google Scholar] [CrossRef]
- Bassnett, S. Lens organelle degradation. Exp. Eye Res. 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Snytnikova, O.A.; Khlichkina, A.A.; Yanshole, L.V.; Yanshole, V.V.; Iskakov, I.A.; Egorova, E.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Metabolomics of the human aqueous humor. Metabolomics 2017, 13, 5. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Yanshole, L.V.; Iskakov, I.A.; Yanshole, V.V.; Chernykh, V.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Quantitative metabolomic analysis of the human cornea and aqueous humor. Metabolomics 2017, 13, 152. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Verkhovod, T.D.; Yanshole, V.V.; Kiryutin, A.S.; Yanshole, L.V.; Fursova, A.Z.; Stepakov, D.A.; Novoselov, V.P.; Sagdeev, R.Z. Metabolomic composition of normal aged and cataractous human lenses. Exp. Eye Res. 2015, 134, 15–23. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Snytnikova, O.A.; Kiryutin, A.S.; Yanshole, L.V.; Sagdeev, R.Z.; Tsentalovich, Y.P. Metabolomics of the rat lens: A combined LC-MS and NMR study. Exp. Eye Res. 2014, 125, 71–78. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Yanshole, L.V.; Zelentsova, E.A.; Tsentalovich, Y.P. Ovothiol A is the Main Antioxidant in Fish Lens. Metabolites 2019, 9, 95. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Snytnikova, O.A.; Yanshole, V.V.; Tsentalovich, Y.P.; Sagdeev, R.Z. Post-mortem changes in the metabolomic compositions of rabbit blood, aqueous and vitreous humors. Metabolomics 2016, 12, 172. [Google Scholar] [CrossRef]
- Cappello, T.; Giannetto, A.; Parrino, V.; Maisano, M.; Oliva, S.; De Marco, G.; Guerriero, G.; Mauceri, A.; Fasulo, S. Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 26, 32–39. [Google Scholar] [CrossRef]
