Extending the Metabolite Diversity of the Endophyte Dimorphosporicola tragani
Abstract
1. Introduction
2. Results
2.1. Strain Characterization
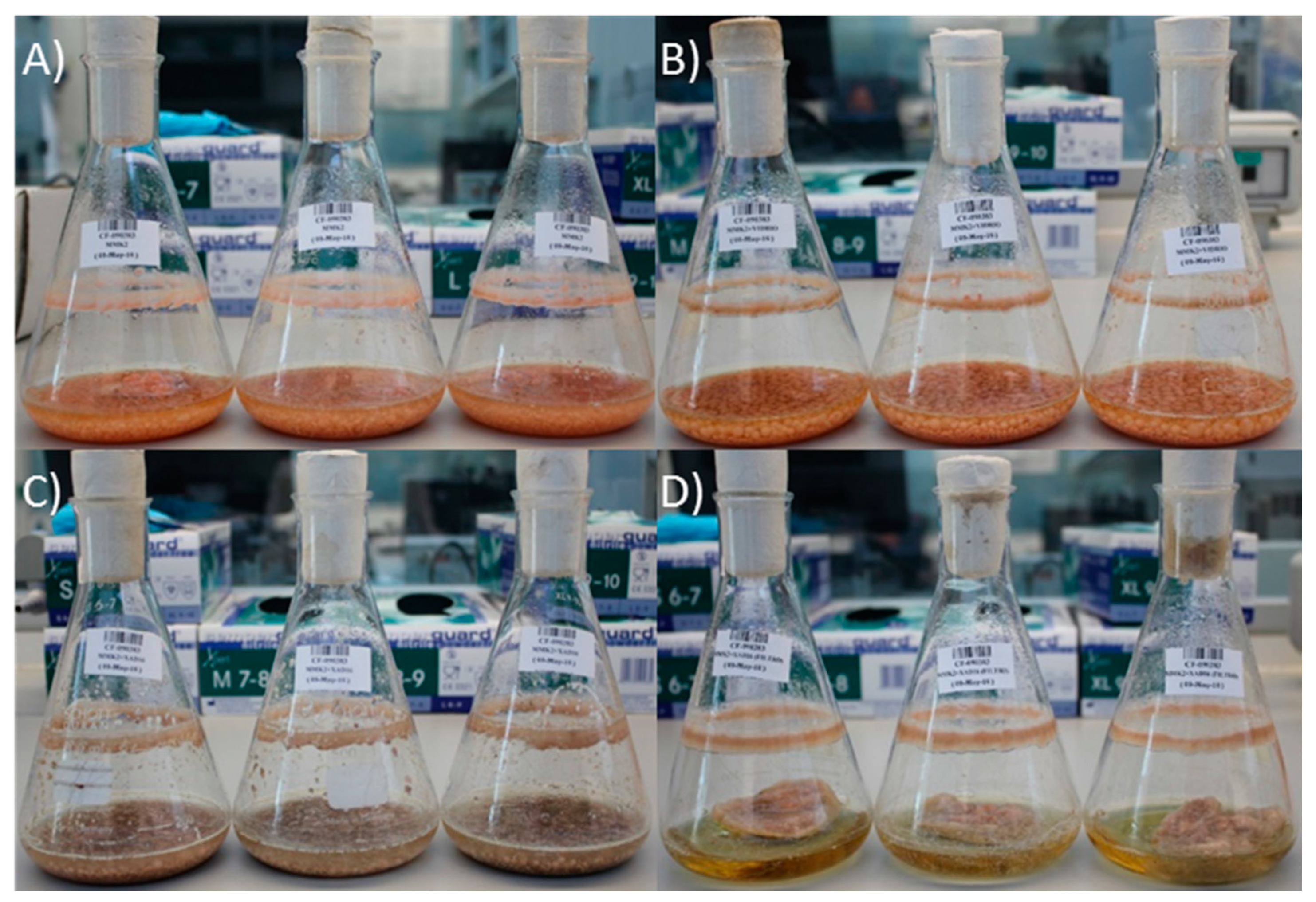
2.2. Effect of Nutritional Conditions and Adsoprtive Polymeric Resin Addition
2.3. Chemical Comparison and Dereplication of Dimorphosporicola tragani Fermentation Conditions
