Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype
Abstract
1. Introduction
2. Results
2.1. Characteristics of Participants
2.2. Correlation of 44 Metabolites Comparing Three Normalization Methods
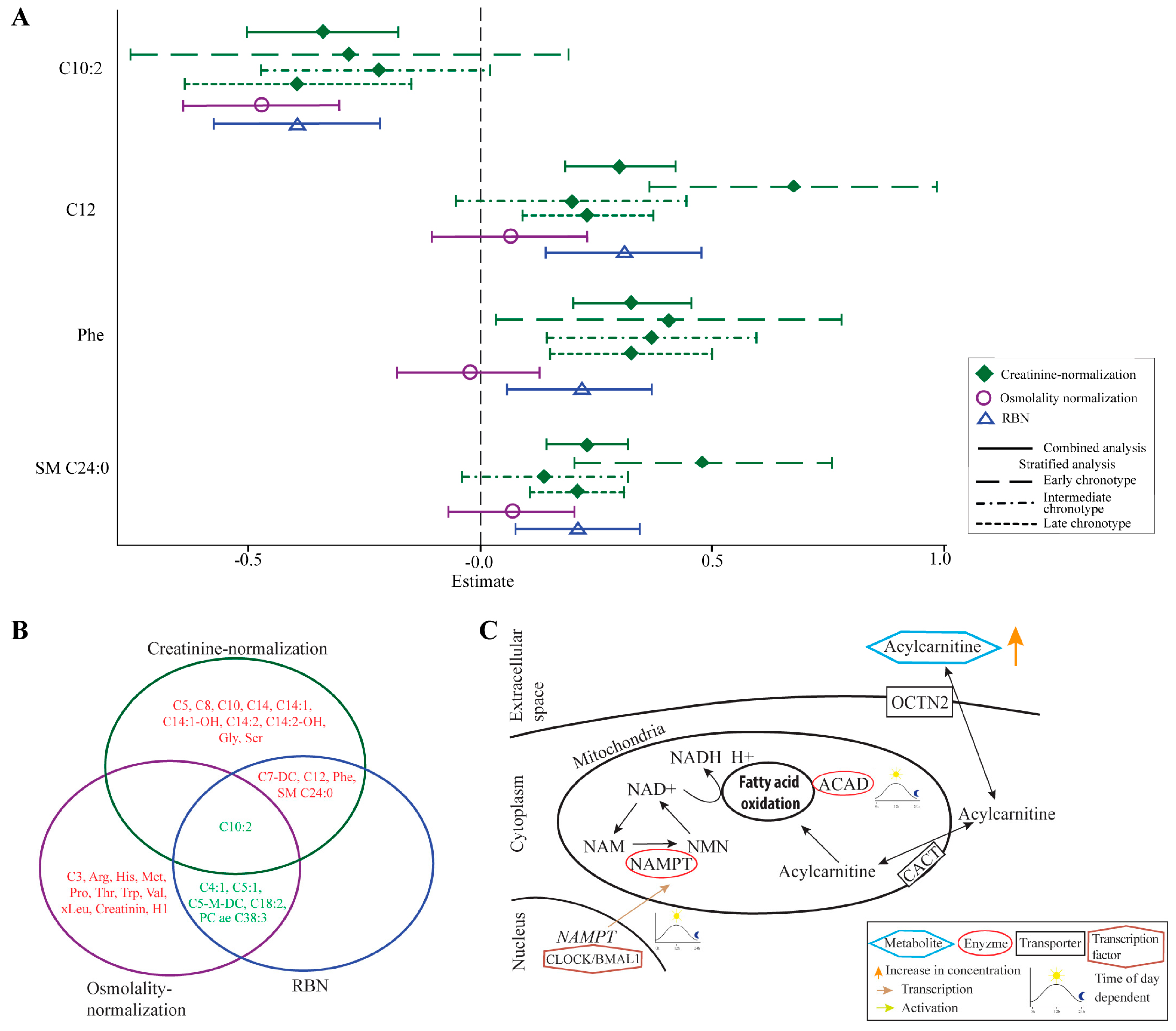
2.3. Metabolites Associated with Night Shift in the Combined Analysis in Three Normalization Methods
2.4. Metabolites Associated with Night Shift in the Chronotype—Stratified Analyses
3. Discussion
3.1. Identified Metabolites Largely Depend on the Applied Normalization Method
3.2. Elevated Levels of Acylcarnitines May Result from Impaired Fatty Acid Oxidation
3.3. Strengths and Limitations
3.4. Summary and Conclusions
4. Material and Methods
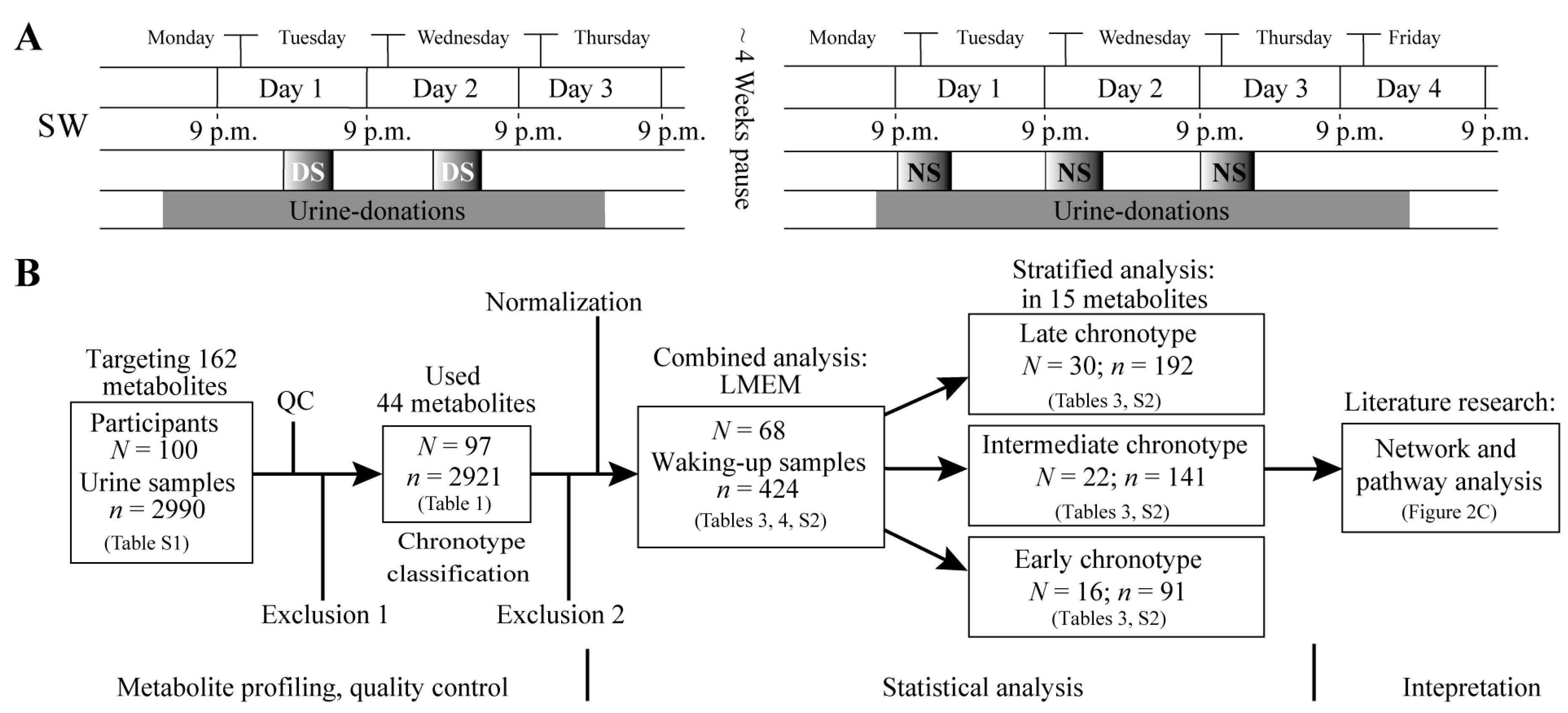
4.1. Study Design and Study Participants
4.2. Chronotype Classification
4.3. Urine Samples
4.4. Targeted Metabolite Profiling
4.5. Osmolality Measurement
4.6. Normalization Approaches
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proper, K.I.; van de Langenberg, D.; Rodenburg, W.; Vermeulen, R.C.; van der Beek, A.J.; van Steeg, H.; van Kerkhof, L.W. The relationship between shift work and metabolic risk factors: A systematic review of longitudinal studies. Am. J. Prev. Med. 2016, 50, e147–e157. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.; Goldman-Mellor, S.; Nolan, P.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Lassen, C.F. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup. Environ. Med. 2012, 69, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Batty, G.D.; Hublin, C. Shift work as a risk factor for future type 2 diabetes: Evidence, mechanisms, implications and future research directions. PLoS Med. 2011, 8, e1001138. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Dashti, H.S.; Lane, J.M.; Anderson, S.G.; Schernhammer, E.S.; Rutter, M.K.; Saxena, R.; Scheer, F.A. Night Shift Work, Genetic Risk and Type 2 Diabetes in the UK Biobank. Diabetes Care 2018, 41, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.; Rabstein, S.; Wichert, K.; Erbel, R.; Eisele, L.; Arendt, M.; Dragano, N.; Brüning, T.; Jöckel, K.-H. Shift work and the incidence of prostate cancer: A 10-year follow-up of a German population-based cohort study. Scand. J. Work Environ. Health 2017, 43, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality and social jet lag in shift-workers. J. Biol. Rhythms 2013, 28, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.-J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A. A single night of sleep curtailment increases plasma acylcarnitines: Novel insights in the relationship between sleep and insulin resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Derkach, A.; Moore, S.C.; Zheng, W.; Shu, X.