Metabolomic Analysis Reveals Unique Biochemical Signatures Associated with Protection from Radiation Induced Lung Injury by Lack of cd47 Receptor Gene Expression
Abstract
1. Introduction
2. Results
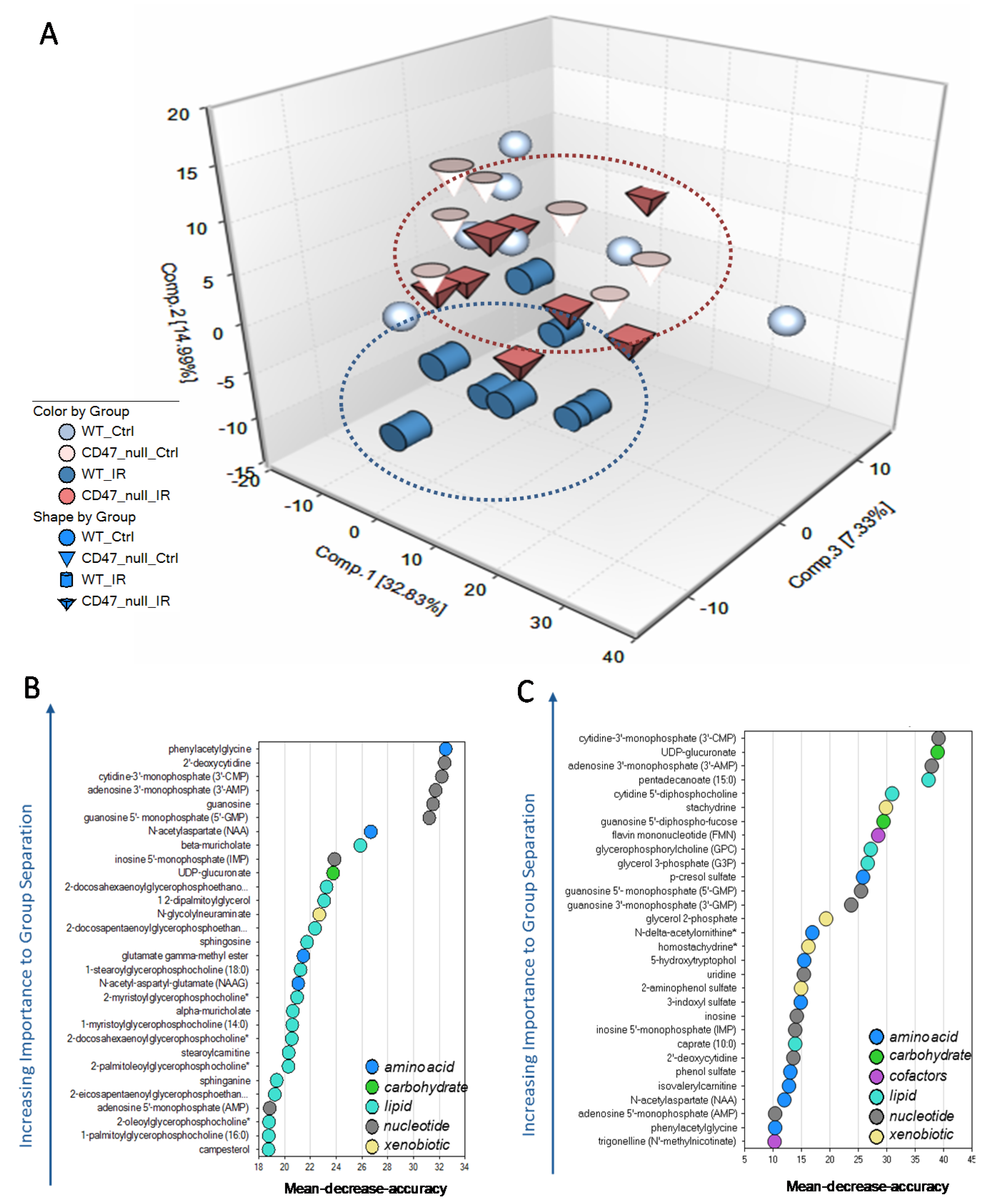
2.1. Unique Biochemical Signatures in the Absence of CD47 after Ionizing Radiation Treatment
2.2. CD47 Modulates Antioxidant Response after Ionizing Radiation
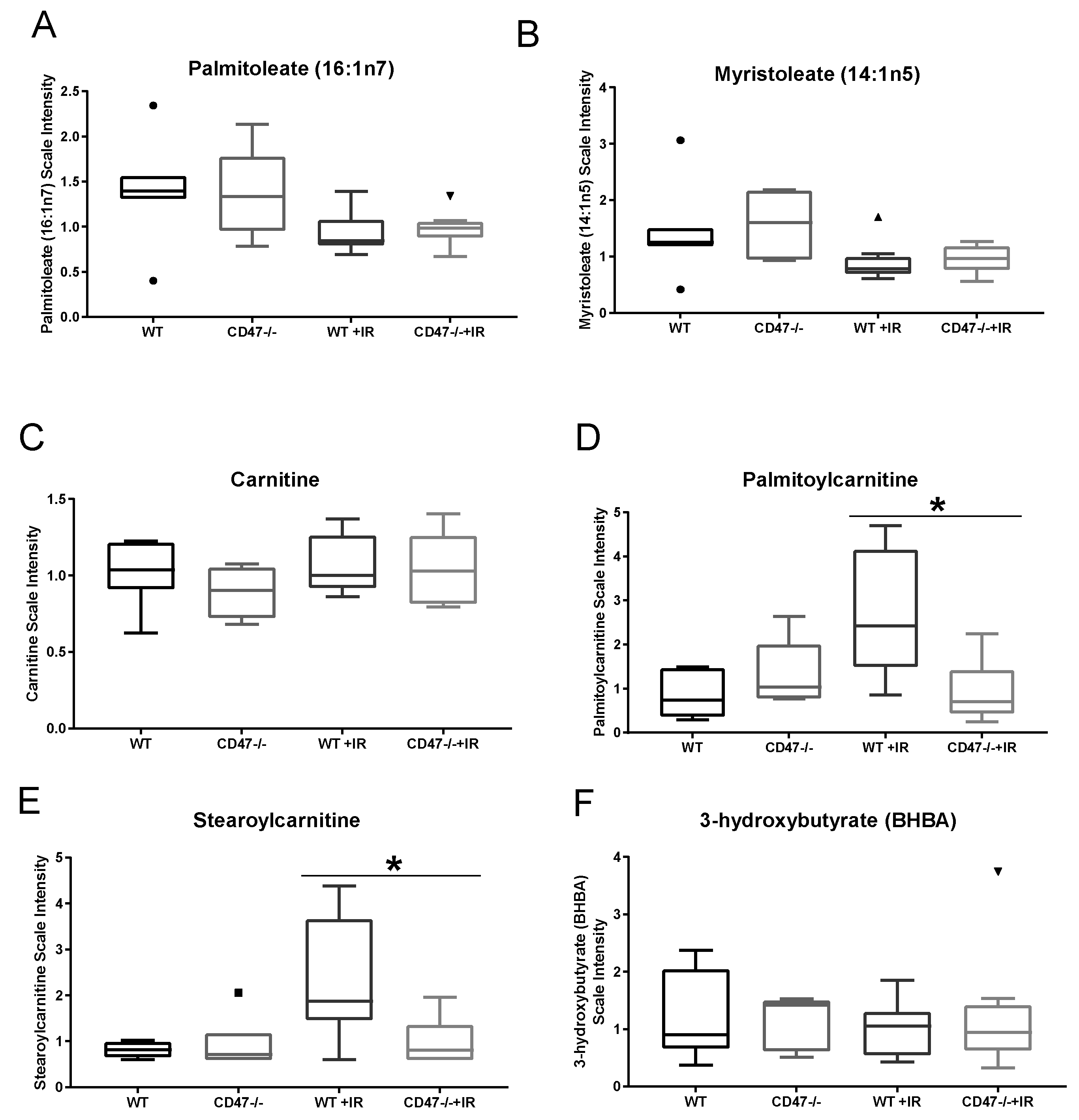
2.3. CD47 Regulates Lipid Metabolism as a Response to Ionizing Radiation
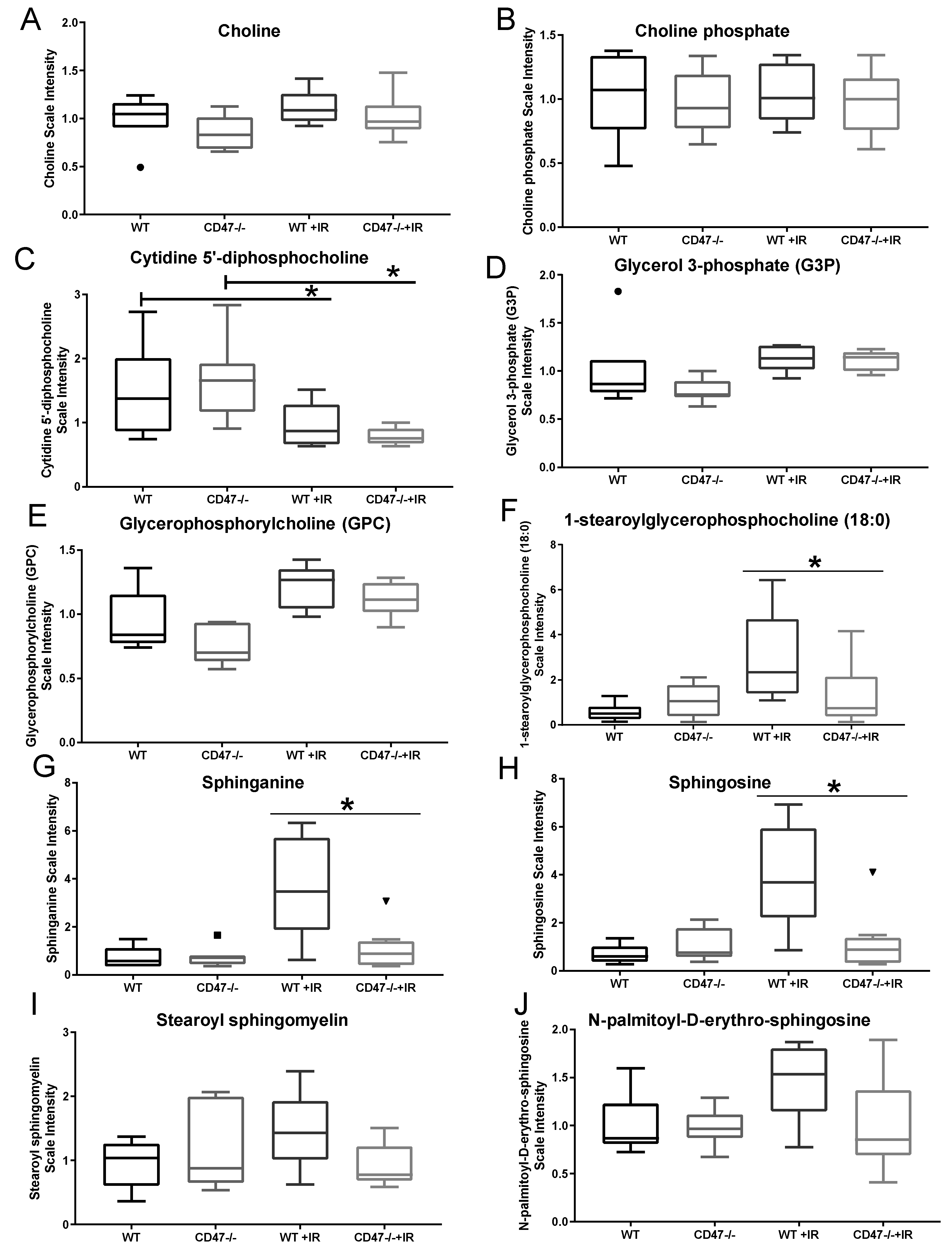
2.4. Differential Regulation of Cell Membrane Phospholipids after Ionizing Radiation
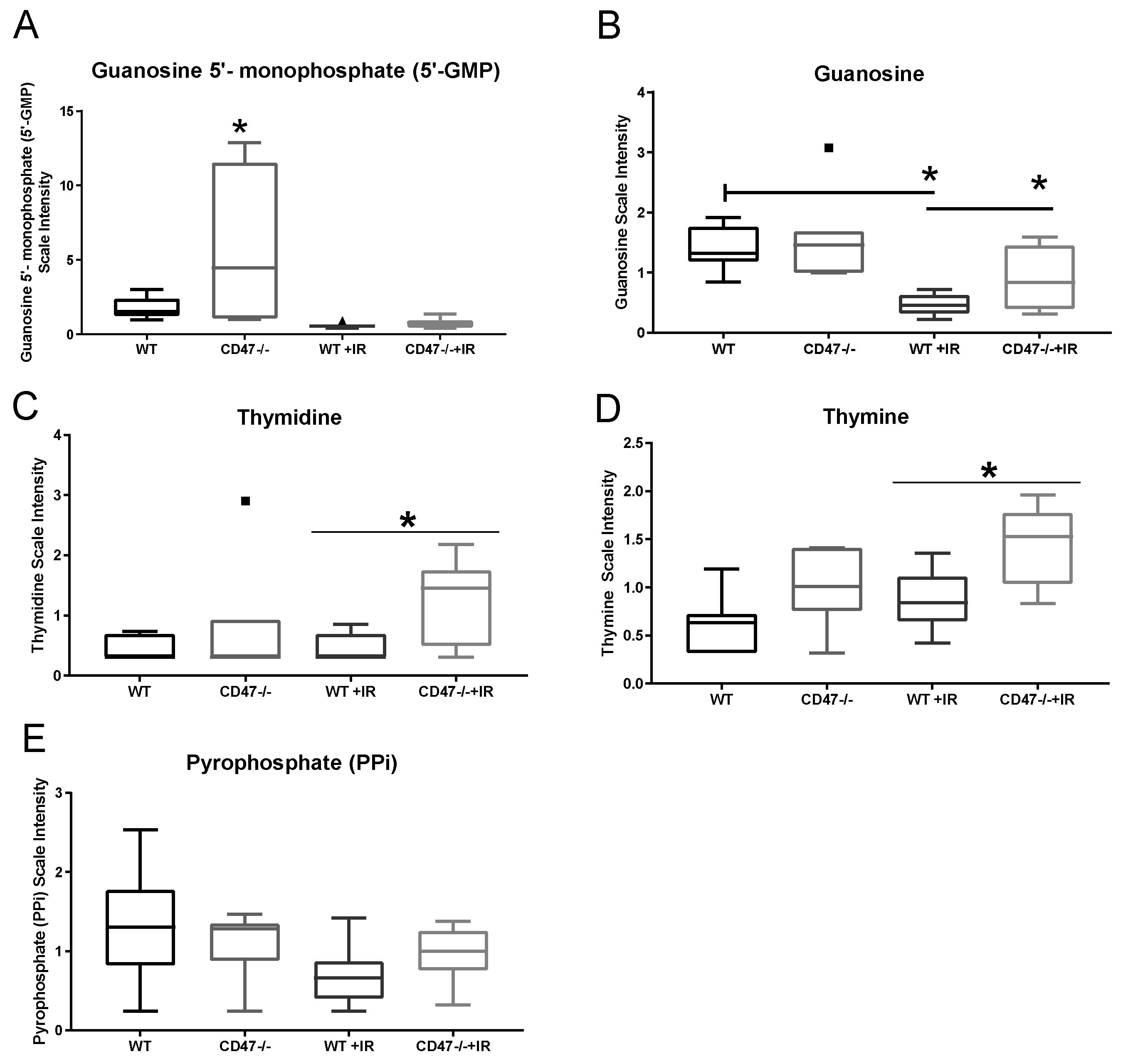
2.5. Absence of CD47 Preserves Nucleotide Metabolism after Ionizing Radiation
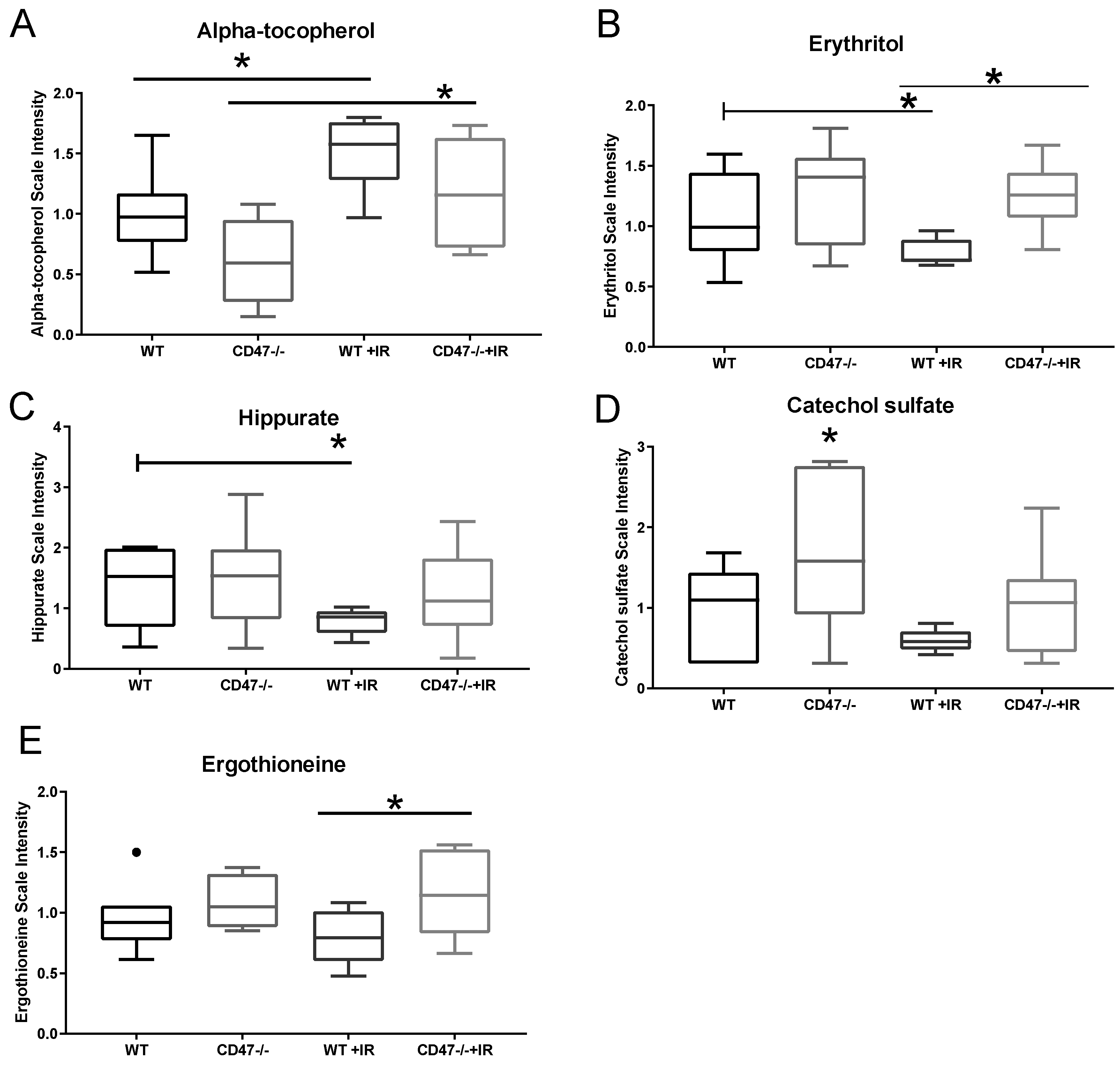
2.6. Differential Regulation of Nutrient Processing after Ionizing Radiation
3. Discussion
4. Materials and Methods
4.1. Mouse Irradiation
4.2. Metabolite Analysis
4.3. Liquid Chromatography/Mass Spectrometry (LC/MS, LC/MS2)
4.4. Gas Chromatography/Mass Spectrometry (GC/MS)
4.5. Bioinformatics
4.6. Statistical Calculation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pannkuk, E.L.; Laiakis, E.C.; Girgis, M.; Dowd, S.E.; Dhungana, S.; Nishita, D.; Bujold, K.; Bakke, J.; Gahagen, J.; Authier, S.; et al. Temporal Effects on Radiation Responses in Nonhuman Primates: Identification of Biofluid Small Molecule Signatures by Gas Chromatography(-)Mass Spectrometry Metabolomics. Metabolites 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Fatanmi, O.O.; Beattie, L.A.; Ducey, E.J.; Seed, T.M. Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation combined injury in mice. J. Radiat. Res. 2014, 55, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Down, J.D.; Yanch, J.C. Identifying the high radiosensitivity of the lungs of C57L mice in a model of total-body irradiation and bone marrow transplantation. Radiat. Res. 2010, 174, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Xu, P.; Hadley, C.; Katz, B.P.; McGurk, R.; Down, J.D.; Vujaskovic, Z. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 2012, 103, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Williams, J.P.; Hernady, E.; Reed, C.; Fenton, B.; Love, T.; Finkelstein, J.N.; Johnston, C.J. A Potential Biomarker for Predicting the Risk of Radiation-Induced Fibrosis in the Lung. Radiat. Res. 2018, 190, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Maxhimer, J.B.; Soto-Pantoja, D.R.; Ridnour, L.A.; Shih, H.B.; Degraff, W.G.; Tsokos, M.; Wink, D.A.; Isenberg, J.S.; Roberts, D.D. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci. Transl. Med. 2009, 1, 3ra7. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Stein, E.V.; Rogers, N.M.; Sharifi-Sanjani, M.; Isenberg, J.S.; Roberts, D.D. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin. Ther. Targets 2013, 17, 89–103. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Terabe, M.; Ghosh, A.; Ridnour, L.A.; DeGraff, W.G.; Wink, D.A.; Berzofsky, J.A.; Roberts, D.D. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014, 74, 6771–6783. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Ridnour, L.A.; Wink, D.A.; Roberts, D.D. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci. Rep. 2013, 3, 1038. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Isenberg, J.S.; Roberts, D.D. Therapeutic Targeting of CD47 to Modulate Tissue Responses to Ischemia and Radiation. J. Genet. Syndr. Gene Ther. 2011, 2, 1000105. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Miller, T.W.; Pendrak, M.L.; DeGraff, W.G.; Sullivan, C.; Ridnour, L.A.; Abu-Asab, M.; Wink, D.A.; Tsokos, M.; Roberts, D.D. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy 2012, 8, 1628–1642. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Soto-Pantoja, D.R.; Schwartz, A.L.; Sipes, J.M.; DeGraff, W.G.; Ridnour, L.A.; Wink, D.A.; Roberts, D.D. CD47 Receptor Globally Regulates Metabolic Pathways That Control Resistance to Ionizing Radiation. J. Biol. Chem. 2015, 290, 24858–24874. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, A.L.; Jackson, I.L.; Shah, J.R.; Czarniecki, C.W.; Maidment, B.W.; Williams, J.P. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiat. Res. 2012, 177, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Li, H.; Eichler, G.S.; Krausz, K.W.; Weinstein, J.N.; Fornace, A.J., Jr.; Gonzalez, F.J.; Idle, J.R. UPLC-ESI-TOFMS-based metabolomics and gene expression dynamics inspector self-organizing metabolomic maps as tools for understanding the cellular response to ionizing radiation. Anal. Chem. 2008, 80, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.L.; Clark, J.R.; Green, S.I.; Maresso, A.W. A dual component heme biosensor that integrates heme transport and synthesis in bacteria. J. Microbiol. Methods 2015, 118, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, V.A.; Rabender, C.S.; Sankala, H.; Gauter-Fleckenstein, B.; Fleckenstein, K.; Batinic-Haberle, I.; Jackson, I.; Vujaskovic, Z.; Anscher, M.S.; Mikkelsen, R.B.; et al. Proteomic analysis of radiation-induced changes in rat lung: Modulation by the superoxide dismutase mimetic MnTE-2-PyP(5+). Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Tyurina, Y.Y.; Tyurin, V.A.; Epperly, M.W.; Greenberger, J.S.; Kagan, V.E. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic. Biol. Med. 2008, 44, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Dizdaroglu, M. Clemens von Sonntag and the early history of radiation-induced sugar damage in DNA. Int. J. Radiat. Biol. 2014, 90, 446–458. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of sodium benzoate, a widely used food preservative, on glucose homeostasis and metabolic profiles in humans. Mol. Genet. Metab. 2015, 114, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Balestrieri, M.L. Ergothioneine Antioxidant Function: From Chemistry to Cardiovascular Therapeutic Potential. J. Cardiovasc. Pharmacol. 2017, 69, 183–191. [Google Scholar] [CrossRef] [PubMed]
- White, E. Role of the metabolic stress responses of apoptosis and autophagy in tumor suppression. In Oncogenes Meet Metabolism; Ernst Schering Foundation Symposium Proceedings; Springer: Berlin/Heidelberg, Germany, 2008; Volume 2007, pp. 23–34. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Frazier, W.A.; Krishna, M.C.; Wink, D.A.; Roberts, D.D. Enhancing cardiovascular dynamics by inhibition of thrombospondin-1/CD47 signaling. Curr. Drug Targets 2008, 9, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Romeo, M.J.; Abu-Asab, M.; Tsokos, M.; Oldenborg, A.; Pappan, L.