2.4. Effect of Different Epigenetic Treatments on the Dimorphosporicola tragani Bioactivity Profile
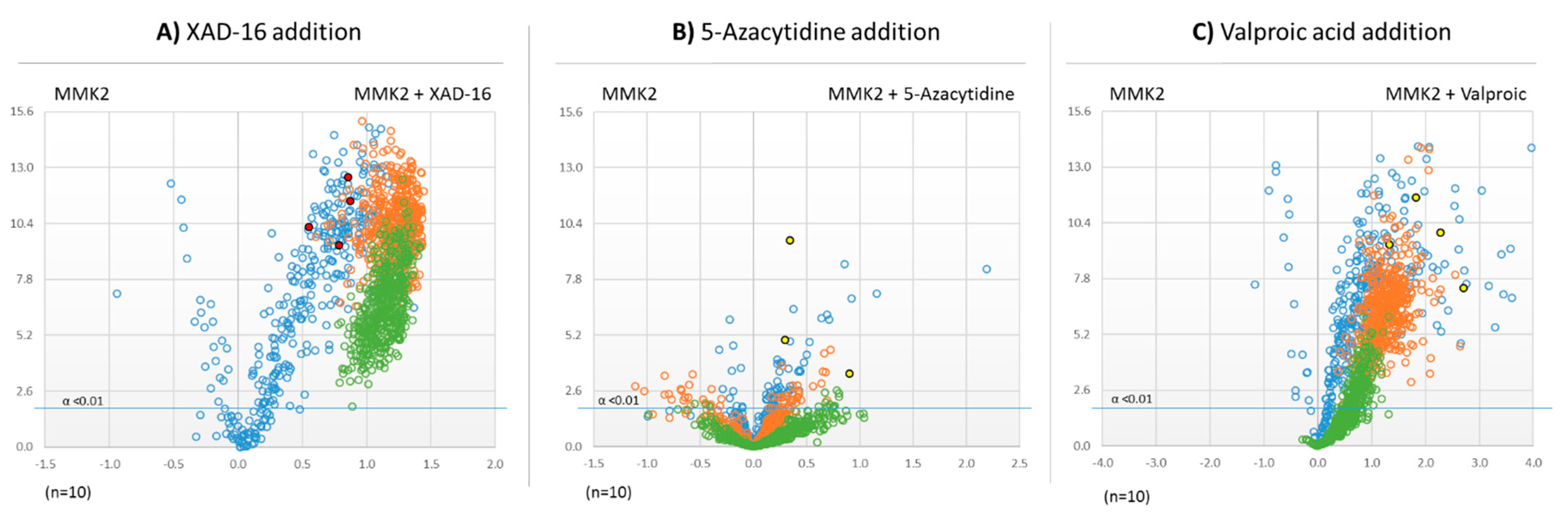
2.5. Untargeted Metabolomic Evaluation of the Differential Bioactive Secondary Metabolite (SM) Profiles of Dimorphosporicola tragani