- Gowda, G.A.; Raftery, D. Quantitating metabolites in protein precipitated serum using NMR spectroscopy. Anal. Chem. 2014, 86, 5433–5440. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Yanshole, L.V.; Snytnikova, O.A.; Tsentalovich, Y.P. Quantitative metabolomic analysis of changes in the lens and aqueous humor under development of age-related nuclear cataract. Metabolomics 2019, 15, 29. [Google Scholar] [CrossRef]
- Terzhevik, A.; Golosov, S. Dissolved Oxygen in Ice-Covered Lakes. In Encyclopedia of Lakes and Reservoirs; Bengtsson, L., Herschy, R.W., Fairbridge, R.W., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 220–222. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.T.; Kistler, J.; Donaldson, P. The lens circulation. J. Membr. Biol. 2007, 216, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.T.; Rae, J.L. Transport properties of the lens. Am. J. Physiol. 1985, 249, C181–C190. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.T.; Rae, J.L. The lens: Local transport and global transparency. Exp. Eye Res. 2004, 78, 689–698. [Google Scholar] [CrossRef]
- McNulty, R.; Wang, H.; Mathias, R.T.; Ortwerth, B.J.; Truscott, R.J.W.; Bassnett, S. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004, 559, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Hand, C.E.; Honek, J.F. Biological chemistry of naturally occurring thiols of microbial and marine origin. J. Nat. Prod. 2005, 68, 293–308. [Google Scholar] [CrossRef]
