1. Introduction
The application of “omics” sciences for deciphering the mechanisms involved in complex diseases has succeeded, showing a great impact in the recent years not only in the number of publications, but also in the advance of medical treatments [
1,
2]. Metabolomics has gained attention due to its capability to inquire into the metabolism of living organisms.
Asthma is a prevalent disease that currently affects almost 25% of the population worldwide [
3]. The number of cases increases every year, as well as its severity, creating a high economic burden and severely impacting health, especially in children. It is a multifactorial disease in which the clinical phenotypes are not clearly defined; partially because the underlying molecular mechanisms are still largely unknown [
3]. In addition, it is associated with secondary comorbidities such as obesity and allergy.
Metabolomics experiments rely on the use of high-throughput analytical techniques, such as nuclear magnetic resonance (NMR) and mass spectrometry (MS) based techniques. The use of high-resolution MS, particularly with a quadrupole-time of flight analyzer coupled to liquid chromatography (LC-QToF-MS), has dramatically increased the sensitivity and specificity of the technique enabling the extension of the metabolome coverage in a single run. Therefore, it obtains a very complete picture of the metabolic fingerprint of a patient.
Usually, the instrumental analysis consists on the measurement of a small number of samples—less than a hundred—in a single batch. However, in large-scale studies samples need to be analyzed across multiple batches. This is required due to different reasons including the shift in the instrument response associated with the contamination of the ionization source after repeated injections of samples. Every time a batch is run, the instrument is cleaned, calibrated and conditioned, leading to minor changes in the analytical response. This introduces a between-batch systematic error that needs to be properly addressed to enable the joint analysis of multi-batch data.
For this purpose, the development of algorithms for a post-acquisition correction of both intra- and inter-batch effects is an active field of research [
4,
5,
6]. On the other hand, additional aspects in large-scale studies, such as the use of internal standards (ISs), the preparation of the quality control samples (QCs), the strategy for sample preparation, the instrumental analysis and the batch design are an integral part of the study design and must be clearly established in advance [
7].
The “IS” is a compound (or a group of them) with the closest chemical nature possible to the target metabolites found in the sample and that is not naturally present in it. ISs are widely used in quantitative (targeted) LC-MS methods to assess the effects of the matrix on ion suppression or enhancement, as well as to test the extraction efficiency [
8]. In untargeted metabolomic studies, ISs should be carefully selected to avoid interference with the a priori unknown metabolites. Thus, isotopically labeled analogues (often with
2H or
13C) are frequently selected due to their nearly identical physico-chemical properties to the unlabeled metabolites. The ISs selected should provide a broad coverage of the different classes of metabolites that are expected to be found in the samples. However, their use in untargeted LC-QToF-MS is limited to the assessment of instrument performance, as the IS intensity should not be used to correct between batch systematic errors since the metabolites present in the samples can influence the IS estimates by cross contribution [
4,
7,
9]. Currently, different normalization strategies to correct the instrumental drift have been developed, and most of them rely on the information contented in QCs [
10,
11,
12].
Furthermore, the preparation of the QCs in large-scale studies is not obvious. Ideally, the QC should be prepared by pooling a small volume of all samples; however, in large-scale studies sometimes this is not feasible. Moreover, even when this is possible, if the time of sample thawing for QC preparation is extensive, it may induce the enzymes in blood samples to activate, leading to a change in the original sample. Therefore, alternative strategies have been suggested, i.e., the use of a pool of randomly selected samples that could represent the sample population [
13]. Nonetheless, the exact number of samples needed to represent the whole population should be defined beforehand. Additional factors increasing the complexity of large-scale studies have not been addressed, such as if samples should be prepared all at once or in different sets, and how could this impact the retrieved metabolomic profiles. These “naïve” aspects are essential. Additionally, randomization of the complete set of samples into the batches is the statistically correct approach; however, there is no warrant that batches will be accurately normalized after integration.
Large-scale studies are mostly unavoidable when working with heterogeneous diseases like asthma, where hundreds of samples are collected, analyzed together and later compared to extract useful biological information. In this work, we propose a series of strategies and troubleshooting suggestions for large-scale LC-QToF-MS metabolomic studies. Special attention is given to the experimental design and to methodological checkpoints required to achieve a proper analysis and integration of a large set of samples.
This work exemplifies from begging to the end the process to be followed in a large-scale analysis in metabolomics. We show for the first time the inclusion of a deuterated lysophosphocholine, sphingolipid and fatty acid, apart from carnitine and isoleucine in the IS mix to cover the complete retention time span in a reverse phase analysis, which is one of the most common analysis in metabolomic fingerprinting studies. Regarding this IS mix, we describe its response in a large-scale sequence in different ways, and we show its utility in the analysis. In addition, we present the comparison of different normalization strategies such as IS, total useful signal (TUS), QC-SVRC normalization and QC-norm. The novelty of this work relies on an experimental strategy to prove that a robust normalization is possible in large-scale studies by using replicates of case samples. This paper highlights the issues found when working with hundreds of samples and shows a way to overcome them and succeed in the integration of the data from multiple batches.
3. Discussion
The execution of large-scale metabolomic studies is a complex task, specially using LC-QToF-MS, as the analysis of samples is typically distributed across different batches. The main issue is that differences in the instrument response across batches introduce a systematic error in the datasets that may lead to underpowered or even incorrect results in downstream analysis, leading ultimately to false discoveries. The aim of this study was to present a strategy to troubleshoot the integration of the data from different batches.
In this study, analytical conditions for the large-scale experiment were described. We considered several aspects that should be taken into consideration before the analysis, such as the total mobile phase volume needed and their placement. Complete volume preparation will avoid changes in the ionization and chromatographic separation during the experiment. Additional factors we consider important and are mentioned in this study are: rebooting the computer before the experiment to ensure enough memory to complete the whole experiment, avoiding communication errors between computer and equipment; preparation of samples in subsets and centrifugation of sample vials before each analysis. Batches should also be designed to ensure that study groups are evenly distributed across batches. Accordingly, in this study, samples were split in two batches, and each batch included approximately the same number of cases and controls.