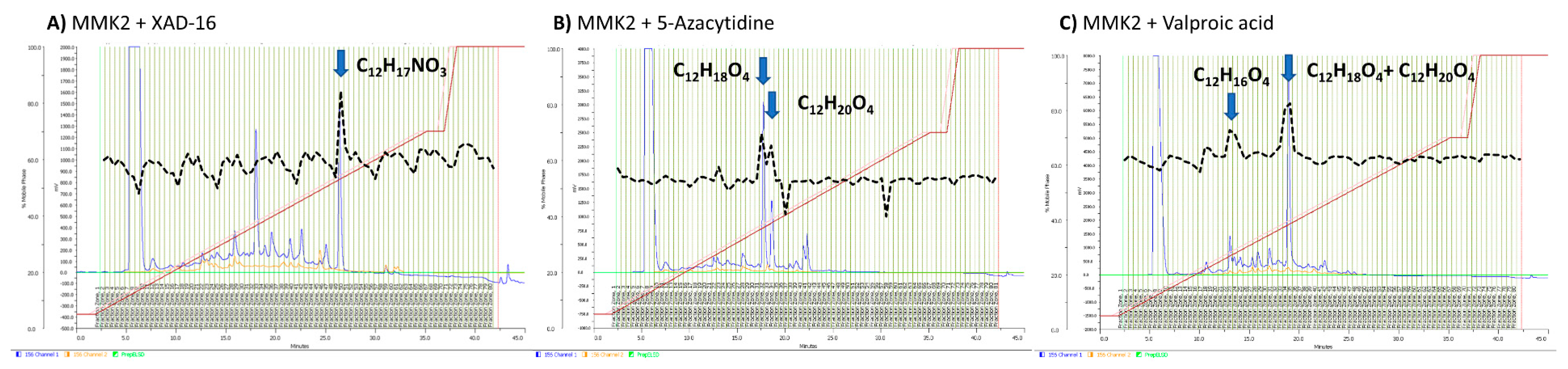
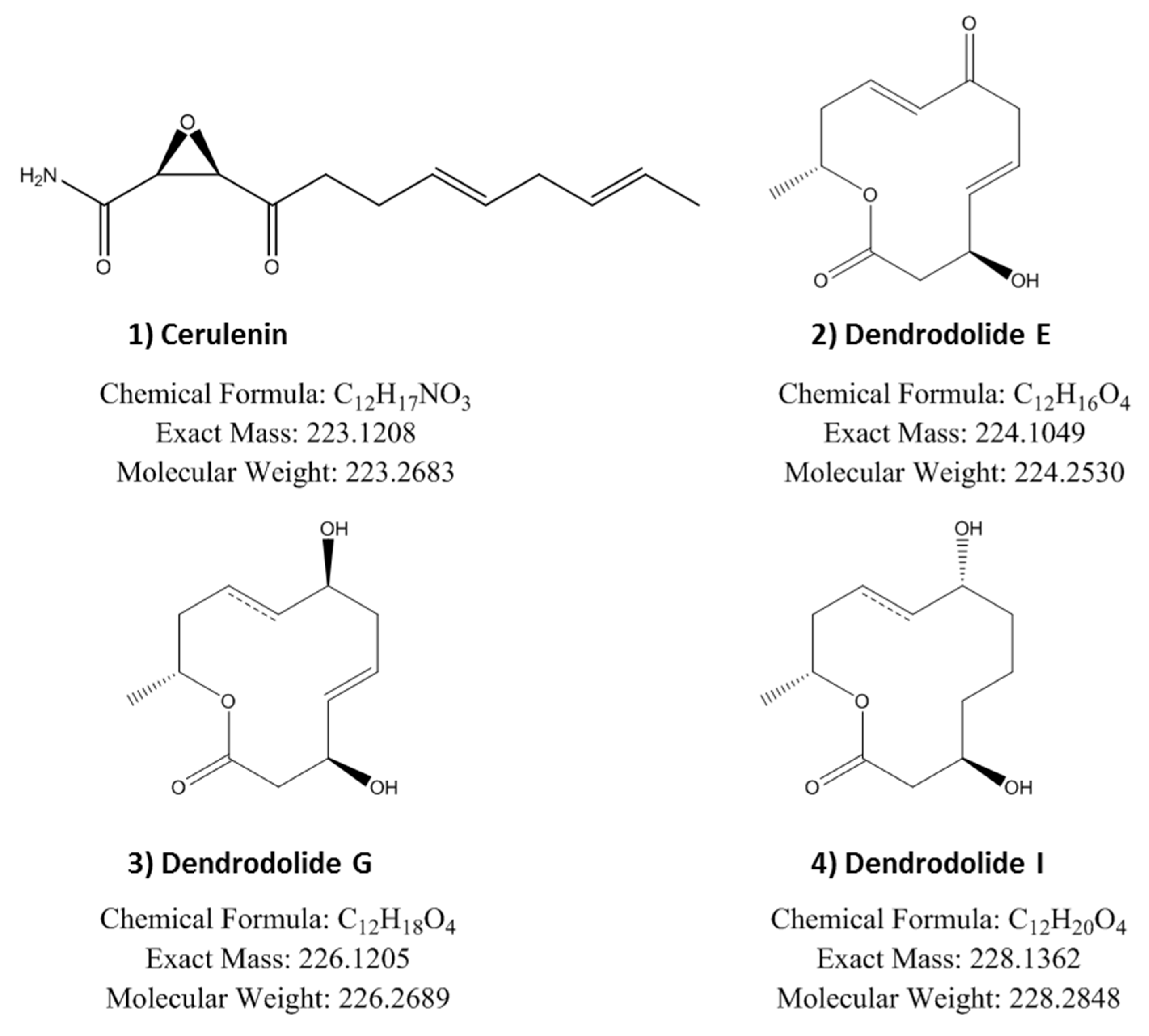
2.6. Identification of Induced Bioactive Compounds of Dimorphosporicola tragani
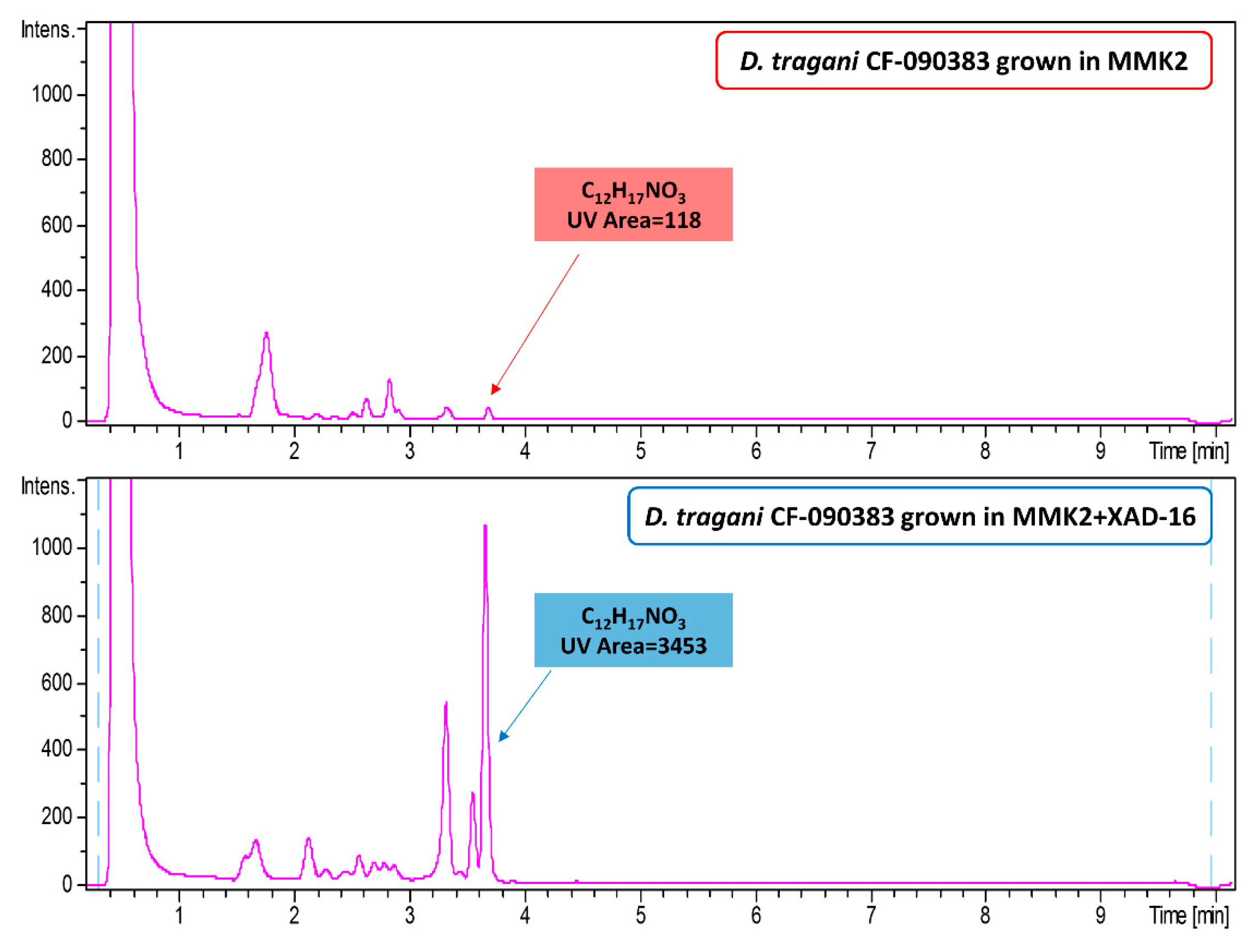
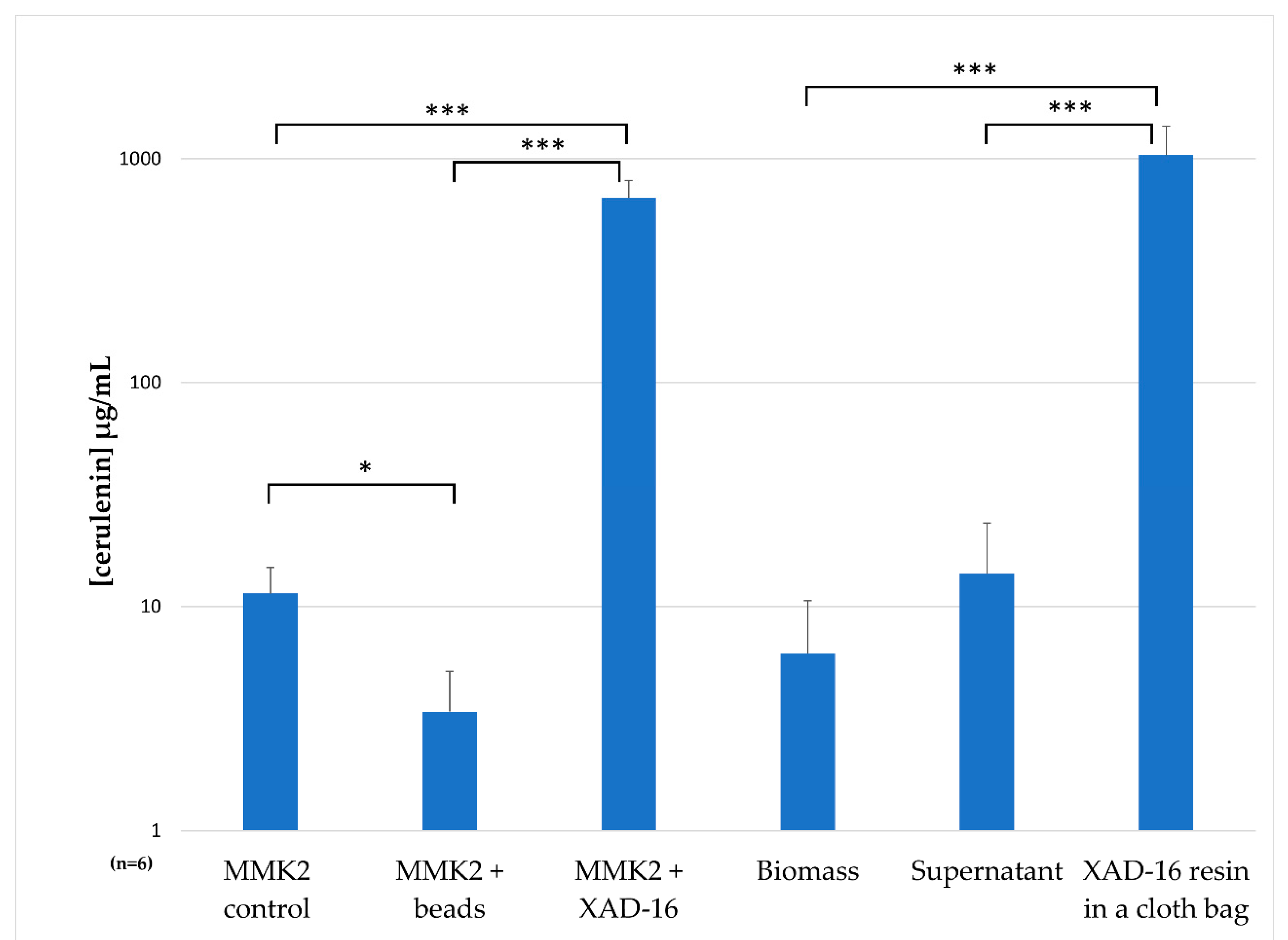
2.7. Understanding the Effect of Adding XAD-16 on the Production of Cerulenin by D. tragani
3. Discussion
4. Materials and Methods
4.1. Strain Isolation and Characterization.
4.2. Fungal Fermentations.
4.3. HPLC-UV-LRMS Profile Analysis, Metabolomics and Quantification
4.4. HPLC-HRMS Database Matching Dereplication of Known Metabolites
4.5. Assay Guided Purification
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef]
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi (Basel) 2018, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 9, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Qu, X.; Chen, F.; Bao, J.; Zhang, G.; Luo, Y. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 2013, 21, 9365–9375. [Google Scholar] [CrossRef]
- Peláez, F.; Cabello, A.; Platas, G.; Díez, M.T.; González del Val, A.; Basilio, A.; Martan, I.; Vicente, F.; Bills, G.; Giacobbe, R.A.; et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000, 3, 333–343. [Google Scholar] [CrossRef]
- Basilio, A.; Justice, M.; Harris, G.; Bills, G.; Collado, J.; de la Cruz, M.; Diez, M.T.; Hernandez, P.; Liberator, P.; Nielsen Kahn, J.; et al. The discovery of moriniafungin, a novel sordarin derivative produced by Morinia pestalozzioides. Bioorganic Med. Chem. 2006, 2, 560–566. [Google Scholar] [CrossRef]