-O.; Gu, F.; Caporaso, N.E.; Sampson, J.N.; Matthews, C.E. Habitual sleep and human plasma metabolomics. Metabolomics 2017, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Holzapfel, C.; Dong, X.; Bader, E.; Yu, Z.; Prehn, C.; Perstorfer, K.; Jaremek, M.; Roemisch-Margl, W.; Rathmann, W. Effects of smoking and smoking cessation on human serum metabolite profile: Results from the KORA cohort study. BMC Med. 2013, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Jaremek, M.; Yu, Z.; Mangino, M.; Mittelstrass, K.; Prehn, C.; Singmann, P.; Xu, T.; Dahmen, N.; Weinberger, K.; Suhre, K. Alcohol-induced metabolomic differences in humans. Transl. Psychiatry 2013, 3, e276. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Rotter, M.; Brandmaier, S.; Prehn, C.; Adam, J.; Rabstein, S.; Gawrych, K.; Brüning, T.; Illig, T.; Lickert, H.; Adamski, J. Stability of targeted metabolite profiles of urine samples under different storage conditions. Metabolomics 2017, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S. Investigations of the effects of gender, diurnal variation and age in human urinary metabolomic profiles. Anal. Chem. 2007, 79, 6995–7004. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, I.; Gronwald, W.; Behrens, G.; Baumeister, S.E.; Hertel, J.; Hochrein, J.; Zacharias, H.U.; Fischer, B.; Oefner, P.J.; Leitzmann, M.F. Visceral adipose tissue but not subcutaneous adipose tissue is associated with urine and serum metabolites. PLoS ONE 2017, 12, e0175133. [Google Scholar] [CrossRef] [PubMed]
- Warrack, B.M.; Hnatyshyn, S.; Ott, K.-H.; Reily, M.D.; Sanders, M.; Zhang, H.; Drexler, D.M. Normalization strategies for metabonomic analysis of urine samples. J. Chromatogr. B 2009, 877, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Mabila, S.L.; Lash, J.P.; Turyk, M.E.; Argos, M. Arsenic and Obesity: A Comparison of Urine Dilution Adjustment Methods. Environ. Health Perspect. 2017, 125, 087020. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, E.; Blackburn, G.; Kalna, G.; Zhang, T.; Anthony, N.; Watson, D.G. A study of the effects of exercise on the urinary metabolome using normalisation to individual metabolic output. Metabolites 2015, 5, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Juda, M.; Vetter, C.; Roenneberg, T. The Munich chronotype questionnaire for shift-workers (MCTQShift). J. Biol. Rhythms 2013, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Simpson, F.; Nye, E.; Bolli, P.; Waal-Manning, H.; Goulding, A.; Phelan, E.; De Hamel, F.; Stewart, R.; Spears, G.; Leek, G. The Milton survey: Part 1, General methods, height, weight and 24-hour excretion of sodium, potassium, calcium, magnesium and creatinine. N. Z. Med. J. 1978, 87, 379–382. [Google Scholar] [PubMed]