Regarding QC preparation, this is not an obvious task. We considered that a quarter of the samples from each experimental group were necessary to obtain a representative sample of our experimental population (i.e., 46 samples in this study). Following this approach, the resultant QC was representative of the tested population and fulfilled the objective of evaluating the performance and stability of the analytical technique. Moreover, in order to avoid repeated freeze-thaw cycles, the selected experimental samples for the QC were assigned to be measured in the first batch and as the first set of samples, with the idea of preparing at the same time each experimental sample and QC. This QC was pooled into different tubes with the aim to thaw new QCs as needed. After the preparation of this first set of samples, we calculated the samples analyzed per day. As a consequence, we prepared a new set of samples each day to maintain a comparable time span to the first sample set.
Peak areas from the IS mix were assessed for a preliminary analysis of data quality after acquisition. Traditionally, the main use of a IS in metabolic fingerprinting in other separation techniques (such as such as gas chromatography and capillary electrophoresis) has been to correct the volume of injection and the MS signal of each sample [
14,
15,
16]. In the case of LC-QToF-MS, especially in large-scale experiments, IS are useful for the monitoring of performance of the system [
7]. In this study, a combination of 5 ISs was employed: L-carnitine-D
3, isoleucine-
13C,
15N, sphingosine-D
7, LPC 18:1-D
7 and stearic acid-D
5. The aim of this IS mix was to cover not only the complete RTs of the chromatogram (from 0.6 to 34.5 min) but also a wide spectrum of biochemical and physicochemical properties. The results markedly showed that each signal from the IS mix followed a different trend for the same sample in both ionization modes. Moreover, these independent trends did not correlate with compound abundances, the RT in the chromatogram or the nature of the IS. This fact suggests the idea that each biological sample has an intrinsic matrix effect.
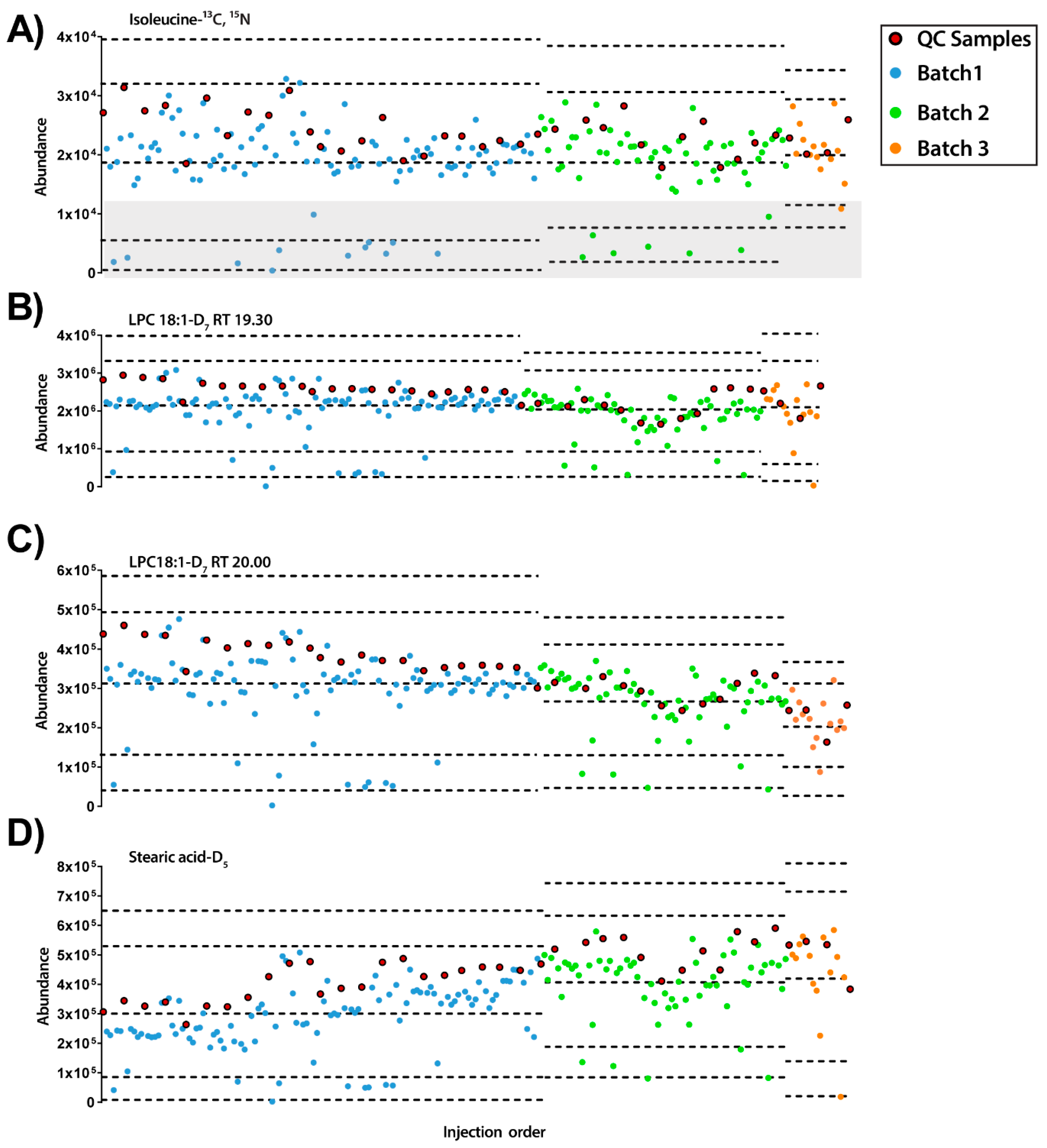
In case of the IS mix in ESI− mode, we found that some samples presented very low intensities of all IS peaks. These samples were not present together in a specific time point of the experiment but were throughout all the batches. This issue is intriguing since it might suggest technical issues in metabolite extraction, but no differences were observed on the TUS values of these samples. Additional experiments are needed to understand this effect.