- Singh, S.B.; Ondeyka, J.; Harris, G.; Herath, K.; Zink, D.; Vicente, F.; Bills, G.; Collado, J.; Platas, G.; González del Val, A.; et al. Isolation, structure, and biological activity of Phaeofungin, a cyclic lipodepsipeptide from a Phaeosphaeria sp. using the Genome-Wide Candida albicans Fitness Test. J. Nat. Prod. 2013, 3, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-Derived Bioactive Compounds Produced by Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chem. Biochem. 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Venugopalan, A.; Potunuru, U.R.; Dixit, M.; Srivastava, S. Reprint of: Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresour. Technol. 2016, 213, 311–318. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, V.; Crespo, G.; de Pedro, N.; Diaz, C.; Martín, J.; Serrano, R.; Mackenzie, A.T.; Justicia, C.; González-Tejero, M.R.; Casares, M.; et al. Fungal endophytes from arid areas of Andalusia: High potential sources for antifungal and antitumoral agents. Sci. Rep. 2018, 8, 9729. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, V.; Pérez-Bonilla, M.; Pérez-Victoria, I.; Martín, J.; Muñoz, F.; Reyes, F.; Tormo, J.R.; Genilloud, O. Multicomponent Analysis of the Differential Induction of Secondary Metabolite Profiles in Fungal Endophytes. Molecules. 2016, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, T.A.J.; Medema, M.H. Computational strategies for genome-based natural product discovery and engineering in fungi. Fungal Genet. Biol. 2016, 89, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Masuma, R.; Tanaka, Y.; Omura, S. Enhancement of cerulenin production by a natural zeolite, an ammonium ion-trapping agent. J. Antibiot. (Tokyo) 1982, 35, 1184–1193. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.D. They seldom occur alone. Fungal Biol. 2016, 120, 1392–1415. [Google Scholar] [CrossRef]