- Varma, S.D. Ascorbic Acid and the Eye with Special Reference to the Lens. Ann. N. Y. Acad. Sci. 1987, 498, 280–306. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Holler, T.P.; Hopkins, P.B. Ovothiols as biological antioxidants. The thiol groups of ovothiol and glutathione are chemically distinct. J. Am. Chem. Soc. 1988, 110, 4837–4838. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Noszál, B. Species-specific thiol-disulfide equilibrium constants of ovothiol A and penicillamine with glutathione. RSC Adv. 2016, 6, 26757–26764. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Orgován, G.; Hosztafi, S.; Noszál, B. The complete microspeciation of ovothiol A, the smallest octafarious antioxidant biomolecule. Anal. Bioanal. Chem. 2014, 406, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.H.; Rabenstein, D.L. Thiol/Disulfide Exchange Reactions of Ovothiol A with Glutathione. J. Org. Chem. 1995, 60, 1904–1907. [Google Scholar] [CrossRef]
- Marjanovic, B.; Simic, M.G.; Jovanovic, S.V. Heterocyclic thiols as antioxidants: Why ovothiol C is a better antioxidant than ergothioneine. Free Radic. Biol. Med. 1995, 18, 679–685. [Google Scholar] [CrossRef]
- Ariyanayagam, M.R.; Fairlamb, A.H. Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol. Biochem. Parasitol. 2001, 115, 189–198. [Google Scholar] [CrossRef]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Isolation and structure of a new sulphur-containing aminoacid from sea urchin eggs. Tetrahedron Lett. 1982, 23, 3207–3208. [Google Scholar] [CrossRef]
- Palumbo, A.; Misuraca, G.; d’Ischia, M.; Donaudy, F.; Prota, G. Isolation and distribution of 1-methyl-5-thiol-l-histidine disulphide and a related metabolite in eggs from echinoderms. Comp. Biochem. Physiol. B 1984, 78, 81–83. [Google Scholar] [CrossRef]
- Turner, E.; Klevit, R.; Hager, L.J.; Shapiro, B.M. Ovothiols, a family of redox-active mercaptohistidine compounds from marine invertebrate eggs. Biochemistry 1987, 26, 4028–4036. [Google Scholar] [CrossRef]