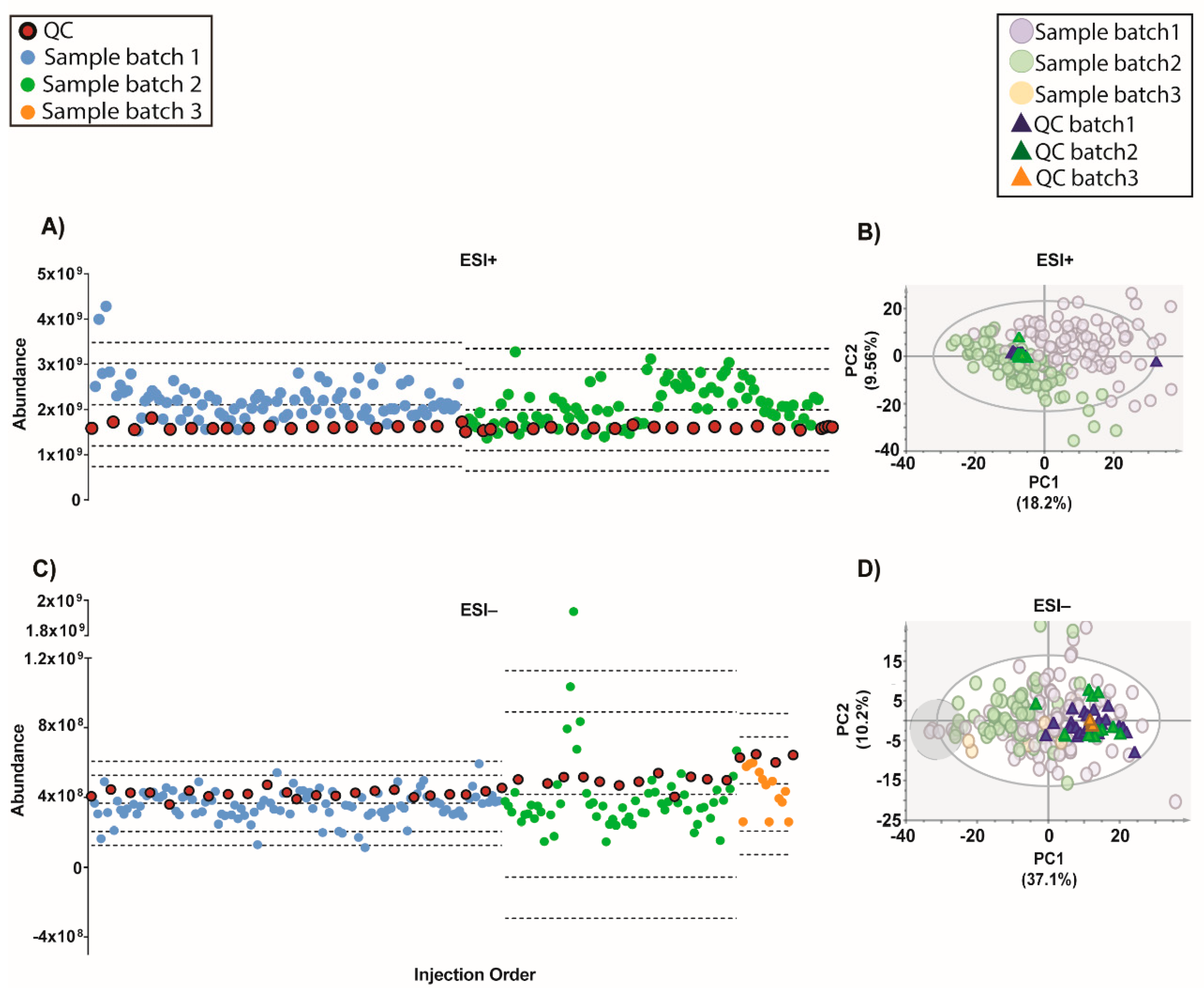
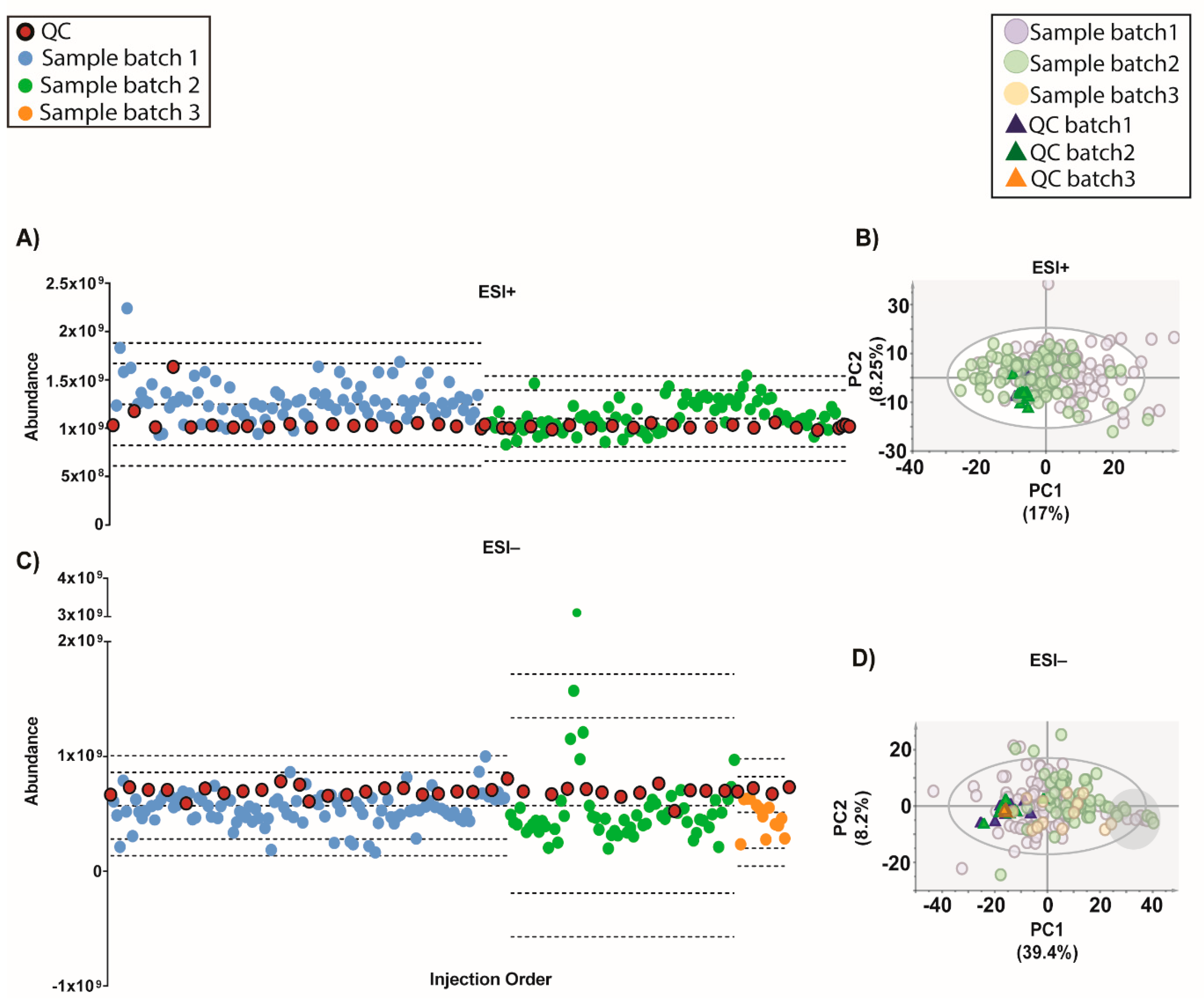
Moving forward, the quality of the initial data (after filtration of blanks, missing values and %RSD in QCs) showed that although on the TUS were comparable among batches, they were different indeed in the PCA plots (
Figure 4 and
Figure S4). This evidence proves that a normalization strategy is necessary to integrate the data from the whole experiment.
In the case of data normalization, we tried two common strategies applied in metabolomics: the normalization by IS (in this case the sum of all IS compounds) and the normalization by TUS. The results show that both approaches did not achieve the integration of the data and, in the case of TUS normalization, increased the drift of the QC by the injection order.
However, in the latest years the normalization methods based on the information contained on QCs has gain great interest to correct the instrumental drift, especially in large-scale studies [
10,
11,
12]. Most of these, have showed the application of support vector regression (SVR) for the modeling of the instrumental drift, such as the strategy proposed by Kuligowski et al. in 2015 [
10], which uses a radial basis function (RBF) kernel to correct the instrumental drift. This algorithm applied to our data, resulted in a good clustering of QCs from all batches in both modes. Moreover, following Sanchéz- Illana et al. in 2018 an inter-batch correction was applied [
4]. The resulting outcome improved the complete integration of QCs in both modes (
Figure 6). The samples with low IS mix in ESI− mode grouped together in one end of most PCAs (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7,
Figures S5–S7 on grey circles) from the different normalization strategies. This demonstrates the importance of adding multiple IS to the samples. Otherwise, it could be thought that these samples shared a common biological condition.
The assessment of the QC-SVRC normalization followed by QC-norm, compared to raw data and with QC-SVRC alone, was tested by the analysis of the PCA models of complete set of samples and the comparison of samples measured in multiple batches (