- Calles-Escandon, J.; Cunningham, J.J.; Snyder, P.; Jacob, R.; Huszar, G.; Loke, J.; Felig, P. Influence of exercise on urea, creatinine and 3-methylhistidine excretion in normal human subjects. Am. J. Physiol. Endocrinol. Metab. 1984, 246, E334–E338. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Kotchmar, D.J.; Fadrowski, J.J.; Silbergeld, E.K. Challenges for environmental epidemiology research: Are biomarker concentrations altered by kidney function or urine concentration adjustment? J. Expo. Sci. Environ. Epidemiol. 2016, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zittema, D.; Casteleijn, N.F.; Bakker, S.J.; Boesten, L.S.; Duit, A.M.; Franssen, C.F.; Gaillard, C.A.; Gansevoort, R.T. Urine concentrating capacity, vasopressin and copeptin in ADPKD and IgA nephropathy patients with renal impairment. PLoS ONE 2017, 12, e0169263. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Testa, F.; Zacchia, M.; Crispo, A.A.; Di Iorio, V.; Capolongo, G.; Rinaldi, L.; D’Antonio, M.; Fioretti, T.; Iadicicco, P. Genetic characterization of Italian patients with Bardet-Biedl syndrome and correlation to ocular, renal and audio-vestibular phenotype: Identification of eleven novel pathogenic sequence variants. BMC Med. Genet. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Chadha, V.; Garg, U.; Alon, U.S. Measurement of urinary concentration: A critical appraisal of methodologies. Pediatr. Nephrol. 2001, 16, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Lin, Y.-S.; Kuo, C.-C.; Weidemann, D.; Weaver, V.; Fadrowski, J.; Neu, A.; Navas-Acien, A. Urine osmolality in the US population: Implications for environmental biomonitoring. Environ. Res. 2015, 136, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Oppedisano, F.; Indiveri, C. Reconstitution into liposomes and functional characterization of the carnitine transporter from renal cell plasma membrane. Biochim. Biophys. Acta 2004, 1661, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Mirzoyan, K.; Klavins, K.; Koal, T.; Gillet, M.; Marsal, D.; Denis, C.; Klein, J.; Bascands, J.-L.; Schanstra, J.P.; Saulnier-Blache, J.-S. Increased urine acylcarnitines in diabetic ApoE-/-mice: Hydroxytetradecadienoylcarnitine (C14: 2-OH) reflects diabetic nephropathy in a context of hyperlipidemia. Biochem. Biophys. Res. Commun. 