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Increasing survival of ischemic tissue by targeting CD47. Circ. Res. 2007, 100, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Romeo, M.J.; Maxhimer, J.B.; Smedley, J.; Frazier, W.A.; Roberts, D.D. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: Implications for human disease. Ann. Surg. 2008, 247, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Shiva, S.; Gladwin, M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide 2009, 21, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.M.; Yao, M.; Novelli, E.M.; Thomson, A.W.; Roberts, D.D.; Isenberg, J.S. Activated CD47 regulates multiple vascular and stress responses: Implications for acute kidney injury and its management. Am. J. Physiol. Renal Physiol. 2012, 303, F1117–F1125. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Maimaitiyiming, H.; Norman, H.; Zhou, Q.; Wang, S. CD47 deficiency protects mice from diet-induced obesity and improves whole body glucose tolerance and insulin sensitivity. Sci. Rep. 2015, 5, 8846. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Jia, Y.; Fukuyama, J.; Switzer, C.H.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J. Biol. Chem. 2007, 282, 15404–15415. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, L.; Azzam, R.; Nemer, G.; Bielawski, J.; Nasser, M.; Bitar, F.; Dbaibo, G.S. Modulation of total ceramide and constituent ceramide species in the acutely and chronically hypoxic mouse heart at different ages. Prostaglandins Other Lipid Mediat. 2008, 86, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Jacobson, J.R.; Berdyshev, E.; Huang, Y.; Sun, X.; Zhao, Y.; Gerhold, L.M.; Siegler, J.; Evenoski, C.; Wang, T.; et al. Role of sphingolipids in murine radiation-induced lung injury: Protection by sphingosine 1-phosphate analogs. FASEB J. 2011, 25, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, I.; Zhou, T.; Mathew, B.; Jacobson, J.R.; Takekoshi, D.; Bhattacharya, P.; Smith, B.; Aydogan, B.; Weichselbaum, R.R.; Natarajan, V.; et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J. Lipid Res. 2012, 53, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yu, N.; Zhang, Z.; Zhang, P.; Yang, Y.; Wu, N.; Xu, L.; Zhang, J.; Ge, J.; Yu, K.; et al. Thrombospondin-1 might be a therapeutic target to suppress RB cells by regulating the DNA double-strand breaks repair. Oncotarget 2016, 7, 6105–6120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef]