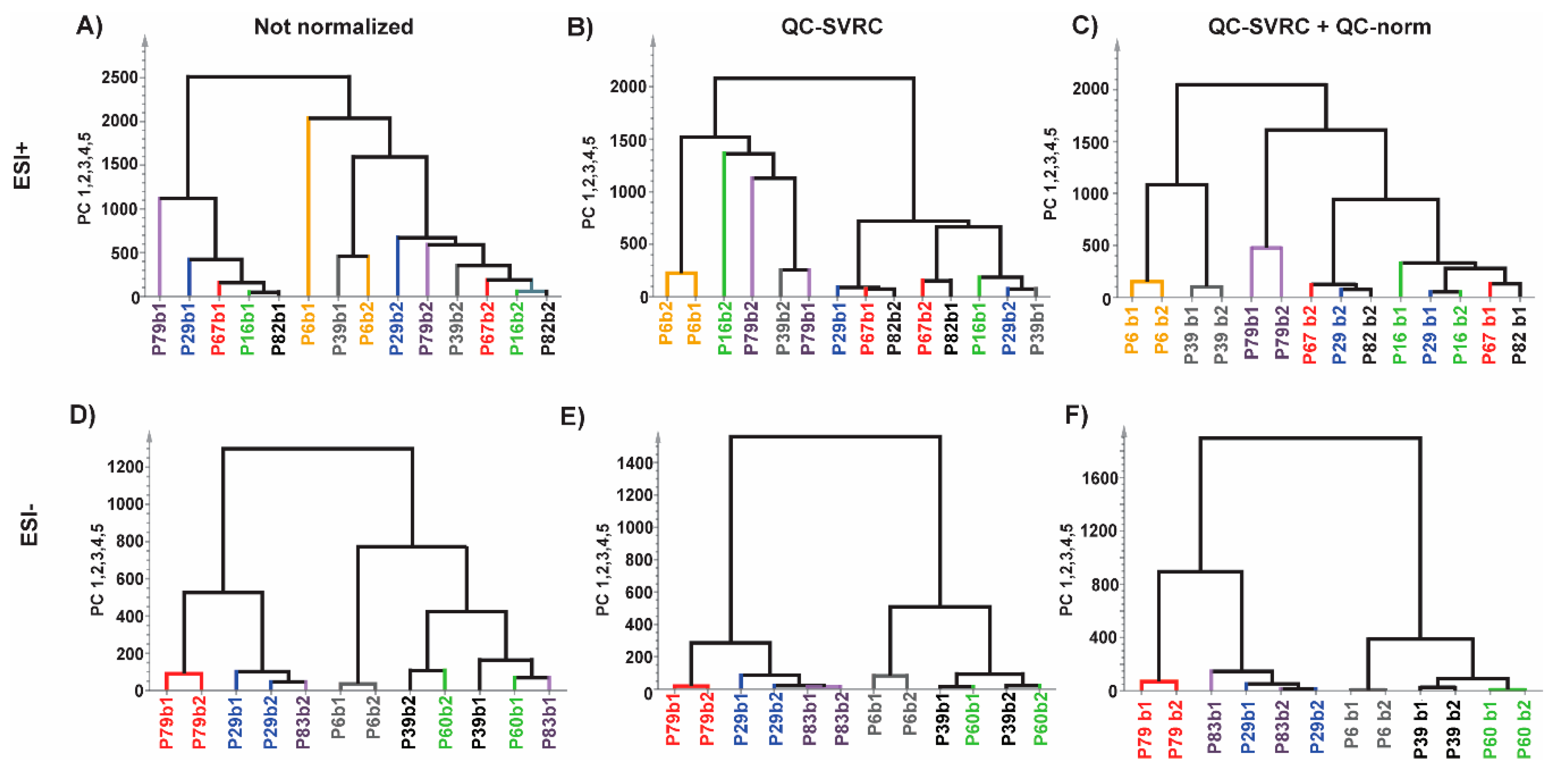
Figures S10 and S11, respectively). This last strategy has not been explored previously and proves the feasibility of the normalization strategy. After testing the reduction in distance of the replicates by the normalization we corroborate these findings by HCA where the clustered pairs increased after every normalization (intra and inter) for both modes (
Figure 7). Additionally, the extent of normalization was checked by exploring the outcome in one of the metabolites common to both modes. We detected that the normalization process only corrects the effects that are necessary as in ESI+ mode there was no visual change compared to ESI− mode. To sum up, these tests demonstrate the full integration and the high quality of the data from multiple batches. This would result in the further reliable comparison of the clinical groups of the study. This was depicted by the significant number of features increased after normalization between two clinical groups, which otherwise would lead to false negative results.
To conclude, this work illustrates a strategy to generate high-quality data from untargeted large-scale metabolomics studies. This strategy considers the stability of the equipment and the normalization of the data to assess a correct integration of the batches. We have addressed different issues that should be taken into consideration in these types of studies. Together, we have generated a guideline to integrate data for different batches that could be useful in any metabolomics study with more than a single batch.
Author Contributions
M.M.E. was the PI and together with D.B., A.V. designed and supervised the research. A.V., J.R.-C., M.D.-H., and D.O. performed the metabolomics analysis and data treatment. M.I.D.-D. performed data treatment and analysis of results. T.C. recruited the patients from the study. S.A. and G.Q. supervised data treatment and the statistical analysis. All authors contributed to the writing of the manuscript and have given approval to the final version of the manuscript.