- Heiligtag, S.J.; Bredehorst, R.; David, K.A. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death Differ. 2002, 9, 1017–1025. [Google Scholar] [CrossRef]
- Bills, G.F.; Platas, G.; Gams, W. Conspecificity of the cerulenin and helvolic acid producing ‘Cephalosporium caerulens’, and the hypocrealean fungus Sarocladium oryzae. Mycol. Res. 2004, 108, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W.; Kappe, R.; Paululat, T.; Mösker, E.; Anke, H. Anti-Candida metabolites from endophytic fungi. Phytochemistry 2007, 68, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Vasantha Devi, T.; Gnanamanickam, S. Toxin produced by Sacrocladium oryzae involved in inducing sheath rot (ShR) symptoms in rice. Int. Rice Res. Notes (Philippines) 1993, 18, 30–31. [Google Scholar]
- Hittalmani, S.; Mahesh, H.B.; Mahadevaiah, C.; Prasannakumar, M.K. De novo genome assembly and annotation of rice sheath rot fungus Sarocladium oryzae reveals genes involved in Helvolic acid and Cerulenin biosynthesis pathways. BMC Genom. 2016, 17, 271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frykman, S.; Tsuruta, H.; Galazzo, J.; Licari, P. Characterization of product capture resin during microbial cultivations. J. Ind. Microbiol. Biotechnol. 2006, 33, 445–453. [Google Scholar] [CrossRef]
- González-Menéndez, V.; Asensio, F.; Moreno, C.; de Pedro, N.; Monteiro, M.C.; de la Cruz, M.; Vicente, F.; Bills, G.F.; Reyes, F.; Genilloud, O.; et al. Assessing the effects of adsorptive polymeric resin additions on fungal secondary metabolite chemical diversity. Mycology 2014, 5, 179–191. [Google Scholar] [CrossRef]