2017, 487, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism control by the circadian clock and vice versa. Nat. Struct. Mol. Biol. 2009, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Udoh, U.S.; Young, M.E. Circadian regulation of metabolism. J. Endocrinol. 2014, 222, R75–R96. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer 2009, 9, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Manzella, N.; Copertaro, A.; Staffolani, S.; Strafella, E.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; Santarelli, L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin and serum 17-β-estradiol levels. Scand. J. Work Environ. Health 2014, 40, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.; Panda, S. Circadian clock, nutrient quality and eating pattern tune diurnal rhythms in the mitochondrial proteome. Proc. Natl. Acad. Sci. USA 2016, 113, 3127–3129. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.; Duran, M.; Loupatty, F.J. Enzymology of the branched-chain amino acid oxidation disorders: The valine pathway. J. Inherit. Metab. Dis. 2012, 35, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Das, P.; Hashimoto, I.; Nakao, T.; Deguchi, Y.; Gouraud, S.S.; Waki, H.; Muragaki, Y.; Maeda, M. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS ONE 2014, 9, e112811. [Google Scholar] [CrossRef] [PubMed]
- Klootwijk, E.D.; Reichold, M.; Helip-Wooley, A.; Tolaymat, A.; Broeker, C.; Robinette, S.L.; Reinders, J.; Peindl, D.; Renner, K.; Eberhart, K. Mistargeting of peroxisomal EHHADH and inherited renal Fanconi’s syndrome. N. Engl. J. Med. 2014, 370, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.-Y.; Ren, S.; Liu, Y. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Kantermann, T.; Sung, H.; Burgess, H.J. Comparing the morningness-eveningness questionnaire and munich chronotype questionnaire to the dim light melatonin onset. J. Biol. Rhythms 2015, 30, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Hida, A.; Aritake, S.; Higuchi, S.; Enomoto, M.; Kato, M.; Vetter, C.; Roenneberg, T.; Mishima, K. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol. Int. 2014, 31, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Engleman, H.; Douglas, N. Sleep·4: Sleepiness, cognitive function and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004, 59, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Römisch-Margl, W.; Prehn, C.; Bogumil, R.; Röhring, C.; Suhre, K.; Adamski, J. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 2012, 8, 133–142. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Marcos, J.; Aguilera, P.; To-Figueras, J.; Pozo, O.J. Comprehensive analysis of the tryptophan metabolome in urine of patients with acute intermittent porphyria. J. Chromatogr. B 2017, 1060, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Middleton, D.R.; Watts, M.J.; Lark, R.M.; Milne, C.J.; Polya, D.A. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ. Health 2016, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Rotter, M.; Frenzel, S.; Zacharias, H.U.; Krumsiek, J.; Rathkolb, B.; Hrabe de Angelis, M.; Rabstein, S.; Pallapies, D.; Brüning, T.; Grabe, H.J.; Wang-Sattler, R. Dilution correction for dynamically influenced urinary analyte data. Anal. Chim. Acta 2018. [Google Scholar] [CrossRef]
- Morris, C.; O’Grada, C.; Ryan, M.; Roche, H.M.; Gibney, M.J.; Gibney, E.R.; Brennan, L. The relationship between BMI and metabolomic profiles: A focus on amino acids. Proc. Nutr. Soc. 2012, 71, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Röjdmark, S.; Berg, A.; Rössner, S.; Wetterberg, L. Nocturnal melatonin secretion in thyroid disease and in obesity. Clin. Endocrinol. 1991, 35, 61–65. [Google Scholar] [CrossRef]
| Clinical Parameters | All Participants | Shift Working Participants (Combined Analysis) | Stratified Analysis | ||
|---|---|---|---|---|---|
| Early Chronotype | Intermediate Chronotype | Late Chronotype | |||
| N | 97 | 68 | 16 | 22 | 30 |
| Chronotype (SD) *, a.m. | 04:02 (01:17) | 04:21 (01:14) | 02:50 (00:43) | 03:59 (00:14) | 05:26 (00:48) |
| Mean age (range), years | 39.5 (25.0–60.0) | 37.2 (25.0–57.0) | 41.3 (25.0–50.0) | 40.5 (25.0–57.0) | 32.5 (25.0–56.0) |
| BMI, kg/m2 | 26.2 (5.2) | 26.2 (5.0) | 26.6 (4.5) | 26.7 (5.5) | 25.7 (5.0) |
| Regular smoker (%) | 27 (27.8) | 26 (38.2) | 3 (18.8) | 10 (45.5) | 13 (43.3) |
| Thyroid disease (%) | 20 (20.1) | 12 (17.6) | 4 (25.0) | 2 (9.1) | 6 (19.4) |
| Hypertension (%) | 16 (16.5) | 10 (14.7) | 2 (12.5) | 3 (13.6) | 5 (16.7) |
| Respiratory disease (%) | 14 (14.4) | 9 (13.4) | 0 (0.0) | 5 (23.8) | 4 (13.3) |
| Cases of Allergy (%) | 53 (54.6) | 37 (54.4) | 6 (37.5) | 11 (50.0) | 20 (66.7) |
| Kidney disease (%) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Metabolite | Creatinine Normalization vs. Osmolality Normalization | Osmolality Normalization vs. RBN | RBN vs. Creatinine Normalization |
|---|---|---|---|
| C0 | 0.76 | 0.84 | 0.97 |
| C2 | 0.84 | 0.85 | 0.98 |
| C3 | 0.68 | 0.76 | 0.96 |
| C4:1 | 0.93 | 0.93 | 0.99 |
| C5 | 0.73 | 0.89 | 0.93 |
| C5-M-DC | 0.75 | 0.83 | 0.96 |
| C5:1 | 0.62 | 0.79 | 0.93 |
| C5:1-DC | 0.68 | 0.76 | 0.95 |
| C6:1 | 0.13 | 0.55 | 0.71 |
| C7-DC | 0.21 | 0.56 | 0.79 |
| C8 | −0.01 | 0.49 | 0.61 |
| C8:1 | 0.70 | 0.72 | 0.96 |
| C9 | 0.65 | 0.77 | 0.93 |