- Turner, E.; Klevit, R.; Hopkins, P.B.; Shapiro, B.M. Ovothiol: A novel thiohistidine compound from sea urchin eggs that confers NAD(P)H-O2 oxidoreductase activity on ovoperoxidase. J. Biol. Chem. 1986, 261, 13056–13063. [Google Scholar]
- Castellano, I.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Pipino, C.; Formoso, G.; Napolitano, A.; Palumbo, A.; Pandolfi, A. Anti-Inflammatory Activity of Marine Ovothiol A in an In Vitro Model of Endothelial Dysfunction Induced by Hyperglycemia. Oxid. Med. Cell Longev. 2018, 2018, 2087373. [Google Scholar] [CrossRef]
- Tamara, S.O.; Yanshole, L.V.; Yanshole, V.V.; Fursova, A.Z.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Spatial distribution of metabolites in the human lens. Exp. Eye Res. 2016, 143, 68–74. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Baslow, M.H.; Guilfoyle, D.N. N-acetyl-l-histidine, a Prominent Biomolecule in Brain and Eye of Poikilothermic Vertebrates. Biomolecules 2015, 5, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Naowarojna, N.; Huang, P.; Cai, Y.; Song, H.; Wu, L.; Cheng, R.; Li, Y.; Wang, S.; Lyu, H.; Zhang, L.; et al. In Vitro Reconstitution of the Remaining Steps in Ovothiol A Biosynthesis: C-S Lyase and Methyltransferase Reactions. Org. Lett. 2018, 20, 5427–5430. [Google Scholar] [CrossRef] [PubMed]
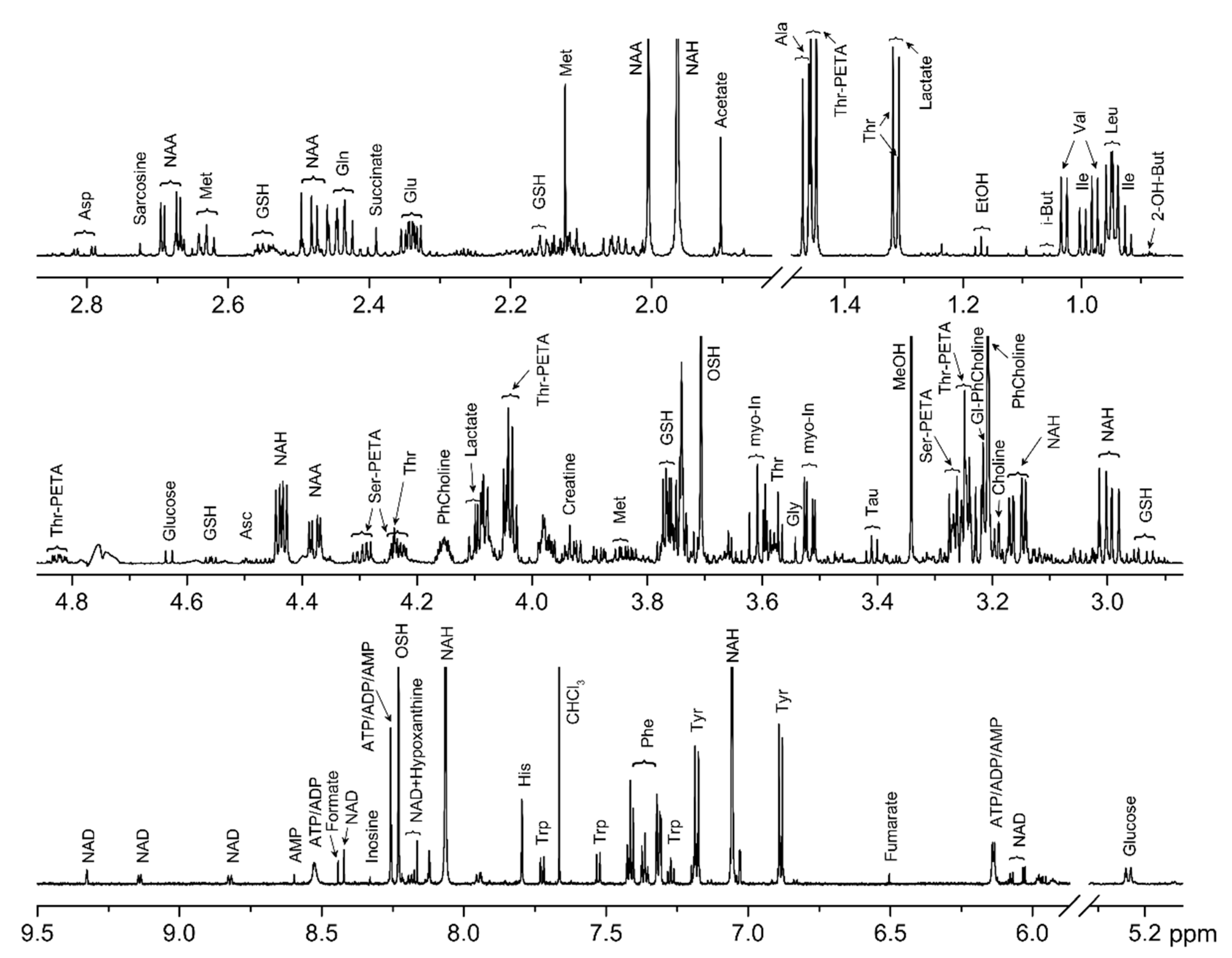

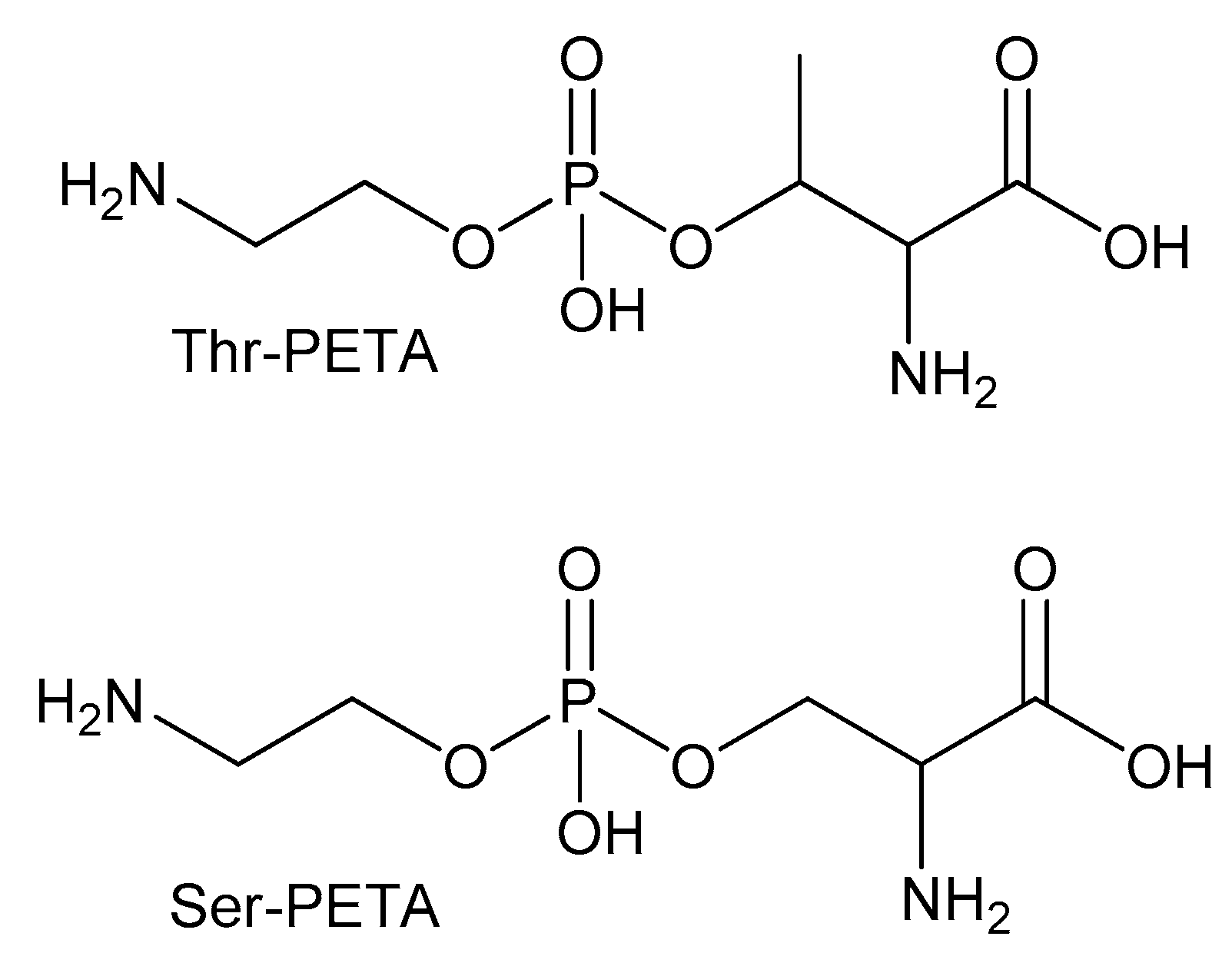
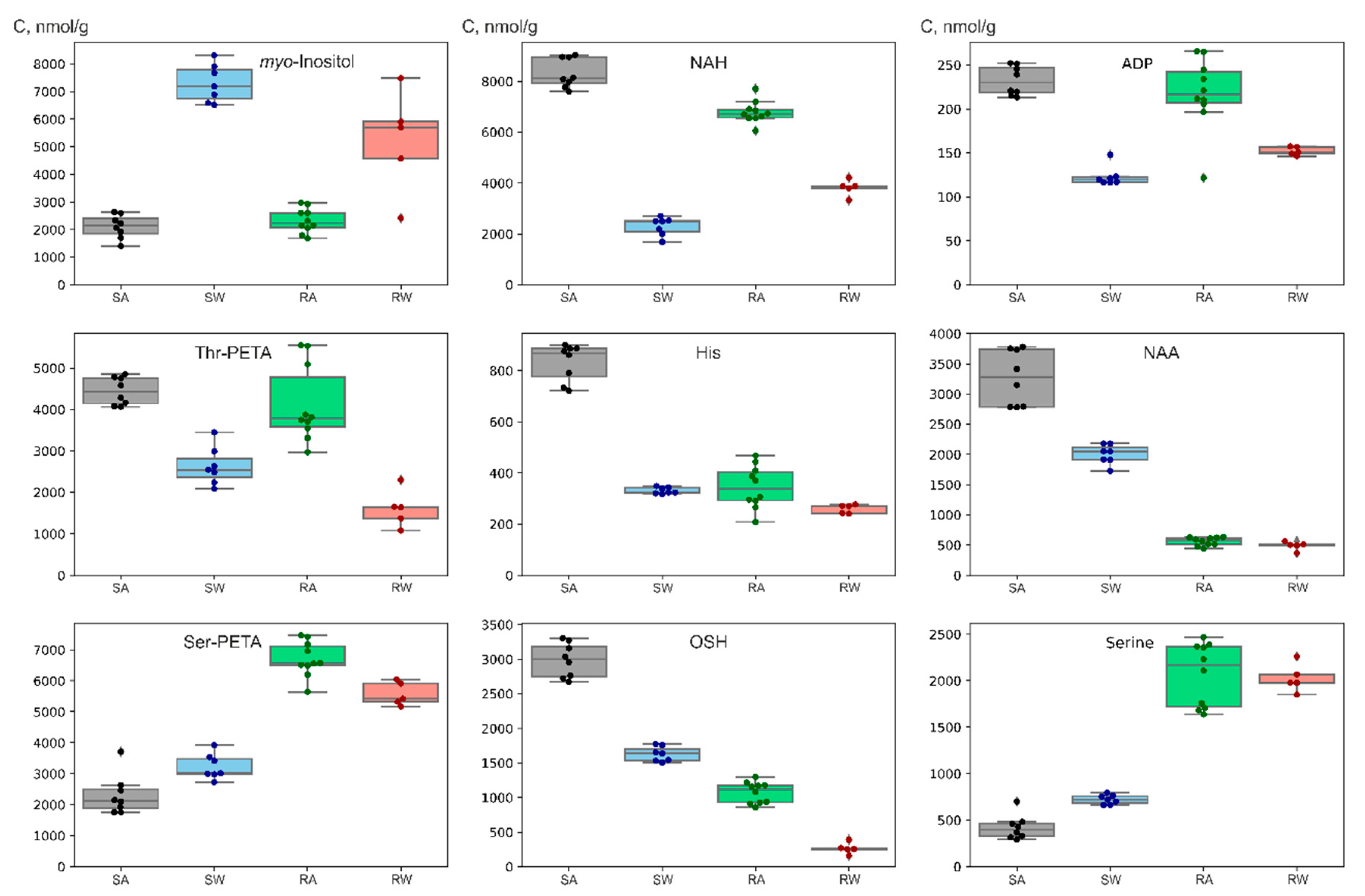
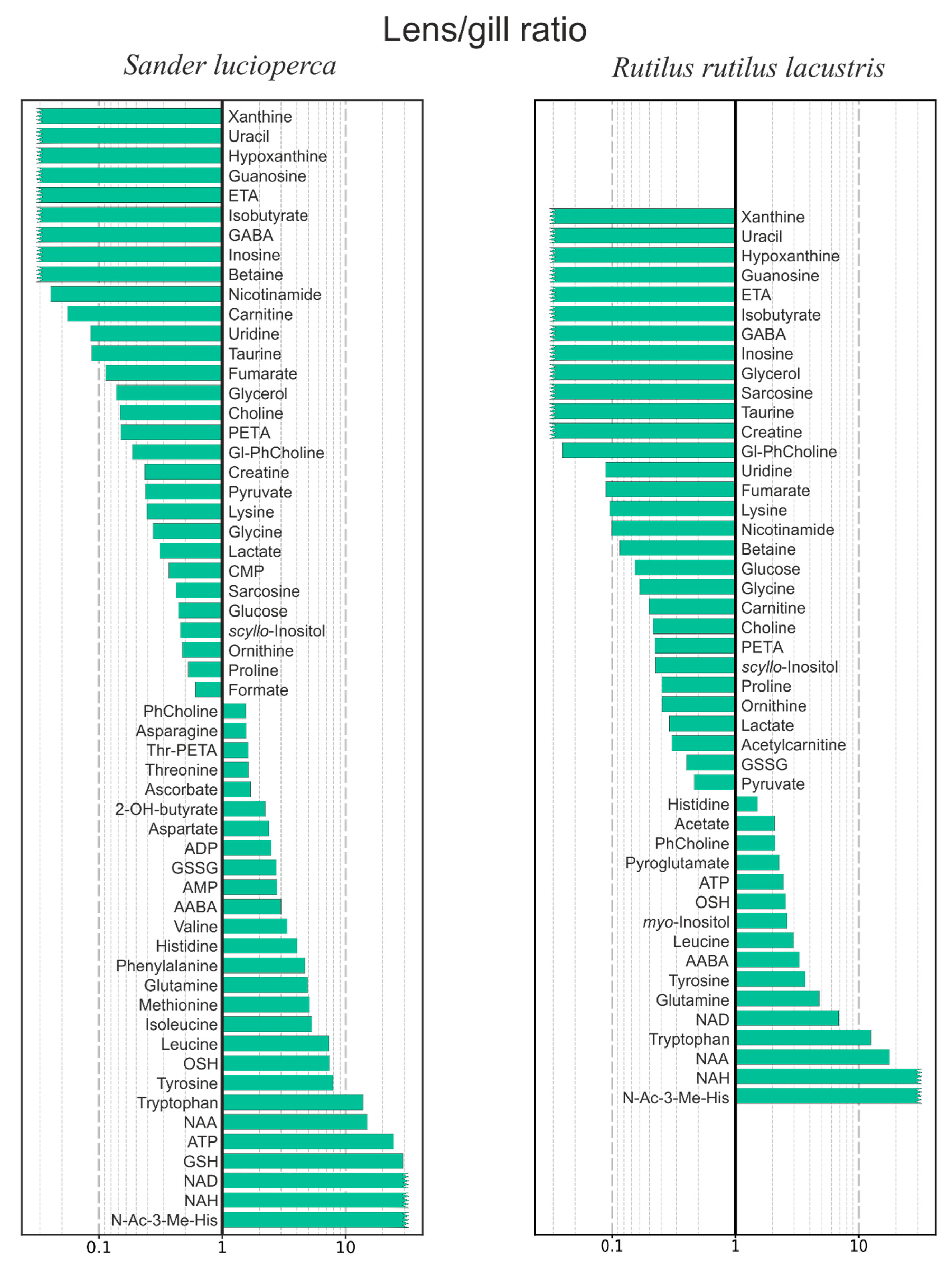
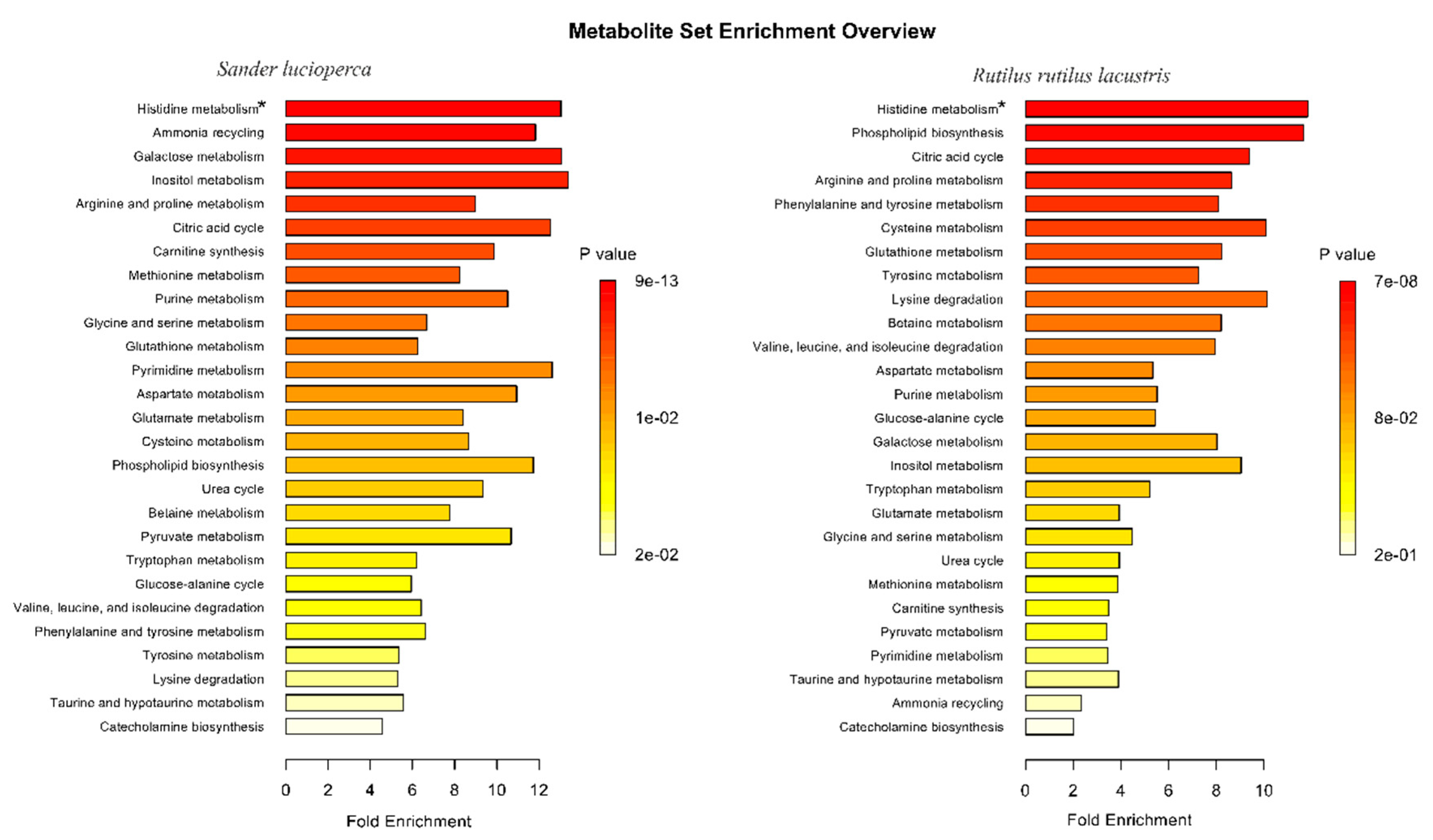
| Metabolite | Method | Sander lucioperca | Rutilus rutilus lacustris | ||||
|---|---|---|---|---|---|---|---|