- Zutz, C.; Bacher, M.; Parich, A.; Kluger, B.; Gacek-Matthews, A.; Schuhmacher, R.; Wagner, M.; Rychli, K.; Strauss, J. Valproic Acid Induces Antimicrobial Compound Production in Doratomyces microspores. Front. Microbiol. 2016, 7, 510. [Google Scholar] [CrossRef]
- Sun, P.; Xu, D.X.; Mándi, A.; Kurtán, T.; Li, T.J.; Schulz, B.; Zhang, W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the sea cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef]
- Marshall, V.P.; McWethy, J.S.; Sirotti, J.M.; Cialdella, J.I. The effect of neutral resins on the fermentation production of rubradirin. J. Ind. Microbiol. Biotechnol. 1990, 5, 283–288. [Google Scholar] [CrossRef]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an antiplasmodial betaine lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef]
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR strategies for the dereplication of marine natural products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, N.; Cautain, B.; Melguizo, A.; Cortes, D.; Vicente, F.; Genilloud, O.; Tormo, J.R.; Peláez, F. Analysis of cytotoxic activity at short incubation times reveals profound differences among Annonaceus acetogenins, inhibitors of mitochondrial Complex I. J. Bioenerg. Biomembr. 2013, 45, 145–152. [Google Scholar] [CrossRef] [PubMed]


| Fermentation Condition (n = 10) | HepG2 (%) ( ± SD) | MCF-7 (%) ( ± SD) | MiaPaca2 (%) ( ± SD) |
|---|---|---|---|
| MMK2 | −13 ± 15 | −16 ± 19 | −17 ± 23 |
| MMK2+ XAD-16 | −100 ± 0 | −100 ±2 | −92 ± 24 |