Funding
This work was supported by ISCIII (PI15/02256, PI16/00249 and PI18/01467) cofounded by FEDER for the thematic network and co-operative research centers ARADyAL RD16/0006/0015, RD16/0006/009. D.O., M.I.D.-D. and J.R.-C. are supported by FPI-CEU predoctoral fellowships and A.V. is funded by a postdoctoral research fellowship from ARADyAL.
Acknowledgments
We would like to thank all institutions and hospitals involved: Centre for Metabolomics and Bioanalysis (CEMBIO, San Pablo CEU University, Madrid), Institute of Applied Molecular Medicine (IMMA, San Pablo CEU University, Madrid), Negrin Universitary Hospital (Las Palmas de G.C.). Authors also acknowledge Tomás Clive Barker-Tejeda, for his asserted comments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Puchades-Carrasco, L.; Pineda-Lucena, A. Metabolomics Applications in Precision Medicine: An Oncological Perspective. Curr. Top. Med. Chem. 2017, 17, 2740–2751. [Google Scholar] [CrossRef] [PubMed]
- Phelps, D.L.; Balog, J.; Gildea, L.F.; Bodai, Z.; Savage, A.; El-Bahrawy, M.A.; Speller, A.V.; Rosini, F.; Kudo, H.; McKenzie, J.S.; et al. The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Br. J. Cancer 2018, 118, 1349–1358. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Available online: http://ginasthma.org/gina-reports/ (accessed on 27 July 2019).
- Sánchez-Illana, Á.; Piñeiro-Ramos, J.D.; Sanjuan-Herráez, J.D.; Vento, M.; Quintás, G.; Kuligowski, J. Evaluation of batch effect elimination using quality control replicates in LC-MS metabolite profiling. Anal. Chim. Acta 2018, 1019, 38–48. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Pearce, J.T.; Spagou, K.; Green, M.; Dona, A.C.; Yuen, A.H.; David, M.; Berry, D.J.; Chappell, K.; Horneffer-van der Sluis, V.; et al. Development and Application of Ultra-Performance Liquid Chromatography-TOF MS for Precision Large Scale Urinary Metabolic Phenotyping. Anal. Chem. 2016, 88, 9004–9013. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- U.S. FOOD&DRUG ADMINISTRATION. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry (accessed on 27 June 2018).
