Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication
Abstract
1. Introduction
2. Results
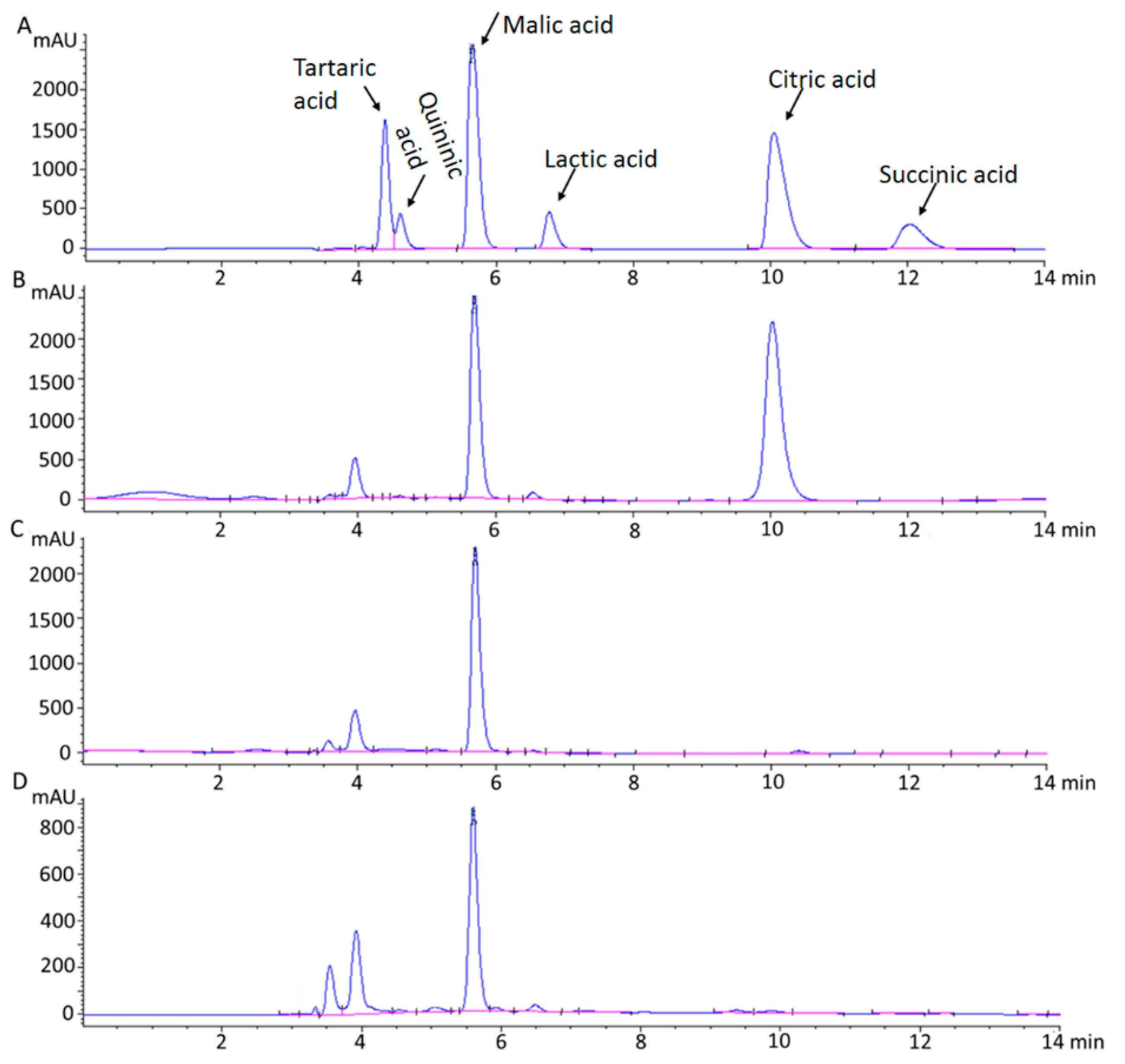
2.1. HPLC Analysis of Chemical Standards for Organic Acids
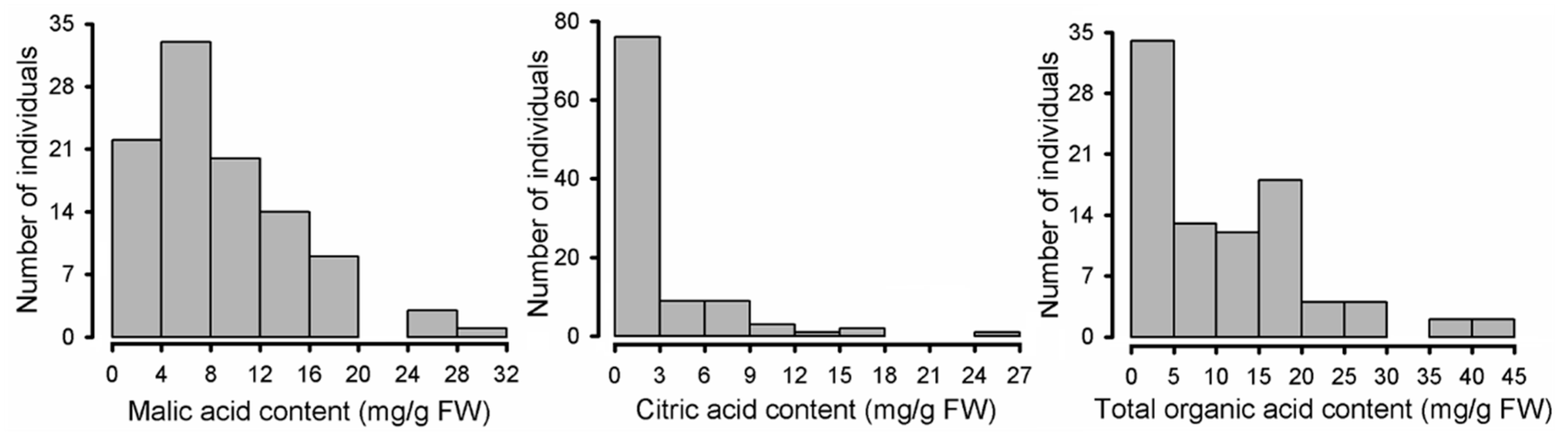
2.2. Variation in Organic Acid Components among Malus Species
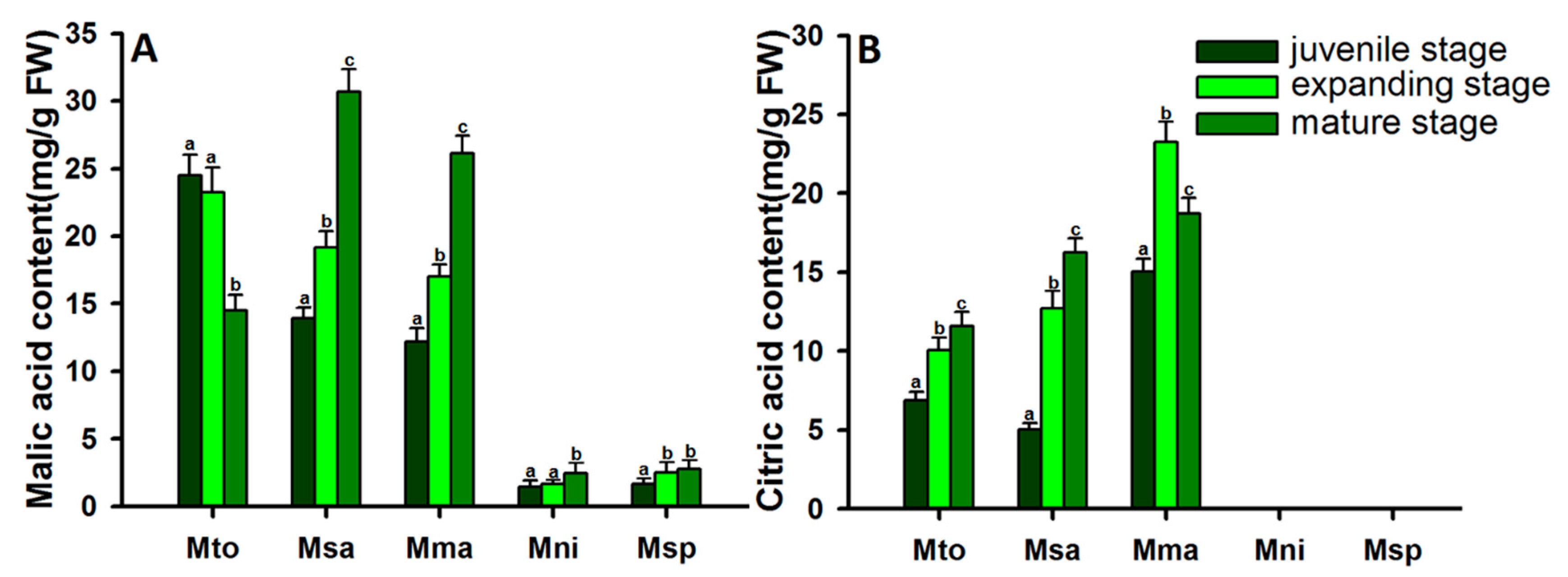
2.3. Dynamic Changes in Malic Acid and Citric Acid Content in Apples at Different Developmental Stages
2.4. Correlation among Organic Acid Contents in Malus Species
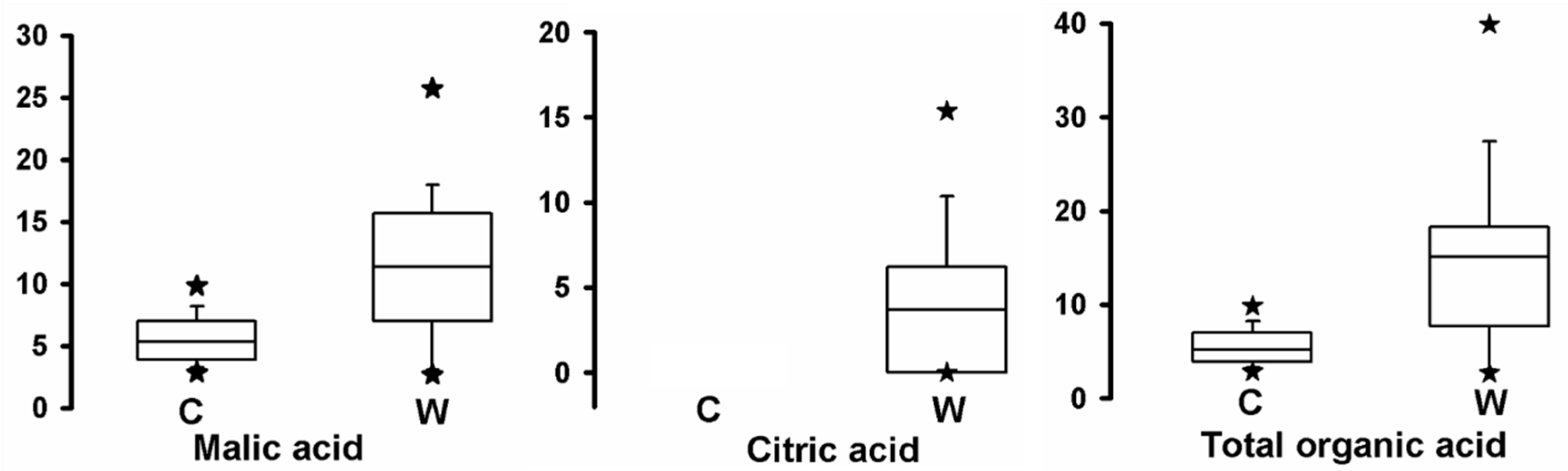
2.5. Comparison of Organic Acid Contents between Cultivated Apples and Wild Relatives
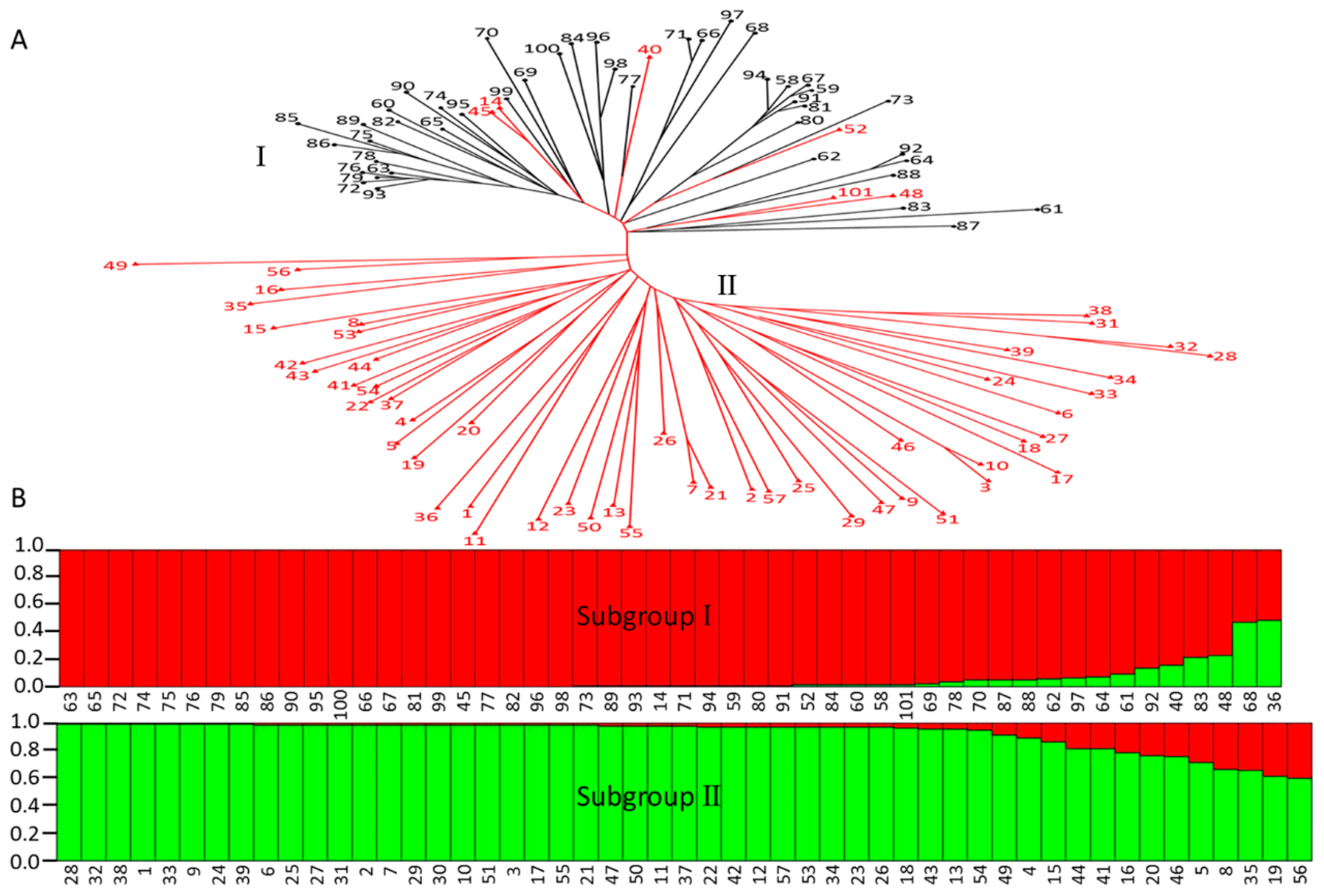
2.6. Estimation of Genetic Relationship and Diversity of Organic Acid Components between Apple Cultivars and Wild Relatives
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction and Determination of Organic Acids
4.3. Data Analysis
4.4. Genotyping of Apple Germ Plasm
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Diakou, P.; Svanella, L.; Raymond, P.; Gaudillère, J.-P.; Moing, A. Phosphoenolpyruvate carboxylase during grape berry development: Protein level, enzyme activity and regulation. Aust. J. Plant Physiol. 