The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction
Abstract
1. Introduction
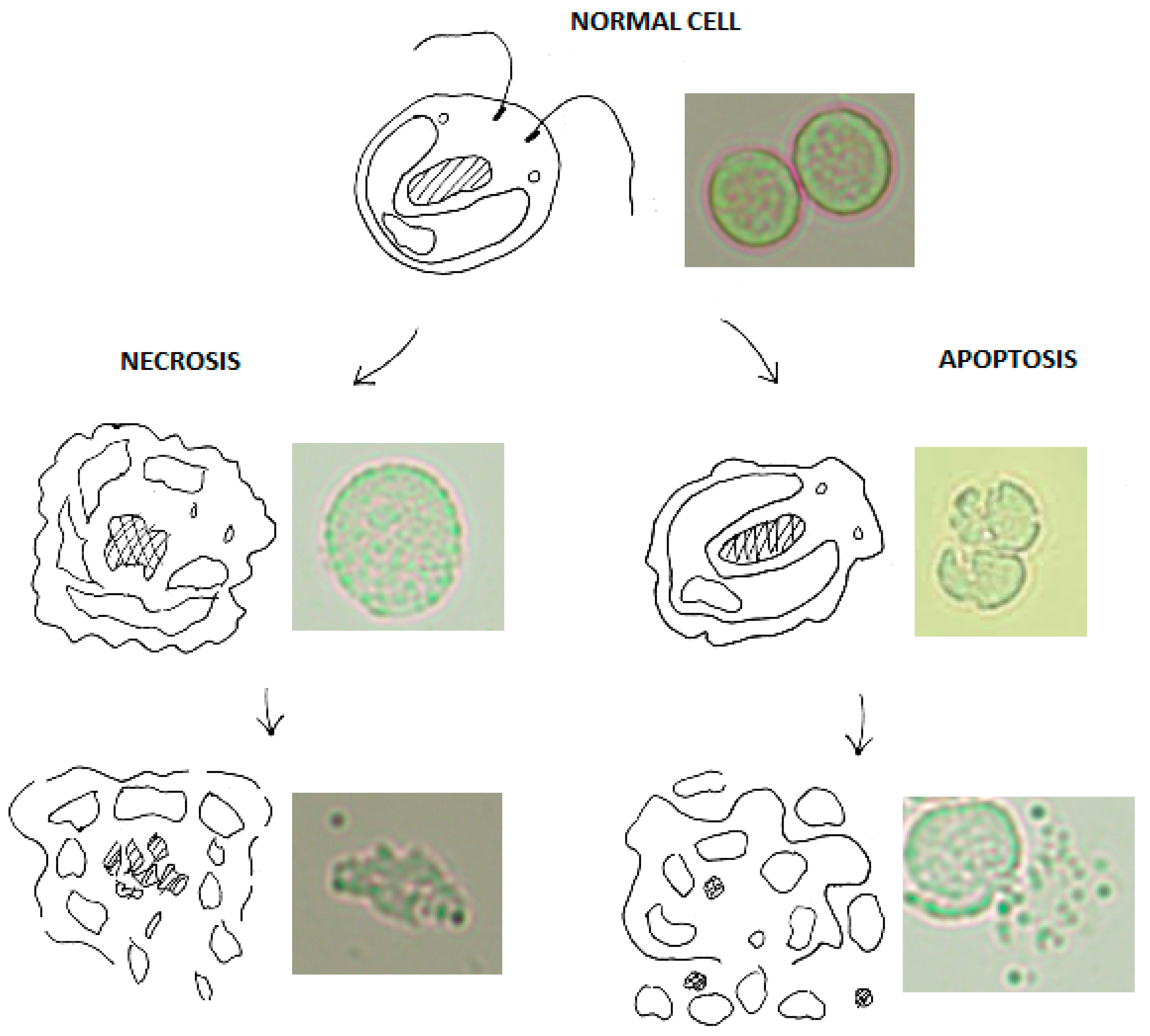
1.1. Cellular Signalling after Ultraviolet Irradiation
1.2. Ultraviolet Light Compared to Conventional Disruption
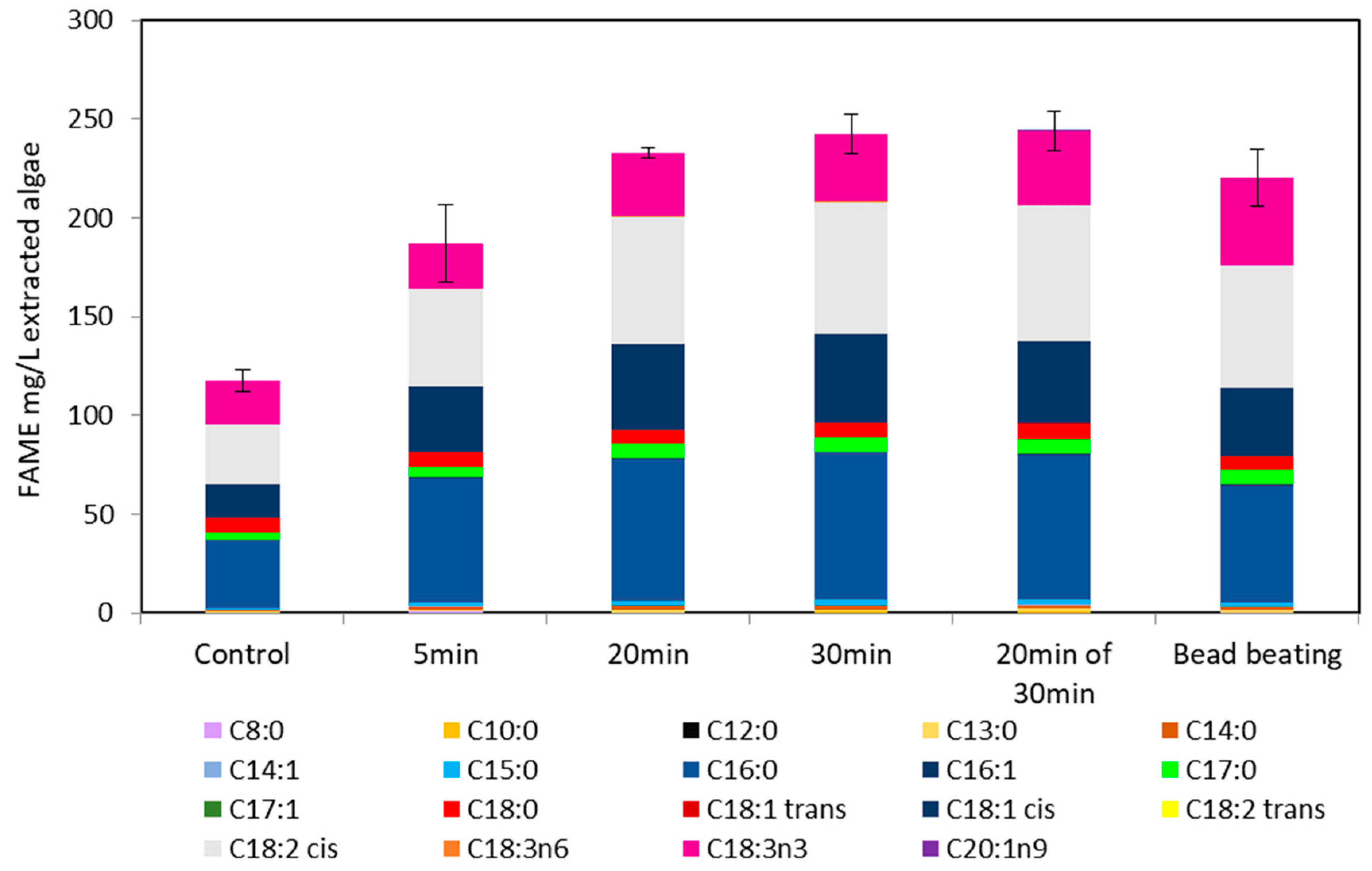
2. Results and Discussion
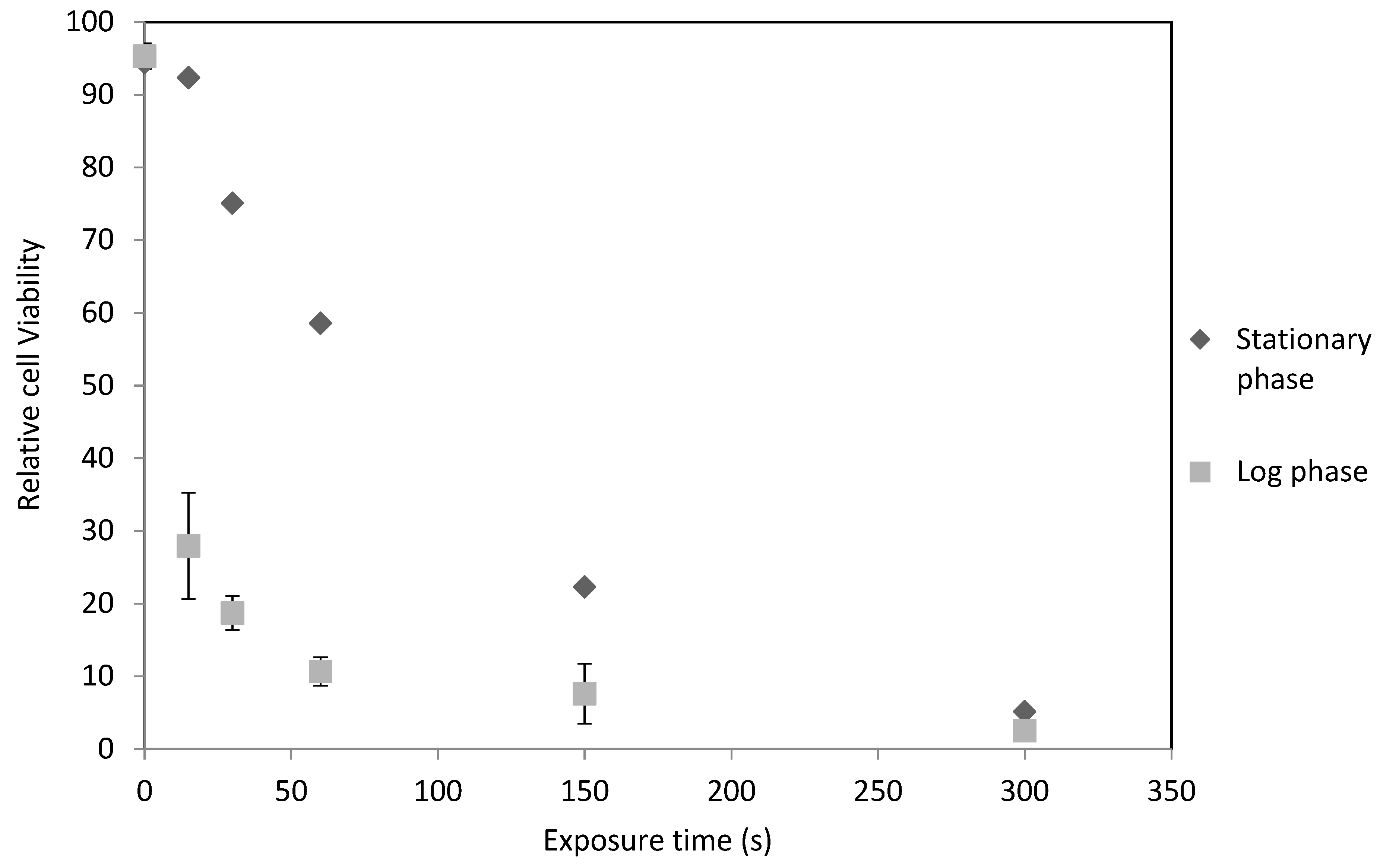
2.1. C. reinhardtii Irradiation
2.2. D. salina Irradiation
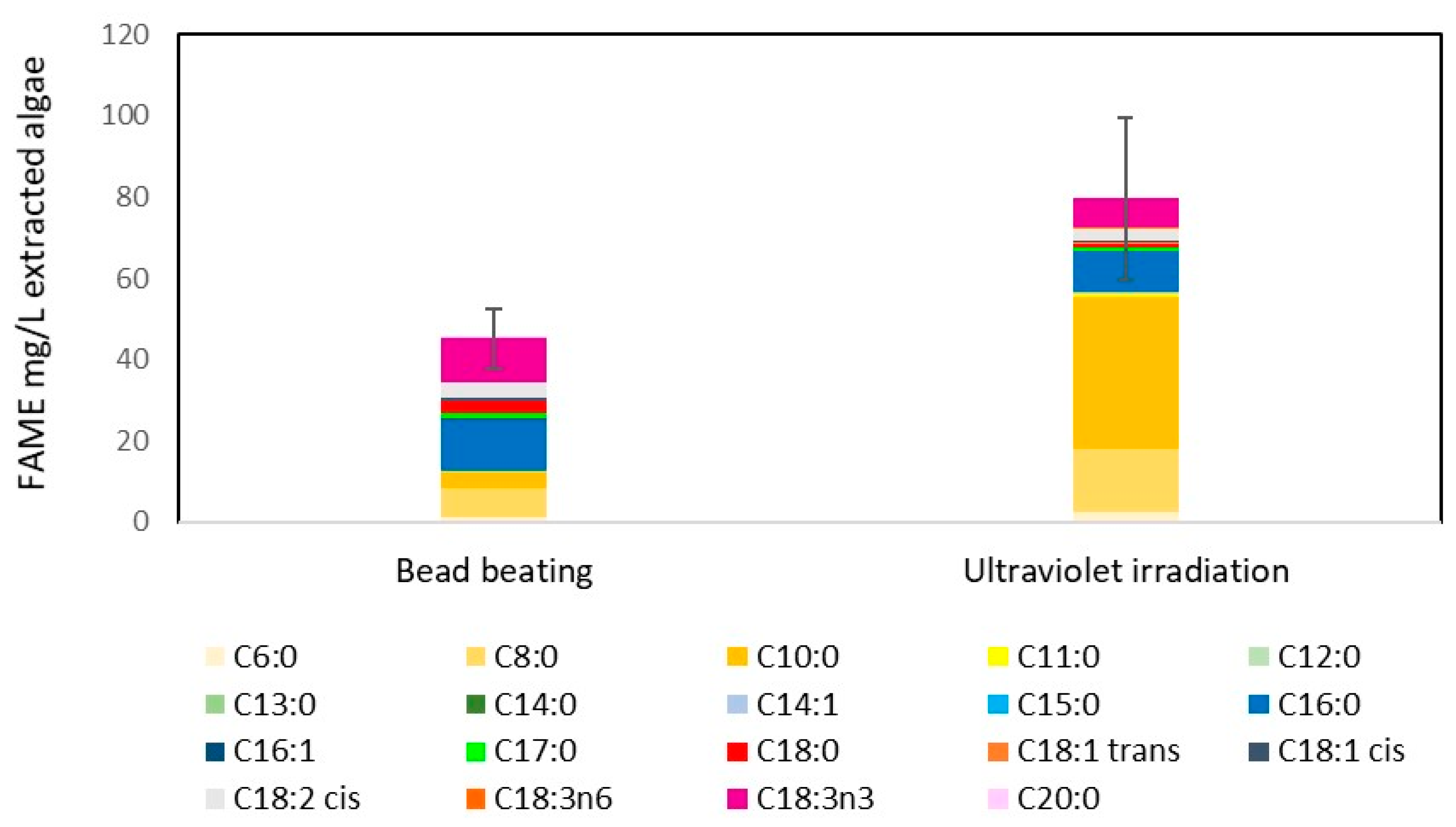
2.3. UV Radiation as a Disruption Method for Biodiesel Production
2.4. Effect of Nitrate Stress on FAME Yield
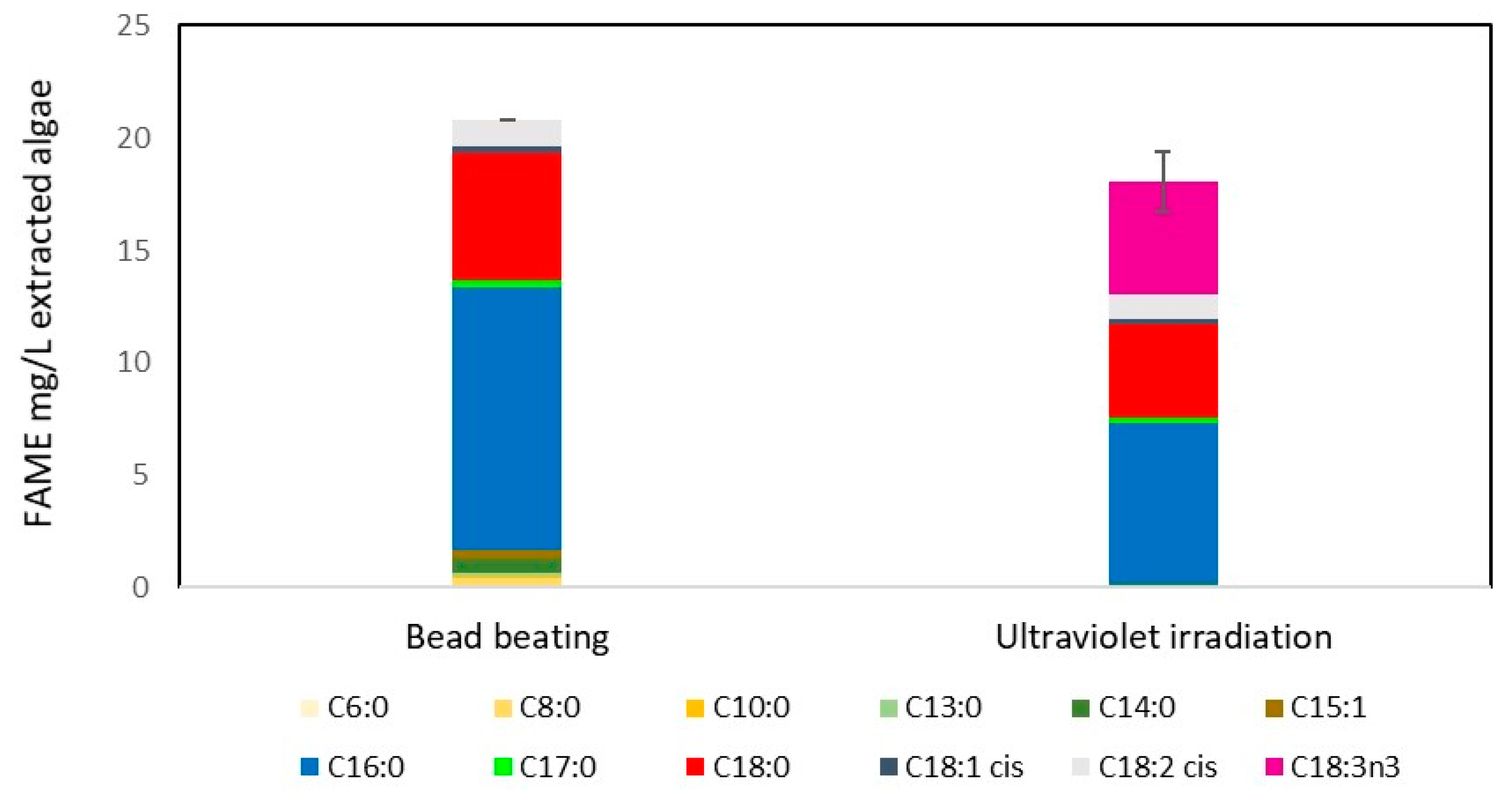