| C10 | −0.08 | 0.49 | 0.57 |
| C10:1 | 0.18 | 0.49 | 0.79 |
| C10:2 | 0.74 | 0.82 | 0.96 |
| C12 | 0.09 | 0.63 | 0.64 |
| C14 | 0.16 | 0.66 | 0.56 |
| C14:1 | 0.23 | 0.70 | 0.70 |
| C14:1-OH | 0.16 | 0.70 | 0.61 |
| C14:2 | 0.12 | 0.64 | 0.62 |
| C14:2-OH | 0.05 | 0.61 | 0.57 |
| C16 | 0.47 | 0.78 | 0.77 |
| C16-OH | 0.68 | 0.90 | 0.86 |
| C16:2 | 0.19 | 0.75 | 0.48 |
| C18:2 | 0.32 | 0.76 | 0.49 |
| Arg | 0.33 | 0.44 | 0.80 |
| Gln | 0.44 | 0.50 | 0.86 |
| Gly | 0.64 | 0.60 | 0.92 |
| His | 0.58 | 0.51 | 0.90 |
| Met | −0.09 | 0.35 | 0.61 |
| Phe | 0.42 | 0.52 | 0.87 |
| Pro | 0.22 | 0.42 | 0.71 |
| Ser | 0.44 | 0.55 | 0.85 |
| Thr | 0.50 | 0.53 | 0.89 |
| Trp | 0.21 | 0.41 | 0.81 |
| Tyr | 0.48 | 0.49 | 0.88 |
| Val | 0.28 | 0.36 | 0.75 |
| Leu/Isoleu | 0.45 | 0.36 | 0.76 |
| Creatinine | - | 0.20 | - |
| PC ae C38:3 | 0.44 | 0.83 | 0.61 |
| PC ae C38:6 | 0.11 | 0.55 | 0.66 |
| SM C24:0 | 0.44 | 0.80 | 0.72 |
| H1 | 0.50 | 0.59 | 0.88 |
| Combined Analysis N = 68; n = 424 | Early Chronotype N = 16; n = 91 | Intermediate Chronotype N = 22; n = 141 | Late Chronotype N = 30; n = 192 | |||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | β–Estimate (95% CI) | FDR p-value | β–Estimate (95% CI) | FDR p-value | β-Estimate (95% CI) | FDR p-Value | β-Estimate (95% CI) | FDR p-Value |
| C5 | 0.09 (0.03, 0.15) | 1.8 × 10−2 | 0.24 (0.10, 0.38) | 6.3 × 10−3 | −0.02 (−0.12, 0.09) | 0.87 | 0.10 (0.01, 0.19) | 0.12 |
| C7-DC | 0.23 (0.12, 0.35) | 7.1 × 10−4 | 0.53 (0.25, 0.81) | 4.3 × 10−3 | 0.14 (−0.08, 0.35) | 0.47 | 0.15 (−0.01, 0.31) | 0.23 |
| C8 | 0.15 (0.05, 0.26) | 1.7 × 10−2 | 0.51 (0.23, 0.79) | 4.3 × 10−3 | 0.00 (−0.21, 0.20) | 0.99 | 0.16 (0.02, 0.29) | 0.12 |
| C10 | 0.18 (0.07, 0.28) | 4.7 × 10−3 | 0.57 (0.26, 0.88) | 4.3 × 10−3 | 0.05 (−0.15, 0.25) | 0.83 | 0.14 (0.01, 0.27) | 0.12 |
| C10:2 | −0.34 (−0.50, −0.18) | 5.1 × 10-4 | −0.22 (−0.69, 0.24) | 0.46 | −0.22 (−0.47, 0.03) | 0.38 | −0.39 (−0.64, −0.14) | 2.3 × 10−2 |
| C12 | 0.30 (0.18, 0.42) | 1.4 × 10−5 | 0.68 (0.37, 0.99) | 2.0 × 10−3 | 0.19 (−0.06, 0.44) | 0.41 | 0.23 (0.09, 0.37) | 2.3 × 10−2 |
| C14 | 0.16 (0.04, 0.27) | 2.1 × 10−2 | 0.53 (0.19, 0.86) | 8.7 × 10−3 | 0.09 (−0.13, 0.32) | 0.66 | 0.04 (−0.10, 0.19) | 0.78 |
| C14:1 | 0.18 (0.07, 0.28) | 4.7 × 10−3 | 0.52 (0.23, 0.82) | 4.6 × 10−3 | 0.14 (−0.06, 0.35) | 0.45 | 0.04 (−0.09, 0.17) | 0.78 |
| C14:1-OH | 0.21 (0.09, 0.33) | 4.7 × 10−3 | 0.58 (0.23, 0.93) | 6.6 × 10−3 | 0.20 (−0.04, 0.43) | 0.41 | 0.05 (−0.10, 0.20) | 0.78 |
| C14:2 | 0.18 (0.07, 0.29) | 6.6 × 10−3 | 0.50 (0.17, 0.82) | 1.1 × 10−2 | 0.13 (−0.07, 0.34) | 0.47 | 0.08 (−0.06, 0.22) | 0.52 |