- Singh, V.K.; Brown, D.S.; Kao, T.C. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int. J. Radiat. Biol. 2010, 86, 12–21. [Google Scholar] [CrossRef]
- Gatley, S.J.; Sherratt, S.A. The localization of hippurate synthesis in the matrix of rat liver mitochondria. Biochem. Soc. Trans. 1976, 4, 525–526. [Google Scholar] [CrossRef]
- Frazier, E.P.; Isenberg, J.S.; Shiva, S.; Zhao, L.; Schlesinger, P.; Dimitry, J.; Abu-Asab, M.S.; Tsokos, M.; Roberts, D.D.; Frazier, W.A. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011, 30, 154–161. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, Y.; Jiang, C.Y.; Liu, S.J. Benzoate metabolism intermediate benzoyl coenzyme A affects gentisate pathway regulation in Comamonas testosteroni. Appl. Environ. Microbiol. 2014, 80, 4051–4062. [Google Scholar] [CrossRef]
- Williams, J.P. Assessment of radiation-induced lung disease. Clin. Adv. Hematol. Oncol. 2011, 9, 160–162. [Google Scholar] [PubMed]
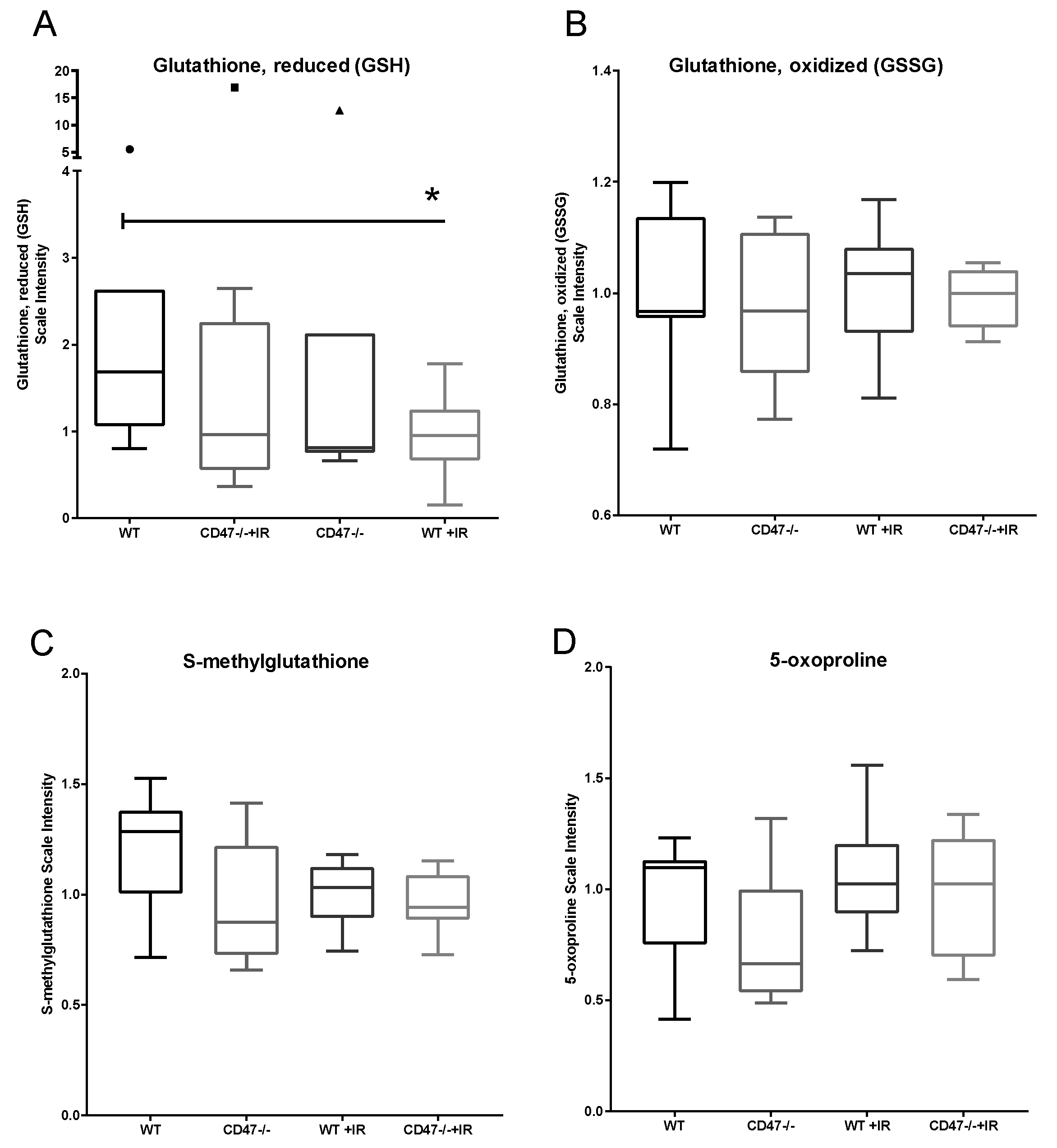
| Glutathione, Reduced (GSH) | Glutathione, Oxidized (GSSG) | GSH:GSSG | |||
|---|---|---|---|---|---|
| Treatment | Average SEM | Treatment | Average SEM | Treatment | Ratio |
| WT | 2.138 ± 0.6102 | WT | 1.007 ± 0.06007 | WT | 2.12 |
| cd47-/- | 2.830 ± 1.666 | cd47-/- | 0.9818 ± 0.05081 | cd47-/- | 2.88 |
| WT + IR | 0.9412 ± 0.1702 | WT + IR | 1.009 ± 0.03887 | WT + IR | 0.93 |
| cd47-/- + IR | 3.025 ± 2.002 | cd47-/- + IR | 0.9909 ± 0.01972 | cd47-/- + IR | 3.05 |
| Lysolipid Metabolites | ANOVA Contrasts | |||
|---|---|---|---|---|
| Fold of Change | ||||
| IR CTRL | CD47-/- WT | |||
| WT | CD47-/- | CTRL | IR | |
| 1-myristoylglycerophosphocholine (14:0) | 4.01 | 1.35 | 1.23 | 0.41 |
| 2-myristoylglycerophosphocholine | 3.99 | 1.09 | 1.29 | 0.35 |
| 1-palmitoylglycerophosphocholine (16:0) | 3.68 | 1.08 | 1.38 | 0.41 |
| 2-palmitoylglycerophosphocholine | 5.29 | 1.12 | 1.57 | 0.33 |
| 1-palmitoleoylglycerophosphocholine (16:1) | 3.86 | 1.18 | 1.21 | 0.37 |
| 2-palmitoleoylglycerophosphocholine | 4.29 | 1.17 | 1.30 | 0.35 |