2.5. Ultraviolet Light Irradiation of M. inermum
2.6. Ultraviolet Light Cell Disruption Energy Efficiency
2.7. Limitations of the Study
2.8. Future Work
3. Materials and Methods
3.1. Algae Strains and Cultivation
3.2. Ultraviolet Light Irradiation of Algae
3.3. Lipid Extraction
3.4. Transesterification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Boer, K.; Moheimani, N.R.; Borowitzka, M.A.; Bahri, P.A. Extraction and conversion pathways for microalgae to biodiesel: A review focused on energy consumption. J. Appl. Phycol. 2012, 24, 1681–1698. [Google Scholar] [CrossRef]
- Shimako, A.H.; Tiruta-Barna, L.; Pigné, Y.; Benetto, E.; Navarrete-Gutiérrez, T.; Guiraud, P.; Ahmadi, A. Environmental assessment of bioenergy production from microalgae based systems. J. Clean. Prod. 2016, 139, 51–60. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lee, T.M.; Chang, J.S. Microalgal drying and cell disruption-Recent advances. Bioresour. Technol. 2015, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qin, S.; Zhang, D.; Li, L.; Mu, Y. Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication. Energies 2016, 9, 173. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Karentz, D. Ecological considerations of Antarctic ozone depletion. Antarct. Sci. 1991, 3, 3–11. [Google Scholar] [CrossRef]
- Kuluncsics, Z.; Perdiz, D.; Brulay, E.; Muel, B.; Sage, E. Wavelength dependence of ultraviolet-induced DNA damage distribution: Involvement of direct or indirect mechanisms and possible artefacts. J. Photochem. Photobiol. B Biol. 1999, 49, 71–80. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Moharikar, S.; D’Souza, J.S.; Kulkarni, A.B.; Rao, B.J. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (chlorophyceae) cells following uv irradiation: Detection and functional analyses. J. Phycol. 2006, 42, 423–433. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Haramaty, L.; Berges, J.A.; Falkowski, P.G. Cell death in the unicellular chlorophyte Dunaliella tertiolecta: A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiol. 2003, 132, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, B.; Dera, J. Light Absorption in Sea Water; Springer: New York, NY, USA, 2007. [Google Scholar]
- Water Technology. Catskill–Delaware Ultraviolet Water Treatment Facility, New York, 2015. Available online: http://www.water-technology.net/projects/-catskill-delaware-ultraviolet-water-treatment-facility/ (accessed on 29 May 2017).