- Redestig, H.; Fukushima, A.; Stenlund, H.; Moritz, T.; Arita, M.; Saito, K.; Kusano, M. Compensation for systematic cross-contribution improves normalization of mass spectrometry based metabolomics data. Anal. Chem. 2009, 81, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Sánchez-Illana, Á.; Sanjuán-Herráez, D.; Vento, M.; Quintás, G. Intra-batch effect correction in liquid chromatography-mass spectrometry using quality control samples and support vector regression (QC-SVRC). Analyst 2015, 140, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gong, X.; Cai, Y. Normalization and integration of large-scale metabolomics data using support vector regression. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Kuo, C.-H.; Tseng, Y.J. Batch Normalizer: A Fast Total Abundance Regression Calibration Method to Simultaneously Adjust Batch and Injection Order Effects in Liquid Chromatography/Time-of-Flight Mass Spectrometry-Based Metabolomics Data and Comparison with Current Calibration Met. Anal. Chem. 2013, 85, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Wilson, I.D.; Nicholls, A.W.; Broadhurst, D. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis 2012, 4, 2249–2264. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Barbas, C. Gas chromatography-mass spectrometry (GC-MS)-based metabolomics. Methods Mol. Biol. 2011, 708, 191–204. [Google Scholar] [PubMed]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; Garcia, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalvez, A.; Godzien, J.; Garcia, A.; Barbas, C. Capillary Electrophoresis Mass Spectrometry as a Tool for Untargeted Metabolomics. Methods Mol. Biol. 2019, 1978, 55–77. [Google Scholar] [PubMed]
- Ciborowski, M.; Javier Ruperez, F.; Martínez-Alcázar, M.P.; Angulo, S.; Radziwon, P.; Olszanski, R.; Kloczko, J.; Barbas, C. Metabolomic approach with LC-MS reveals significant effect of pressure on diver’s plasma. J. Proteome Res. 2010, 9, 4131–4137. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Zorawski, M.; Skotnicki, M.; Zarzycki, W.; Kozlowska, G.; Bibik-Malinowska, K.; Vallejo, M.; García, A.; Barbas, C.; Ramos, M.P. Metabolic fingerprint of gestational diabetes mellitus. J. Proteomics 2014, 103, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Haug, K.; Salek, R.M.; Conesa, P.; Hastings, J.; de Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P.; et al. MetaboLights—An open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2013, 41, D781–D786. [Google Scholar] [CrossRef] [PubMed]
- Gromski, P.; Xu, Y.; Kotze, H.; Correa, E.; Ellis, D.; Armitage, E.; Turner, M.; Goodacre, R. Influence of missing values substitutes on multivariate analysis of metabolomics data. Metabolites 2014, 4, 433–452. [Google Scholar] [CrossRef] [PubMed]
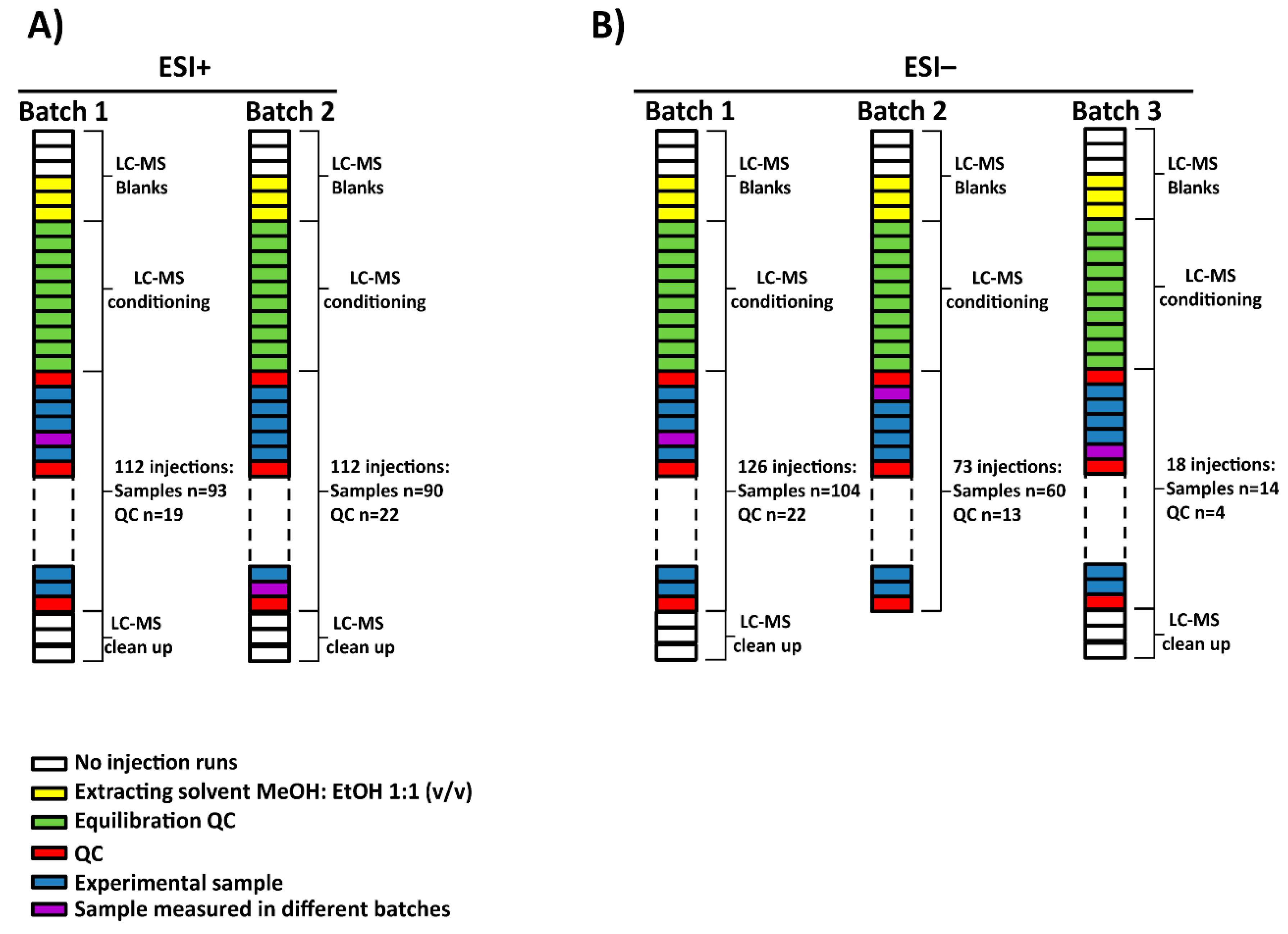
Figure 1.