| 1-stearoylglycerophosphocholine (18:0) | 5.35 | 1.21 | 1.85 | 0.42 |
| 2-stearoylglycerophosphocholine | 4.65 | 1.61 | 1.25 | 0.43 |
| 1-oleoylglycerophosphocholine (18:1) | 4.08 | 1.02 | 1.52 | 0.38 |
| 2-oleoylglycerophosphocholine | 4.66 | 1.10 | 1.31 | 0.31 |
| 1-linoleoylglycerophosphocholine (18:2n6) | 4.19 | 1.13 | 1.43 | 0.39 |
| 2-linoleoylglycerophosphocholine | 4.05 | 1.11 | 1.39 | 0.38 |
| 1-eicosatrienoylglycerophosphocholine (20:3) | 2.28 | 0.94 | 1.10 | 0.45 |
| 1-arachidonoylglycerophosphocholine (20:4n6) | 6.11 | 0.93 | 1.59 | 0.24 |
| 2-arachidonoylglycerophosphocholine | 4.22 | 1.40 | 1.21 | 0.40 |
| 1-docosapentaenoylglycerophosphocholine (22:5n3) | 3.31 | 1.57 | 0.84 | 0.40 |
| 2-docosapentaenoylglycerophosphocholine (22:5n3) | 4.94 | 1.27 | 1.28 | 0.33 |
| 1-docosahexaenoylglycerophosphocholine (22:6n3) | 3.96 | 1.28 | 1.29 | 0.42 |
| 2-docosahexaenoylglycerophosphocholine | 5.23 | 1.34 | 1.45 | 0.37 |
| 1-palmitoylplasmenylethanolamine | 1.57 | 1.21 | 0.84 | 0.64 |
| 1-stearoylplasmenylethanolamine | 0.94 | 1.29 | 0.80 | 1.10 |
| 1-oleoylplasmenylethanolamine | 1.60 | 1.16 | 0.95 | 0.69 |
| 1-palmitoylglycerophosphoethanolamine | 1.34 | 1.14 | 0.82 | 0.70 |
| 2-palmitoylglycerophosphoethanolamine | 4.49 | 1.05 | 1.63 | 0.38 |
| 1-stearoylglycerophosphoethanolamine | 1.80 | 1.15 | 0.97 | 0.62 |
| 1-oleoylglycerophosphoethanolamine | 1.33 | 0.93 | 0.97 | 0.68 |
| 2-oleoylglycerophosphoethanolamine | 1.40 | 1.08 | 1.01 | 0.78 |
| 1-linoleoylglycerophosphoethanolamine | 1.22 | 0.91 | 1.05 | 0.78 |
| 2-linoleoylglycerophosphoethanolamine | 3.05 | 1.01 | 1.39 | 0.46 |
| 1-arachidonoylglycerophosphoethanolamine | 1.22 | 1.04 | 1.07 | 0.91 |
| 2-arachidonoylglycerophosphoethanolamine | 3.22 | 1.09 | 1.33 | 0.45 |
| 2-docosapentaenoylglycerophosphoethanolamine | 7.07 | 1.09 | 1.79 | 0.28 |
| 2-docosahexaenoylglycerophosphoethanolamine* | 4.08 | 1.11 | 1.60 | 0.43 |
| 1-eicosatrienoylglycerophosphoethanolamine | 1.35 | 1.04 | 0.97 | 0.75 |
| 2-eicosapentaenoylglycerophosphoethanolamine | 4.87 | 1.04 | 1.50 | 0.32 |
| 1-docosahexaenoylglycerophosphoethanolamine | 1.29 | 1.18 | 1.02 | 0.93 |