2000, 27, 221–229. [Google Scholar]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Rybka, A.C.P.; Ballus, C.A.; Meinhart, A.D.; Filho, J.T.; Godoy, H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhou, G.; Wei, Q.; Hu, H.; Peng, S. Identification of organic acid-related genes and their expression profiles in two pear (Pyrus pyrofolia) cultivars with difference in predominant acid type at fruit ripening stage. Sci. Hortic. 2011, 129, 680–687. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Daood, H.G.; Biacs, P.A.; Dakar, M.A.; Hajdu, F. Ion-pair chromatography and photodiode-array detection of vitamin C and organic acids. J. Chromatogr. Sci. 1994, 32, 481–487. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Sandín-España, P.; Mateo-Miranda, M.; López-Goti, C.; De Cal, A.; Alonso-Prados, J.L. Development of a rapid and direct method for the determination of organic acids in peach fruit using LC-ESI-MS. Food Chem. 2016, 192, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhao, S.; Wu, B.; Wang, D.; Peng, Q.; Owiti, A.; Fang, T.; Liao, L.; Ogutu, C.; Korban, S.S.; et al. Construction of a high density linkage map and its application in the identification of QTLs for soluble sugar and organic acid components in apple. Tree Genet. Genomes 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Sun, R.; Chang, Y.; Yang, F.; Wang, Y.; Li, H.; Zhao, Y.; Chen, D.; Zhang, X.; Han, Z. A dense SNP genetic map constructed using restriction site-associated DNA sequencing enables detection of QTLs controlling apple fruit quality. BMC Genom. 2015, 16, 747. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.Q.; Liao, L.; Zheng, H.Y.; Chen, J.; Wu, B.H.; Ogutu, C.; Li, S.H.; Korban, S.S.; Han, Y.P. Genes encoding aluminum-activated malate transporter II and their association with fruit acidity in apple. Plant Genome 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Harris, S.; Robinson, J.; Juniper, B. Genetic clues to the origin of the apple. Trends Genet. 2002, 18, 426–430. [Google Scholar] [CrossRef]
- Richards, C.M.; Volk, G.M.; Reilley, A.A.; Henk, A.D.; Lockwood, D.R.; Reeves, P.A.; Forsline, P.L. Genetic diversity and population structure in Malus sieversii, a wild progenitor species of domesticated apple. Tree Genet. Genomes 2009, 5, 339–347. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Gladieux, P.; Smulders, M.J.; Roldánruiz, I.; Laurens, F.; Le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New insight into the history of domesticated apple: Secondary