| Lens Autumn, nmol/g | Lens Winter, nmol/g | Gill Winter, nmol/g | Lens Autumn, nmol/g | Lens Winter, nmol/g | Gill Winter, nmol/g | ||
| Amino acids | |||||||
| Acetylcarnitine | NMR | 30 ± 9 | 17 ± 2 | 16 ± 5 | 10 ± 4 | 18 ± 4 | 60 ± 30 |
| Alanine | NMR | 1600 ± 180 | 1500 ± 140 | 1200 ± 300 | 2300 ± 600 | 2100 ± 300 | 1800 ± 40 |
| Asparagine | MS | 30 ± 4 | 10 ± 1 | 6.7 ± 1.8 | 7.8 ± 1.6 | 4.9 ± 0.4 | 5.3 ± 2.7 |
| Aspartate | NMR | 280 ± 20 | 440 ± 40 | 190 ± 60 | 210 ± 60 | 200 ± 40 | 250 ± 80 |
| Betaine | MS | 6.2 ± 1.9 | 4.0 ± 0.7 | 240 ± 50 | 14 ± 7 | 11 ± 4 | 100 ± 40 |
| Carnitine | MS | 2.3 ± 0.3 | 0.70 ± 0.15 | 13 ± 2 | 12 ± 5 | 6.3 ± 2.7 | 31 ± 17 |
| Creatine | NMR* | 160 ± 40 | 73 ± 7 | 310 ± 40 | 130 ± 30 | 35 ± 6 | 1100 ± 200 |
| Glutamate | NMR | 2100 ± 200 | 2000 ± 140 | 1500 ± 200 | 2200 ± 200 | 1500 ± 130 | 2000 ± 200 |
| Glutamine | NMR | 1900 ± 200 | 990 ± 120 | 200 ± 30 | 2900 ± 540 | 3000 ± 100 | 630 ± 200 |
| Glycine | NMR | 130 ± 20 | 180 ± 40 | 660 ± 70 | 180 ± 80 | 230 ± 40 | 1400 ± 340 |
| Histidine | NMR* | 830 ± 70 | 330 ± 13 | 82 ± 17 | 350 ± 80 | 260 ± 20 | 170 ± 80 |
| Isoleucine | NMR | 550 ± 110 | 470 ± 60 | 88 ± 15 | 140 ± 50 | 60 ± 24 | 100 ± 80 |
| Leucine | NMR | 1600 ± 200 | 1400 ± 200 | 190 ± 30 | 890 ± 200 | 750 ± 170 | 250 ± 190 |
| Lysine | MS | 190 ± 70 | 52 ± 10 | 210 ± 40 | 90 ± 19 | 63 ± 20 | 660 ± 230 |
| Methionine | NMR | 710 ± 160 | 680 ± 160 | 130 ± 30 | 290 ± 80 | 220 ± 60 | 140 ± 70 |
| N-Ac-3-Me-His | NMR | 660 ± 220 | 76 ± 16 | 0 | 110 ± 40 | 44 ± 24 | 0 |
| Ornithine | MS | 46 ± 4 | 23 ± 5 | 50 ± 11 | 32 ± 6 | 18 ± 4 | 73 ± 15 |
| Phenylalanine | NMR* | 800 ± 190 | 440 ± 50 | 93 ± 13 | 240 ± 60 | 200 ± 40 | 130 ± 120 |
| Proline | NMR* | 81 ± 19 | 100 ± 30 | 190 ± 60 | 91 ± 16 | 65 ± 15 | 260 ± 180 |