| 1-eicosenoylglycerophosphoethanolamine (20:1n9) | 1.42 | 0.97 | 1.09 | 0.74 |
| 1-palmitoylglycerophosphoinositol | 1.05 | 0.98 | 0.92 | 0.87 |
| 1-stearoylglycerophosphoinositol | 1.20 | 1.15 | 0.88 | 0.84 |
| 2-stearoylglycerophosphoinositol | 1.55 | 1.04 | 1.11 | 0.75 |
| 1-oleoylglycerophosphoinositol | 0.94 | 0.82 | 1.03 | 0.89 |
| 1-arachidonoylglycerophosphoinositol | 0.96 | 0.96 | 0.87 | 0.86 |
| 2-arachidonoylglycerophosphoinositol | 1.04 | 1.02 | 0.90 | 0.88 |
| 1-stearoylglycerophosphoserine | 1.16 | 1.14 | 0.83 | 0.82 |
| 1-oleoylglycerophosphoserine | 1.10 | 0.93 | 0.90 | 0.76 |
| 2-oleoylglycerophosphoserine | 1.18 | 1.24 | 0.70 | 0.74 |
| 1-palmitoylglycerophosphoglycerol | 1.08 | 1.26 | 0.70 | 0.82 |
| 2-palmitoylglycerophosphoglycerol | 1.12 | 1.17 | 0.91 | 0.95 |
| 1-stearoylglycerophosphoglycerol | 1.15 | 1.48 | 0.66 | 0.85 |
| 2-stearoylglycerophosphoglycerol | 1.07 | 1.56 | 0.64 | 0.93 |
| 1-oleoylglycerophosphoglycerol | 1.14 | 1.14 | 0.83 | 0.83 |
| 2-oleoylglycerophosphoglycerol | 1.38 | 1.49 | 0.70 | 0.76 |
| IR CTRL | CD47-/- WT | ||||
|---|---|---|---|---|---|
| Metabolite | WT | CD47 -/- | CTRL | IR | |
| Purine Metabolism, (Hypo)Xanthine/Inosine containing | inosine 5′-monophosphate (IMP) | 0.11 | 0.08 | 2.80 | 2.09 |
| inosine | 0.72 | 0.84 | 1.00 | 1.17 | |
| hypoxanthine | 0.99 | 1.07 | 0.91 | 0.98 | |
| xanthine | 1.07 | 1.12 | 0.92 | 0.96 | |
| xanthosine | 1.20 | 1.12 | 1.07 | 1.00 | |
| urate | 1.11 | 1.26 | 0.86 | 0.97 | |
| allantoin | 0.82 | 0.79 | 1.08 | 1.05 | |
| Purine Metabolism, Adenine containing | adenosine 5′-diphosphate (ADP) | 1.05 | 0.89 | 1.20 | 1.02 |
| adenosine 5′-monophosphate (AMP) | 0.69 | 0.46 | 1.63 | 1.09 | |
| adenosine 3′-monophosphate (3′-AMP) | 0.45 | 0.50 | 1.00 | 1.12 | |
| adenosine 2′-monophosphate (2′-AMP) | 1.19 | 1.46 | 0.72 | 0.89 | |
| adenosine 3′,5′-cyclic monophosphate (cAMP) | 0.91 | 1.10 | 0.93 | 1.13 | |
| adenosine 3′,5′-diphosphate | 0.99 | 1.24 | 0.88 | 1.11 | |
| adenosine | 1.10 | 0.95 | 1.25 | 1.07 | |
| adenine | 0.89 | 1.13 | 0.75 | 0.95 | |
| N1-methyladenosine | 1.02 | 0.96 | 1.04 | 0.98 | |
| Purine Metabolism, Guanine containing | guanosine 5′- monophosphate (5′-GMP) | 0.32 | 0.15 | 3.01 | 1.37 |