Experimental worklists and batches followed for (A) ESI+ and (B) ESI− modes. (A) Experimental samples were measured into two batches for ESI+ while (B) in the case of ESI− mode, 3 batches were obtained due to an ‘instrument communication error’. NOTE. The source of the equipment was not cleaned up between batches 2 and 3 in ESI− mode.
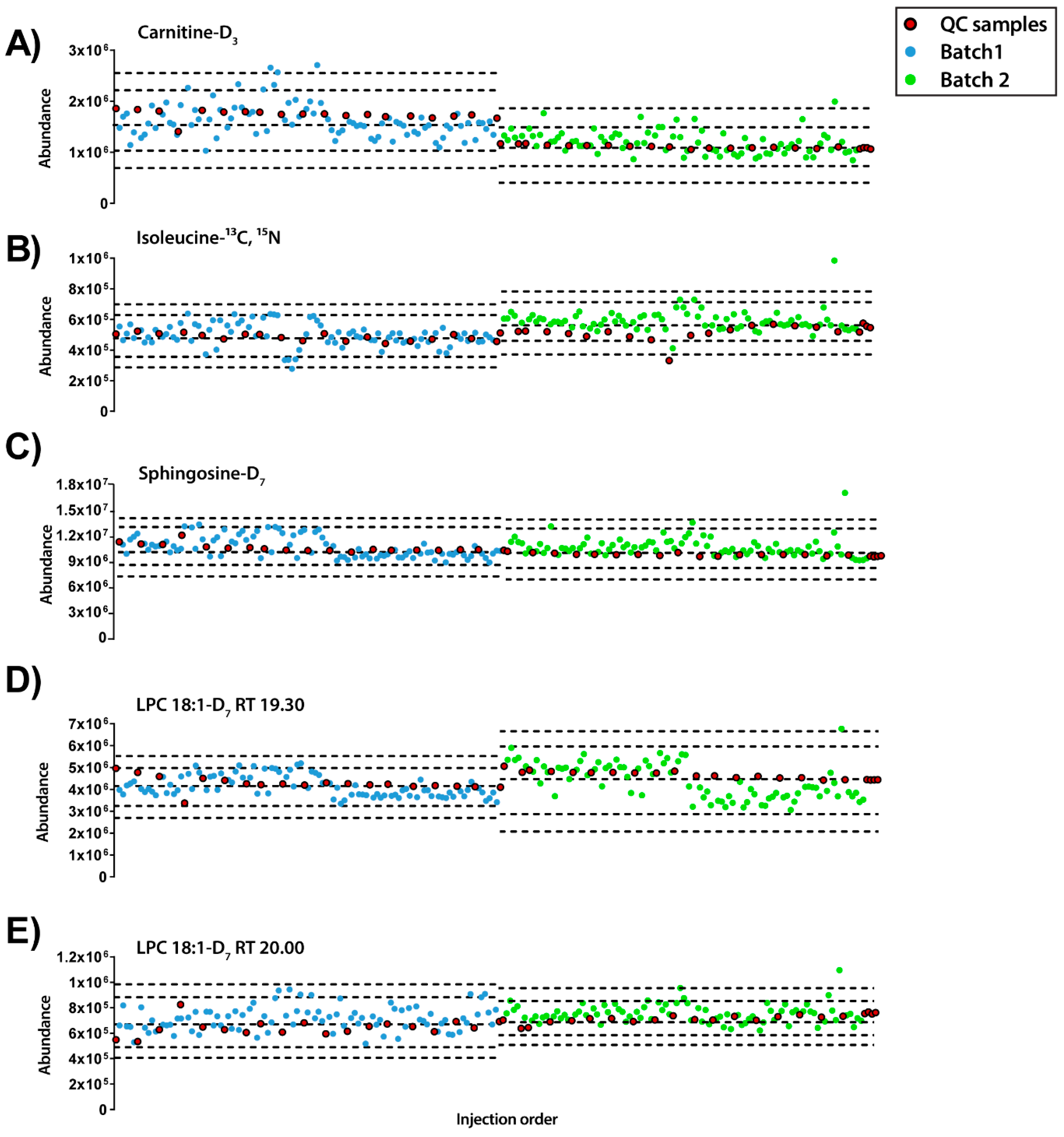
Figure 2.
Quality control charts of the abundance of IS compounds in each sample according to the injection order for ESI+ mode. (A) Carnitine-D3 RT 0.70min, RSD = 22.95%. (B) Isoleucine-13C, 15N RT 0.77 min, RSD = 8.40%. (C) Sphingosine-D7 RT 14.38 min, RSD = 5.15%. (D) LPC18:1-D7 RT 19.30 min, RSD = 7.04%. (E) LPC18:1-D7 RT 20.00 min, RSD = 8.82%. Legend. Y-axis: Abundance. X-axis: Sample order. Red circles: QCs; blue and green circles: Experimental samples measured in batch 1 and batch 2, respectively. Dotted lines represent the mean +/− 2 and 3 SD for each batch independently.
Figure 3.
Quality control charts of the abundance of IS compounds in each sample according to the injection order for ESI− mode. (A) Isoleucine-13C, 15N at RT 0.77 min, RSD =25.01%. Grey rectangle signals samples with low levels of IS mix. (B) LPC18:1-D7 RT 19.30 min, RSD = 23.93%. (C) LPC18:1-D7 RT 20.00 min, RSD = 21.22%. (D) Stearic acid-D5 RT 34.54 min, RSD = 30.03% Legend. Y-axis: Abundance. X-axis: Sample order. Red circles: QCs; blue and green circles: experimental samples measured in batch 1 and batch 2, respectively. Dotted lines represent the mean +/− 2 and 3 SD for each batch independently.
Figure 4.