| C14:2-OH | 0.16 (0.05, 0.28) | 1.8 × 10−2 | 0.56 (0.22, 0.91) | 7.6 × 10−3 | 0.09 (−0.13, 0.30) | 0.66 | 0.04 (−0.10, 0.18) | 0.81 |
| Gly | 0.16 (0.05, 0.28) | 1.8 × 10−2 | −0.09 (−0.35, 0.17) | 0.65 | 0.27 (0.07, 0.46) | 0.10 | 0.21 (0.04, 0.39) | 0.12 |
| Phe | 0.33 (0.20, 0.45) | 1.4 × 10−5 | 0.40 (0.03, 0.78) | 8.4 × 10−2 | 0.37 (0.14, 0.60) | 6.9 × 10−2 | 0.32 (0.15, 0.50) | 8.0 × 10−3 |
| Ser | 0.15 (0.03, 0.27) | 3.8 × 10−2 | −0.06 (−0.35, 0.23) | 0.77 | 0.22 (0.02, 0.43) | 0.29 | 0.17 (−0.02, 0.36) | 0.25 |
| SM C24:0 | 0.23 (0.14, 0.32) | 1.4 × 10−5 | 0.48 (0.20, 0.76) | 5.3 × 10−3 | 0.14 (−0.04, 0.32) | 0.41 | 0.21 (0.11, 0.31) | 4.1 × 10−3 |
| Osmolality-Normalization | Regression Based Normalization | |||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Basic Model | Full model | Basic Model | Full model | ||||
| β-Estimate (95% CI) | FDR | β-Estimate (95% CI) | FDR | β-Estimate (95% CI) | FDR | β-Estimate (95% CI) | FDR | |
| C3 | −0.23 (−0.39, −0.07) | 2.0 × 10−2 | −0.25 (−0.42, −0.07) | 1.9 × 10−2 | −0.14 (−0.29, 0.02) | 0.17 | −0.15 (−0.32, 0.01) | 0.17 |
| C4:1 | −0.10 (−0.16, −0.04) | 3.7 × 10−3 | −0.10 (−0.16, −0.05) | 4.2 × 10−3 | −0.07 (−0.12, −0.02) | 2.1 × 10−2 | −0.07 (−0.12, −0.02) | 4.5 × 10−2 |
| C5-M-DC | −0.26 (−0.37, −0.14) | 2.5 × 10−4 | −0.29 (−0.41, −0.16) | 1.1 × 10−4 | −0.22 (−0.32, −0.11) | 5.9 × 10−4 | −0.24 (−0.35, −0.13) | 4.6 × 10−4 |
| C5:1 | −0.27 (−0.42, −0.12) | 3.5 × 10−3 | −0.32 (−0.48, −0.16) | 9.2 × 10−4 | −0.19 (−0.33, −0.06) | 2.1 × 10−2 | −0.25 (−0.39, −0.11) | 4.7 × 10−3 |
| C7-DC | −0.05 (−0.21, 0.11) | 0.62 | −0.04 (−0.21, 0.14) | 0.72 | 0.26 (0.10, 0.42) | 1.1 × 10−2 | 0.29 (0.12, 0.46) | 6.9 × 10−3 |
| C10:2 | −0.49 (−0.65, −0.33) | 1.6 × 10−7 | −0.47 (−0.64, −0.30) | 4.3 × 10−6 | −0.42 (−0.58, −0.25) | 2.0 × 10−5 | −0.39 (−0.57, −0.22) | 4.1 × 10−4 |
| C12 | 0.03 (−0.12, 0.19) | 0.72 | 0.06 (−0.11, 0.23) | 0.51 | 0.25 (0.09, 0.41) | 1.2 × 10−2 | 0.31 (0.14, 0.48) | 3.5 × 10−3 |
| C18:2 | −0.19 (−0.34, −0.04) | 3.5 × 10−2 | −0.23 (−0.39, −0.07) | 1.8 × 10−2 | −0.25 (−0.40, −0.09) | 1.1 × 10−2 | −0.32 (−0.48, −0.15) | 1.7 × 10−3 |
| Arg | −0.29 (−0.44, −0.15) | 8.8 × 10−4 | −0.31 (−0.46, −0.16) | 9.2 × 10−4 | −0.20 (−0.36, −0.04) | 6.2 × 10−2 | −0.26 (−0.43, −0.08) | 2.1 × 10−2 |
| His | −0.15 (−0.28, −0.03) | 3.8 × 10−2 | −0.16 (−0.29, −0.03) | 4.3 × 10−2 | −0.03 (−0.15, 0.08) | 0.70 | −0.05 (−0.17, 0.08) | 0.56 |
| Met | −0.23 (−0.38, −0.07) | 1.7 × 10−2 | −0.24 (−0.40, −0.08) | 1.4 × 10−2 | −0.13 (−0.30, 0.03) | 0.21 | −0.16 (−0.33, 0.02) | 0.18 |