| Sarcosine | NMR | 36 ± 10 | 13 ± 4 | 30 ± 7 | 4.3 ± 5.7 | 0 | 88 ± 30 |
| Serine | NMR* | 420 ± 130 | 720 ± 50 | 560 ± 80 | 2100 ± 300 | 2000 ± 150 | 1700 ± 1100 |
| Threonine | MS | 340 ± 130 | 320 ± 60 | 200 ± 40 | 420 ± 60 | 230 ± 60 | 180 ± 100 |
| Tryptophan | NMR* | 360 ± 120 | 380 ± 30 | 27 ± 6 | 170 ± 60 | 180 ± 30 | 14 ± 10 |
| Tyrosine | NMR | 1100 ± 400 | 780 ± 110 | 100 ± 20 | 450 ± 160 | 580 ± 170 | 160 ± 130 |
| Valine | NMR* | 790 ± 140 | 620 ± 90 | 190 ± 40 | 170 ± 50 | 93 ± 28 | 190 ± 130 |
| Organic acids | |||||||
| 2-OH-butyrate | MS | 34 ± 8 | 6.0 ± 2.3 | 2.7 ± 1.7 | 9.1 ± 4.8 | 1.5 ± 0.3 | 0.96 ± 0.81 |
| AABA | NMR* | 110 ± 25 | 53 ± 15 | 18 ± 3 | 180 ± 60 | 150 ± 27 | 46 ± 10 |
| Acetate | NMR | 510 ± 30 | 250 ± 20 | 170 ± 90 | 220 ± 60 | 180 ± 20 | 90 ± 40 |
| Formate | NMR | 60 ± 20 | 16 ± 8 | 26 ± 6 | 46 ± 32 | 65 ± 61 | 38 ± 26 |
| Fumarate | NMR | 16 ± 1 | 11 ± 3 | 94 ± 29 | 6.4 ± 3.3 | 4.3 ± 2.7 | 48 ± 12 |
| GABA | MS | 1.4 ± 0.9 | 0.49 ± 0.64 | 71 ± 18 | 12 ± 14 | 8.3 ± 4.9 | 460 ± 160 |
| Isobutyrate | NMR | 7.8 ± 2.4 | 0 | 6.9 ± 3.2 | 0 | 0 | 9.4 ± 5.0 |
| Lactate | NMR | 2500 ± 500 | 1000 ± 200 | 3400 ± 600 | 1700 ± 600 | 1500 ± 140 | 5100 ± 1900 |
| Pyroglutamate | MS | 94 ± 8 | 78 ± 15 | 75 ± 34 | 72 ± 11 | 65 ± 5 | 29 ± 15 |
| Pyruvate | NMR | 0 | 10 ± 2 | 43 ± 14 | 5.5 ± 6.6 | 11 ± 3 | 23 ± 8 |
| Succinate | NMR | 90 ± 15 | 59 ± 8 | 87 ± 40 | 34 ± 7 | 34 ± 8 | 59 ± 50 |
| Alcohols, amines, and sugars | |||||||
| Choline | NMR | 120 ± 20 | 140 ± 30 | 970 ± 200 | 43 ± 9 | 86 ± 25 | 400 ± 80 |
| ETA | NMR | 0 | 0 | 1500 ± 200 | 0 | 0 | 620 ± 440 |
| Glucose | NMR | 290 ± 120 | 140 ± 20 | 320 ± 200 | 540 ± 400 | 250 ± 90 | 1600 ± 1600 |
| Glycerol | NMR | 50 ± 6 | 73 ± 18 | 530 ± 130 | 0 | 0 | 300 ± 200 |