Outcome of the data normalization strategy after QC-SVRC algorithm for both ESI+ and ESI− modes. (A,C) Quality control chart of ESI+ and ESI− modes. Samples with a TUS higher than 3 SD of the mean or lower than −3 SD were removed from PCA model. (B,D) PCA plots of ESI+ and ESI− mode, respectively Signals with %RSD < 30% on QCs were kept, and UV scaling was used. Features in ESI+ and ESI− modes, respectively: 1056 and 394. Legend. TUS plot: Blue and green dots: samples of batch1 and batch2, respectively, red dots: QCs. Dotted lines represent the mean +/− 2 and 3 SD for each batch independently. PCA: Blue dots and dark blue triangles are samples and QCs measured in batch1, respectively; green dots and dark green triangles are samples and QCs from batch2, respectively. Grey circle are samples with low levels of IS mix.
Figure 5.
Outcome of the data normalization strategy after QC-SVRC algorithm and QC-norm for both: ESI+ and ESI− modes. (A,C) Quality control chart of ESI+ and ESI− modes, respectively. Samples with a TUS higher than 3 SD of the mean or lower than −3 SD were removed from the PCA model. (B,D) PCA plots of ESI+ and ESI− mode, respectively. Signals with %RSD < 30% on QCs were kept, and UV scaling was used. Features in ESI+ and ESI− modes respectively: 880 and 525. Legend. TUS plot: Blue and green dots: samples of batch1 and batch2, respectively, red dots: QCs. Dotted lines represent the mean +/− 2 and 3 SD for each batch independently. PCA: Blue dots and dark blue triangles are samples and QCs measured in batch1, respectively; green dots and dark green triangles are samples and QCs from batch2, respectively. Grey circle signals samples with low levels of IS mix.
Figure 6.
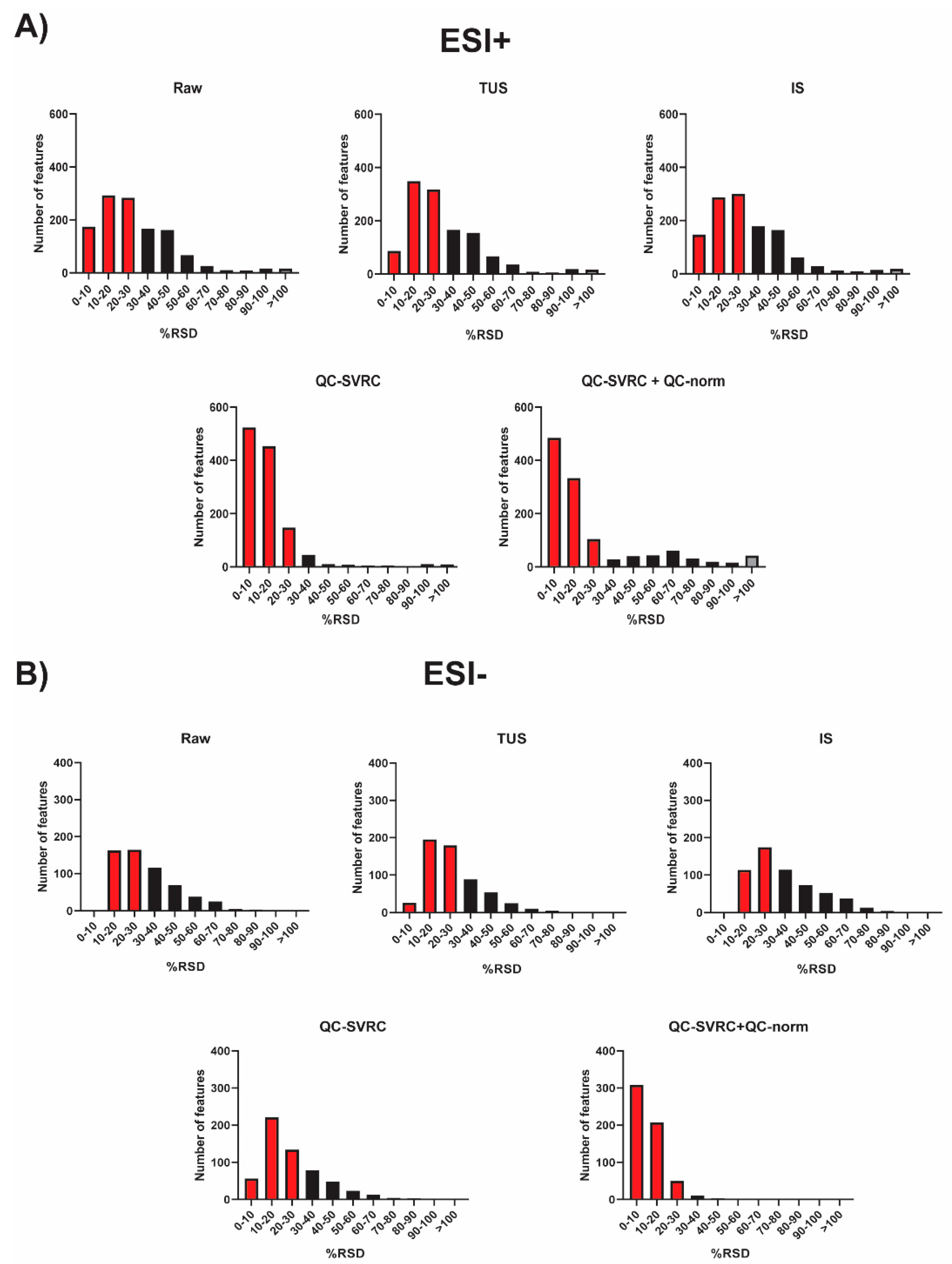
RSD distribution across QC samples from the complete dataset after each normalization method. (A) ESI+ mode and (B) ESI− mode. The red and grey bars indicate peaks that fall under RSD < 30% and RSD > 100%, respectively.
Figure 7.
Normalization impact evaluated by HCA test on repeated experimental samples for (A,D) raw data; (B,E) after normalization by QC-SVRC and; (C,F) QC-SVRC + QC-norm for both, ESI+ and ESI− modes, respectively. Legend. Every pair of samples represents one patient and is depicted in a different color for each polarity mode.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).