contribution of the european wild apple to the genome of cultivated varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Givan, C.V. Evolving concepts in plant glycolysis: Two centuries of progress. Biol. Rev. 1999, 74, 277–309. [Google Scholar] [CrossRef]
- Martinoia, E.; Maeshima, M.; Neuhaus, E.H. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Andrade, P.B.; Mendes, G.C.; Seabra, R.M.; Ferreira, M.A. Study of the organic acids composition of quince (Cydonia oblonga Miller) fruit and jam. J. Agric. Food Chem. 2002, 50, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Sadka, A.; Dahan, E.; Or, E.; Roose, M.L.; Marsh, K.B.; Cohen, L. Comparative analysis of mitochondrial citrate synthase gene structure, transcript level and enzymatic activity in acidless and acid-containing Citrus varieties. Funct. Plant Biol. 2001, 28, 383–390. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Escobar, N.M.; Ross, H.A.; Souleyre, E.J.F.; Hancock, R.D.; Witte, C.-P.; Davies, H.V. Identification, cloning and expression analysis of strawberry (Fragaria × ananassa) mitochondrial citrate synthase and mitochondrial malate dehydrogenase. Physiol. Plant. 2004, 121, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liao, L.; Peng, Q.; Fang, T.; Zhou, H.; Korban, S.S.; Han, Y. Reduced representation genome sequencing reveals patterns of genetic diversity and selection in apple. J. Integr. Plant Biol. 2017, 59, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B.; Lougheed, E.C.; Franklin, E.W.; McMillan, I. The starch iodine test for determining stage of maturation in apples. Can. J. Plant Sci. 1979, 59, 725–735. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
| SSR | Chr. | Motif | Primer (5’–3’) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Hi07d08 | 1 | (GT)21 | TGACATGCTTTTAGAGGTGGAC | GTTTGAGGGGTGTCCGTACAAG |
| CH05e03 | 2 | (GA)26 | CGAATATTTTCACTCTGACTGGG | CAAGTTGTTGTACTGCTCCGAC |
| Hi15h12 | 3 | (GAT)6 | GAACAAGAAGGACGCGAATC | GTTTGGGCCTCGTTATCACTACCA |
| AT000420-SSR | 4 | (GA)8 | TTGGACCAATTATCTCTGCTATT | GATGTGGTCAGGGAGAGGAG |
| Hi11a03 | 5 | (GAA)8 | GGAATTGGAGCTTGATGCAG | GTTTCATACGGAATGGCAAATCG |
| CH03c01 | 6 | (CT)13 | CCTTTTGGCACTAGGCAGAC | CTGCCCTCAAGGAGAATGTC |
| Hi05b09 | 7 | (AG)16 | AAACCCAACCCAAAGAGTGG | GTTTCTAACGTGCGCCTAACGTG |
| Hi04e05 | 8 | (AG)40 | AAGGGTGTTTGCGGAGTTAG | GGTGCGCTGTCTTCCATAAA |
| Hi23d06 | 9 | (ACA)8 | TTGAAACCCGTACATTCAACTC | GTTTCAAGAACCGTGCGAAATG |
| COL | 10 | (GA)17 | AGGAGAAAGGCGTTTACCTG | GACTCATTCTTCGTCGTCACTG |