| Gl-PhCholine | NMR | 260 ± 30 | 340 ± 40 | 1800 ± 400 | 95 ± 26 | 56 ± 8 | 1400 ± 400 |
| PhCholine | NMR | 1500 ± 200 | 810 ± 60 | 520 ± 170 | 2200 ± 200 | 1300 ± 50 | 610 ± 160 |
| PETA | NMR* | 630 ± 230 | 250 ± 43 | 1700 ± 300 | 350 ± 60 | 560 ± 130 | 2500 ± 700 |
| scyllo-Inositol | NMR | 10 ± 3 | 130 ± 40 | 280 ± 70 | 11 ± 3 | 13 ± 11 | 58 ± 30 |
| Osmolytes | |||||||
| myo-Inositol | NMR | 2100 ± 400 | 7300 ± 700 | 8200 ± 1100 | 2300 ± 400 | 5200 ± 1900 | 2000 ± 500 |
| NAA | NMR | 3300 ± 500 | 2000 ± 200 | 130 ± 100 | 560 ± 70 | 490 ± 70 | 30 ± 10 |
| NAH | NMR | 8300 ± 600 | 2300 ± 400 | 50 ± 20 | 6800 ± 400 | 3800 ± 300 | 13 ± 5 |
| Ser-PETA | NMR | 2300 ± 600 | 3200 ± 400 | 3300 ± 500 | 6700 ± 600 | 5600 ± 400 | 3800 ± 1100 |
| Taurine | NMR | 340 ± 80 | 480 ± 110 | 5500 ± 780 | 370 ± 200 | 170 ± 30 | 8700 ± 1200 |
| Thr-PETA | NMR | 4400 ± 300 | 2600 ± 500 | 1600 ± 300 | 4100 ± 900 | 1600 ± 500 | 1400 ± 600 |
| Antioxidants | |||||||
| Ascorbate | NMR | 91 ± 17 | 40 ± 7 | 23 ± 11 | 54 ± 10 | 57 ± 8 | 100 ± 180 |
| GSH | NMR* | 470 ± 150 | 490 ± 80 | 17 ± 15 | 280 ± 130 | 150 ± 100 | 100 ± 140 |
| GSSG | NMR* | 260 ± 80 | 200 ± 30 | 70 ± 20 | 140 ± 60 | 37 ± 17 | 92 ± 46 |
| OSH | NMR | 3000 ± 200 | 1600 ± 108 | 220 ± 70 | 1100 ± 200 | 270 ± 80 | 100 ± 90 |
| Nitrogenous bases, nucleotides, nucleosides | |||||||
| CMP | MS | 17 ± 2 | 6.5 ± 0.3 | 18 ± 5 | 39 ± 4 | 20 ± 3 | 20 ± 5 |
| ADP | NMR | 230 ± 20 | 120 ± 10 | 49 ± 10 | 220 ± 40 | 150 ± 10 | 85 ± 65 |
| AMP | NMR* | 59 ± 9 | 57 ± 17 | 20 ± 16 | 53 ± 16 | 57 ± 6 | 55 ± 50 |
| ATP | NMR | 930 ± 60 | 540 ± 40 | 22 ± 5 | 840 ± 60 | 560 ± 80 | 230 ± 240 |
| Creatinine | NMR | 13 ± 6 | 7.8 ± 1.6 | 15 ± 5 | 19 ± 6 | 28 ± 9 | 34 ± 9 |