| MMK2+ 5-azacytidine | −31 ± 11 | −55 ± 22 | −63 ± 11 |
| MMK2+ hydralazine | 13 ± 2 | −6 ± 4 | −27 ± 6 |
| MMK2+ quercetin | −5 ± 9 | −19 ± 7 | −31 ± 11 |
| MMK2+ SBHA | −29 ± 14 | −34 ±1 6 | 7 ± 7 |
| MMK2+ sodium butyrate | 2 ± 16 | 2 ± 11 | 1 ± 8 |
| MMK2+ valproic acid | −100 ± 0 | −100 ± 1 | −100 ± 28 |
| Compound Purified from D. tragani | HepG2 | MiaPaca2 | MCF-7 |
|---|---|---|---|
| Cerulenin | 23.08 | 18.53 | 10.14 |
| Dendrodolide E | > 40 | > 40 | 10.27 |
| Dendrodolide G | 12.8 | 8.25 | 8.23 |
| Dendrodolide I | 36.10 | 15.12 | 11.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Menéndez, V.; Crespo, G.; Toro, C.; Martín, J.; de Pedro, N.; Tormo, J.R.; Genilloud, O. Extending the Metabolite Diversity of the Endophyte Dimorphosporicola tragani. Metabolites 2019, 9, 197. https://doi.org/10.3390/metabo9100197
González-Menéndez V, Crespo G, Toro C, Martín J, de Pedro N, Tormo JR, Genilloud O. Extending the Metabolite Diversity of the Endophyte Dimorphosporicola tragani. Metabolites. 2019; 9(10):197. https://doi.org/10.3390/metabo9100197
Chicago/Turabian StyleGonzález-Menéndez, Victor, Gloria Crespo, Clara Toro, Jesús Martín, Nuria de Pedro, Jose R Tormo, and Olga Genilloud. 2019. "Extending the Metabolite Diversity of the Endophyte Dimorphosporicola tragani" Metabolites 9, no. 10: 197. https://doi.org/10.3390/metabo9100197
APA StyleGonzález-Menéndez, V., Crespo, G., Toro, C., Martín, J., de Pedro, N., Tormo, J. R., & Genilloud, O. (2019). Extending the Metabolite Diversity of the Endophyte Dimorphosporicola tragani. Metabolites, 9(10), 197. https://doi.org/10.3390/metabo9100197