| CN491050-SSR | 11 | (AG)14 | CGCTGATGCGATAATCAATG | GTTTCACCCACAGAATCACCAGA |
| CH04d02 | 12 | (TC)19 | CGTACGCTGCTTCTTTTGCT | CTATCCACCACCCGTCAACT |
| Hi08e06 | 13 | (TTG)7 | GCAATGGCGTTCTAGGATTC | GTTTGGCTGCTTGGAGATGTG |
| CH03g04 | 14 | (GA)20 | ATGTCCAATGTAGACACGCAAC | TTGAAGATGGCCTAACCTTGTT |
| Hi15c07 | 15 | (CCA)6 | TCACTTCCCATCATCACTGC | GTTTCAATGTCGAGGCTGGTAATG |
| Hi01a08 | 16 | (TCT)28 | AAGTCCAATCGCACTCACG | CGTAGCTCTCTCCCGATACG |
| CH01h01 | 17 | (TC)25 | GAAAGACTTGCAGTGGGAGC | GGAGTGGGTTTGAGAAGGTT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. https://doi.org/10.3390/metabo8040074
Ma B, Yuan Y, Gao M, Li C, Ogutu C, Li M, Ma F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites. 2018; 8(4):74. https://doi.org/10.3390/metabo8040074
Chicago/Turabian StyleMa, Baiquan, Yangyang Yuan, Meng Gao, Cuiying Li, Collins Ogutu, Mingjun Li, and Fengwang Ma. 2018. "Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication" Metabolites 8, no. 4: 74. https://doi.org/10.3390/metabo8040074
APA StyleMa, B., Yuan, Y., Gao, M., Li, C., Ogutu, C., Li, M., & Ma, F. (2018). Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites, 8(4), 74. https://doi.org/10.3390/metabo8040074