- Gleick, P.H. The World’s Water. Volume 7: The Biennial Report on Freshwater Resources; Island Press: Washington, DC, USA, 2011. [Google Scholar]
- Loewus, F.A.; Runeckles, V.C. The Structure, Biosynthesis, and Degradation of Wood, Boston, MA; Springer: New York, NY, USA, 1977. [Google Scholar]
- Hoshina, R.; Fujiwara, Y. Molecular characterization of Chlorella cultures of the National Institute for Environmental Studies culture collection with description of Micractinium inermum sp. nov., Didymogenes sphaerica sp. nov., and Didymogenes soliella sp. nov. (Chlorellaceae, Tr.). Phycol. Res. 2013, 61, 124–132. [Google Scholar]
- Selvarajan, R.; Felföldi, T.; Tauber, T.; Sanniyasi, E.; Sibanda, T.; Tekere, M. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies 2015, 8, 7502–7521. [Google Scholar] [CrossRef]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Vonshak, A. Laboratory Techniques for the cultivation of microalgae. In Handbook of Microalgal Mass Culture; Richmond, A., Ed.; Taylor & Francis: London, UK, 1986; p. 30. [Google Scholar]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Bangert, K.; Wilkinson, S.J.; Gilmour, D.J. Synergistic carbon metabolism in a fast growing mixotrophic freshwater microalgal species Micractinium inermum. Biomass Bioenergy 2015, 82, 73–86. [Google Scholar] [CrossRef]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-hemersdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shen, H.; Ondruschka, B.; Zhang, Y.; Wang, W.; Bremner, D.H. Removal of blue-green algae using the hybrid method of hydrodynamic cavitation and ozonation. J. Hazard. Mater. 2012, 235, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Preetha, K.; John, L.; Subin, C.S.; Vijayan, K.K. Phenotypic and genetic characterization of Dunaliella (Chlorophyta) from Indian salinas and their diversity. Aquat. Biosyst. 2012, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Hounslow, E.; Kapoore, R.V.; Vaidyanathan, S.; Gilmour, D.J.; Wright, P.C. The Search for a Lipid Trigger: The Effect of Salt Stress on the Lipid Profile of the Model Microalgal Species Chlamydomonas reinhardtii for Biofuels Production. Curr. Biotechnol. 2016, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V. Mass Spectrometry Based Hyphenated Techniques for Microalgal and Mammalian Metabolomics. Ph.D. Thesis, University of Sheffield, Sheffield, UK, August 2014. [Google Scholar]
- Pandhal, J.; Choon, W.; Kapoore, R.; Russo, D.; Hanotu, J.; Wilson, I.; Desai, P.; Bailey, M.; Zimmerman, W.J.; Ferguson, A.S. Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment. Biology 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sydney, T.; Marshall-Thompson, J.-A.; Kapoore, R.V.; Vaidyanathan, S.; Pandhal, J.; Fairclough, J.P.A. The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction. Metabolites 2018, 8, 65. https://doi.org/10.3390/metabo8040065
Sydney T, Marshall-Thompson J-A, Kapoore RV, Vaidyanathan S, Pandhal J, Fairclough JPA. The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction. Metabolites. 2018; 8(4):65. https://doi.org/10.3390/metabo8040065
Chicago/Turabian StyleSydney, Thomas, Jo-Ann Marshall-Thompson, Rahul Vijay Kapoore, Seetharaman Vaidyanathan, Jagroop Pandhal, and J. Patrick A. Fairclough. 2018. "The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction" Metabolites 8, no. 4: 65. https://doi.org/10.3390/metabo8040065
APA StyleSydney, T., Marshall-Thompson, J.-A., Kapoore, R. V., Vaidyanathan, S., Pandhal, J., & Fairclough, J. P. A. (2018). The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction. Metabolites, 8(4), 65. https://doi.org/10.3390/metabo8040065