| Phe | 0.00 (−0.15, 0.14) | 0.98 | −0.02 (−0.18, 0.13) | 0.76 | 0.24 (0.09, 0.39) | 1.1 × 10−2 | 0.21 (0.06, 0.37) | 3.6 × 10−2 |
| Pro | −0.24 (−0.38, −0.09) | 9.9 × 10−3 | −0.25 (−0.41, −0.10) | 7.0 × 10−3 | −0.05 (−0.19, 0.09) | 0.61 | −0.09 (−0.24, 0.06) | 0.36 |
| Thr | −0.20 (−0.36, −0.05) | 3.2 × 10−2 | −0.22 (−0.38, −0.06) | 2.7 × 10−2 | −0.09 (−0.23, 0.05) | 0.42 | −0.12 (−0.27, 0.04) | 0.28 |
| Trp | −0.19 (−0.32, −0.06) | 2.0 × 10−2 | −0.21 (−0.36, −0.07) | 1.3 × 10−2 | −0.01 (−0.12, 0.11) | 0.90 | −0.05 (−0.17, 0.07) | 0.56 |
| Val | −0.18 (−0.33, −0.03) | 4.1 × 10−2 | −0.20 (−0.36, −0.04) | 4.1 × 10−2 | −0.07 (−0.22, 0.09) | 0.56 | −0.11 (−0.28, 0.05) | 0.31 |
| Leu/Isoleu | −0.25 (−0.40, −0.10) | 7.6 × 10−3 | −0.27 (−0.43, −0.11) | 5.1 × 10−3 | −0.09 (−0.24, 0.07) | 0.49 | −0.13 (−0.30, 0.04) | 0.28 |
| Creatinine | −0.18 (−0.33, −0.04) | 3.4 × 10−2 | −0.19 (−0.34, −0.04) | 3.5 × 10−2 | −0.15 (−0.30, 0.00) | 0.12 | −0.18 (−0.34, −0.02) | 9.3 × 10−2 |
| PC ae C38:3 | −0.35 (−0.51, −0.18) | 6.8 × 10−4 | −0.34 (−0.51, −0.16) | 2.1 × 10−3 | −0.57 (−0.74, −0.40) | 4.5 × 10−9 | −0.55 (−0.74, −0.37) | 2.0 × 10−7 |
| SM C24:0 | 0.05 (−0.07, 0.18) | 0.45 | 0.07 (−0.07, 0.20) | 0.39 | 0.19 (0.06, 0.31) | 1.9 × 10−2 | 0.21 (0.08, 0.34) | 1.2 × 10−2 |
| H1 | −0.26 (−0.40, −0.11) | 3.7 × 10−3 | −0.25 (−0.40, −0.10) | 7.0 × 10−3 | −0.20 (−0.36, −0.04) | 0.62 | −0.17 (−0.34, 0.01) | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotter, M.; Brandmaier, S.; Covic, M.; Burek, K.; Hertel, J.; Troll, M.; Bader, E.; Adam, J.; Prehn, C.; Rathkolb, B.; et al. Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype. Metabolites 2018, 8, 45. https://doi.org/10.3390/metabo8030045
Rotter M, Brandmaier S, Covic M, Burek K, Hertel J, Troll M, Bader E, Adam J, Prehn C, Rathkolb B, et al. Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype. Metabolites. 2018; 8(3):45. https://doi.org/10.3390/metabo8030045
Chicago/Turabian StyleRotter, Markus, Stefan Brandmaier, Marcela Covic, Katarzyna Burek, Johannes Hertel, Martina Troll, Erik Bader, Jonathan Adam, Cornelia Prehn, Birgit Rathkolb, and et al. 2018. "Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype" Metabolites 8, no. 3: 45. https://doi.org/10.3390/metabo8030045
APA StyleRotter, M., Brandmaier, S., Covic, M., Burek, K., Hertel, J., Troll, M., Bader, E., Adam, J., Prehn, C., Rathkolb, B., Hrabe de Angelis, M., Grabe, H. J., Daniel, H., Kantermann, T., Harth, V., Illig, T., Pallapies, D., Behrens, T., Brüning, T., ... Wang-Sattler, R. (2018). Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype. Metabolites, 8(3), 45. https://doi.org/10.3390/metabo8030045