| guanosine 3′-monophosphate (3′-GMP) | 0.71 | 0.73 | 0.97 | 1.00 | |
| guanosine | 0.33 | 0.58 | 1.13 | 1.99 | |
| Pyrimidine Metabolism, Uracil containing | uridine monophosphate (5′ or 3′) | 0.60 | 0.51 | 1.80 | 1.52 |
| uridine | 1.12 | 1.15 | 1.03 | 1.05 | |
| uracil | 1.10 | 1.29 | 0.80 | 0.94 | |
| pseudouridine | 0.93 | 0.83 | 1.14 | 1.01 | |
| 3-ureidopropionate | 1.18 | 1.08 | 1.03 | 0.94 | |
| beta-alanine | 0.91 | 0.90 | 1.13 | 1.12 | |
| Pyrimidine Metabolism, Cytidine containing | cytidine 5′-monophosphate (5′-CMP) | 0.88 | 0.99 | 0.84 | 0.94 |
| cytidine-3′-monophosphate (3′-CMP) | 0.18 | 0.22 | 0.94 | 1.15 | |
| cytidine | 1.08 | 1.07 | 0.90 | 0.89 | |
| 2′-deoxycytidine | 2.11 | 1.35 | 1.11 | 0.71 | |
| Pyrimidine Metabolism, Thymine containing | thymidine | 1.04 | 1.53 | 1.98 | 2.92 |
| thymine | 1.37 | 1.48 | 1.52 | 1.64 | |
| Purine and Pyrimidine Metabolism | methylphosphate | 0.87 | 1.15 | 0.76 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stirling, E.R.; Cook, K.L.; Roberts, D.D.; Soto-Pantoja, D.R. Metabolomic Analysis Reveals Unique Biochemical Signatures Associated with Protection from Radiation Induced Lung Injury by Lack of cd47 Receptor Gene Expression. Metabolites 2019, 9, 218. https://doi.org/10.3390/metabo9100218
Stirling ER, Cook KL, Roberts DD, Soto-Pantoja DR. Metabolomic Analysis Reveals Unique Biochemical Signatures Associated with Protection from Radiation Induced Lung Injury by Lack of cd47 Receptor Gene Expression. Metabolites. 2019; 9(10):218. https://doi.org/10.3390/metabo9100218
Chicago/Turabian StyleStirling, Elizabeth R., Katherine L. Cook, David D. Roberts, and David R. Soto-Pantoja. 2019. "Metabolomic Analysis Reveals Unique Biochemical Signatures Associated with Protection from Radiation Induced Lung Injury by Lack of cd47 Receptor Gene Expression" Metabolites 9, no. 10: 218. https://doi.org/10.3390/metabo9100218
APA StyleStirling, E. R., Cook, K. L., Roberts, D. D., & Soto-Pantoja, D. R. (2019). Metabolomic Analysis Reveals Unique Biochemical Signatures Associated with Protection from Radiation Induced Lung Injury by Lack of cd47 Receptor Gene Expression. Metabolites, 9(10), 218. https://doi.org/10.3390/metabo9100218