| Guanosine | NMR | 0 | 0 | 77 ± 12 | 0 | 0 | 6.0 ± 4.4 |
| Hypoxanthine | NMR | 0 | 0 | 290 ± 80 | 0 | 0 | 300 ± 250 |
| Inosinate | NMR | 12 ± 2 | 23 ± 6 | 21 ± 12 | 38 ± 16 | 66 ± 19 | 64 ± 69 |
| Inosine | NMR | 28 ± 4 | 19 ± 5 | 600 ± 70 | 0 | 0 | 80 ± 50 |
| NAD | NMR | 180 ± 30 | 81 ± 10 | 1.8 ± 0.9 | 100 ± 30 | 46 ± 9 | 6.7 ± 9.9 |
| Nicotinamide | NMR* | 3.4 ± 0.7 | 1.9 ± 0.6 | 48 ± 6 | 2.7 ± 0.3 | 3.9 ± 1.3 | 39 ± 14 |
| Uracil | NMR | 0 | 0 | 190 ± 30 | 0 | 0 | 160 ± 90 |
| Uridine | MS | 4.6 ± 0.7 | 3.1 ± 1.3 | 36 ± 5 | 3.3 ± 1.6 | 3.8 ± 1.6 | 43 ± 27 |
| Xanthine | NMR | 0 | 0 | 56 ± 14 | 0 | 0 | 130 ± 110 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsentalovich, Y.P.; Yanshole, V.V.; Yanshole, L.V.; Zelentsova, E.A.; Melnikov, A.D.; Sagdeev, R.Z. Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills. Metabolites 2019, 9, 264. https://doi.org/10.3390/metabo9110264
Tsentalovich YP, Yanshole VV, Yanshole LV, Zelentsova EA, Melnikov AD, Sagdeev RZ. Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills. Metabolites. 2019; 9(11):264. https://doi.org/10.3390/metabo9110264
Chicago/Turabian StyleTsentalovich, Yuri P., Vadim V. Yanshole, Lyudmila V. Yanshole, Ekaterina A. Zelentsova, Arsenty D. Melnikov, and Renad Z. Sagdeev. 2019. "Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills" Metabolites 9, no. 11: 264. https://doi.org/10.3390/metabo9110264
APA StyleTsentalovich, Y. P., Yanshole, V. V., Yanshole, L. V., Zelentsova, E. A., Melnikov, A. D., & Sagdeev, R. Z. (2019). Seasonal Variations and Interspecific Differences in Metabolomes of Freshwater Fish Tissues: Quantitative Metabolomic Profiles of Lenses and Gills. Metabolites, 9(11), 264. https://doi.org/10.3390/metabo9110264