Analysis of Sub-Lethal Toxicity of Perfluorooctane Sulfonate (PFOS) to Daphnia magna Using 1H Nuclear Magnetic Resonance-Based Metabolomics
Abstract
:1. Introduction
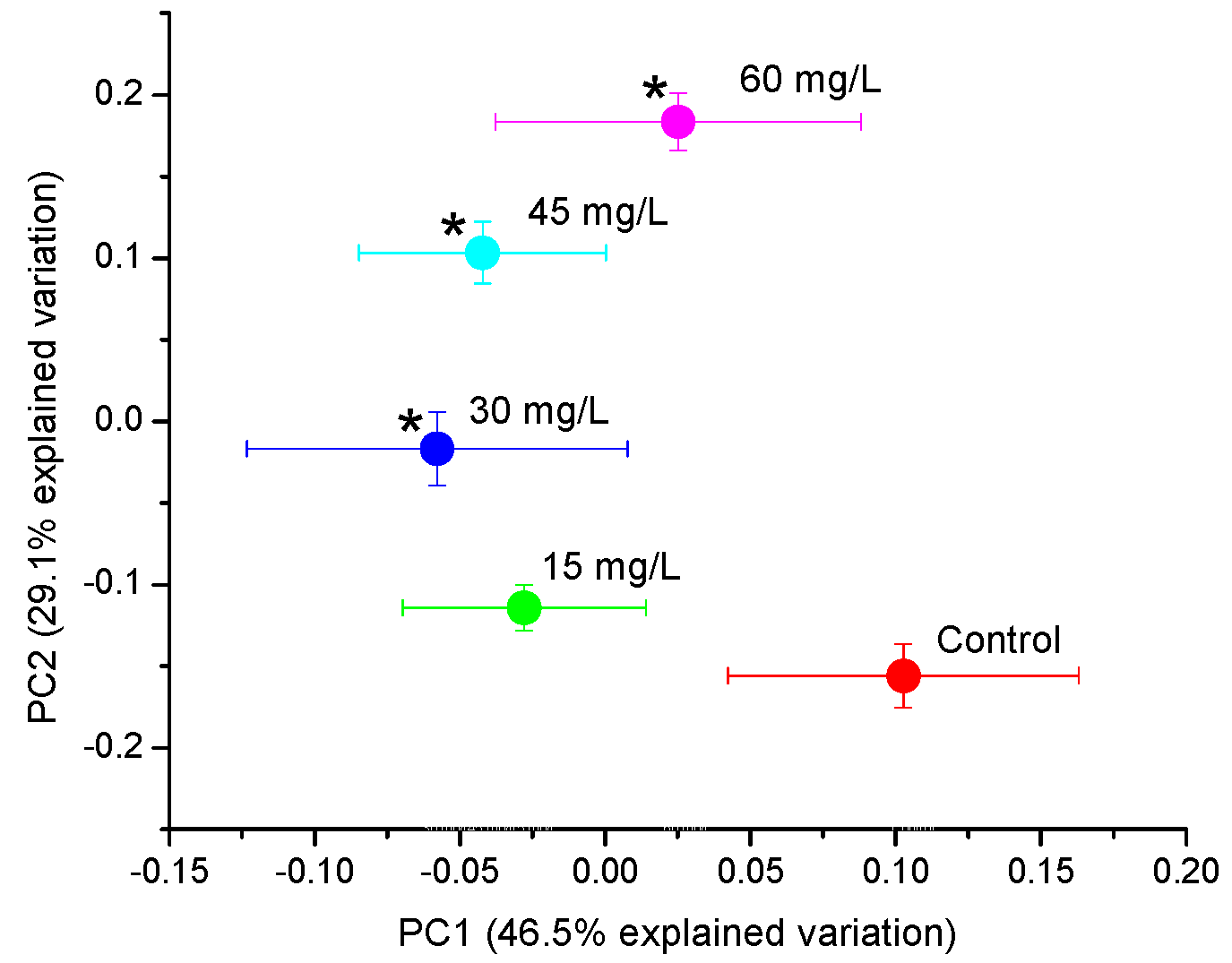
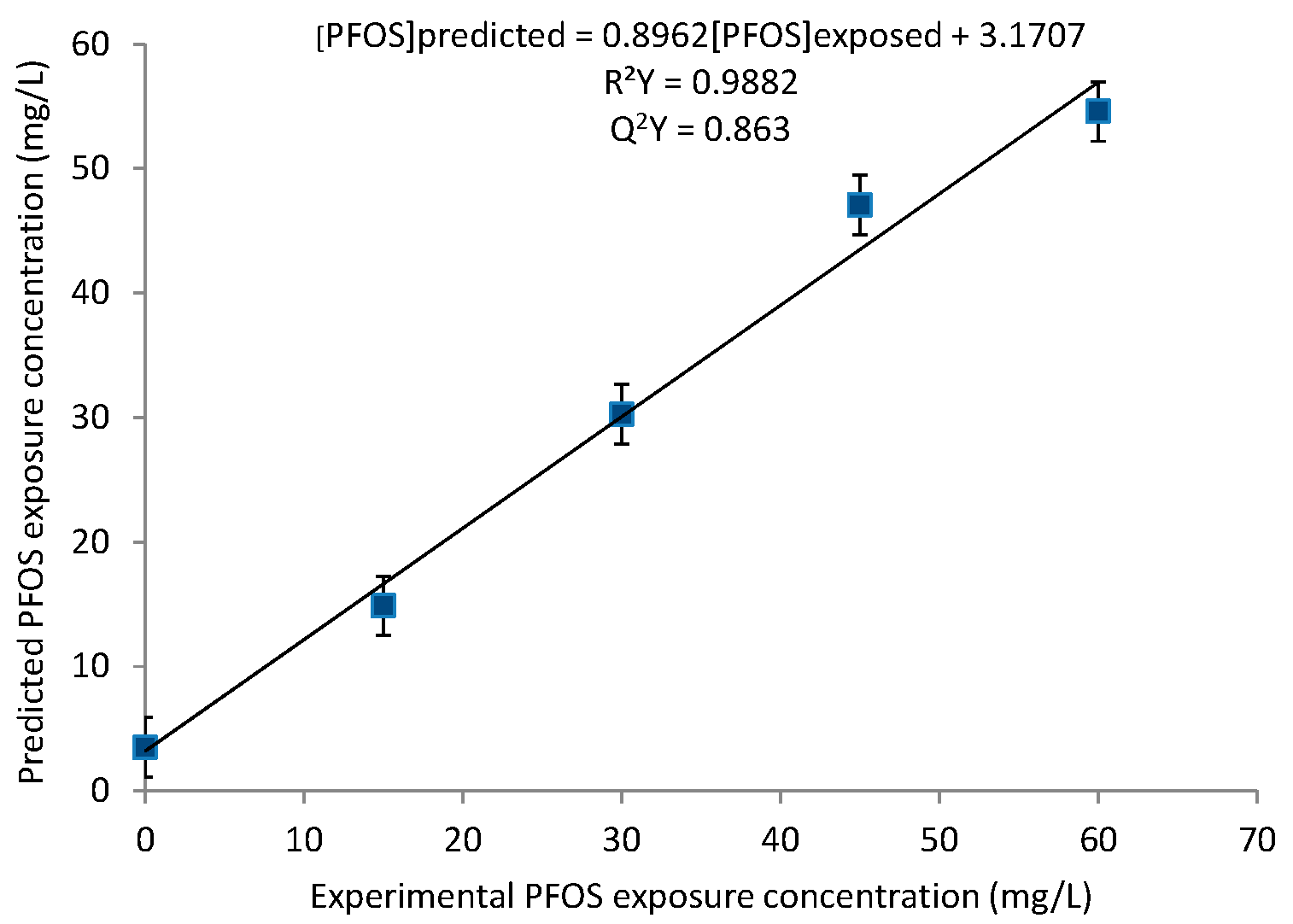
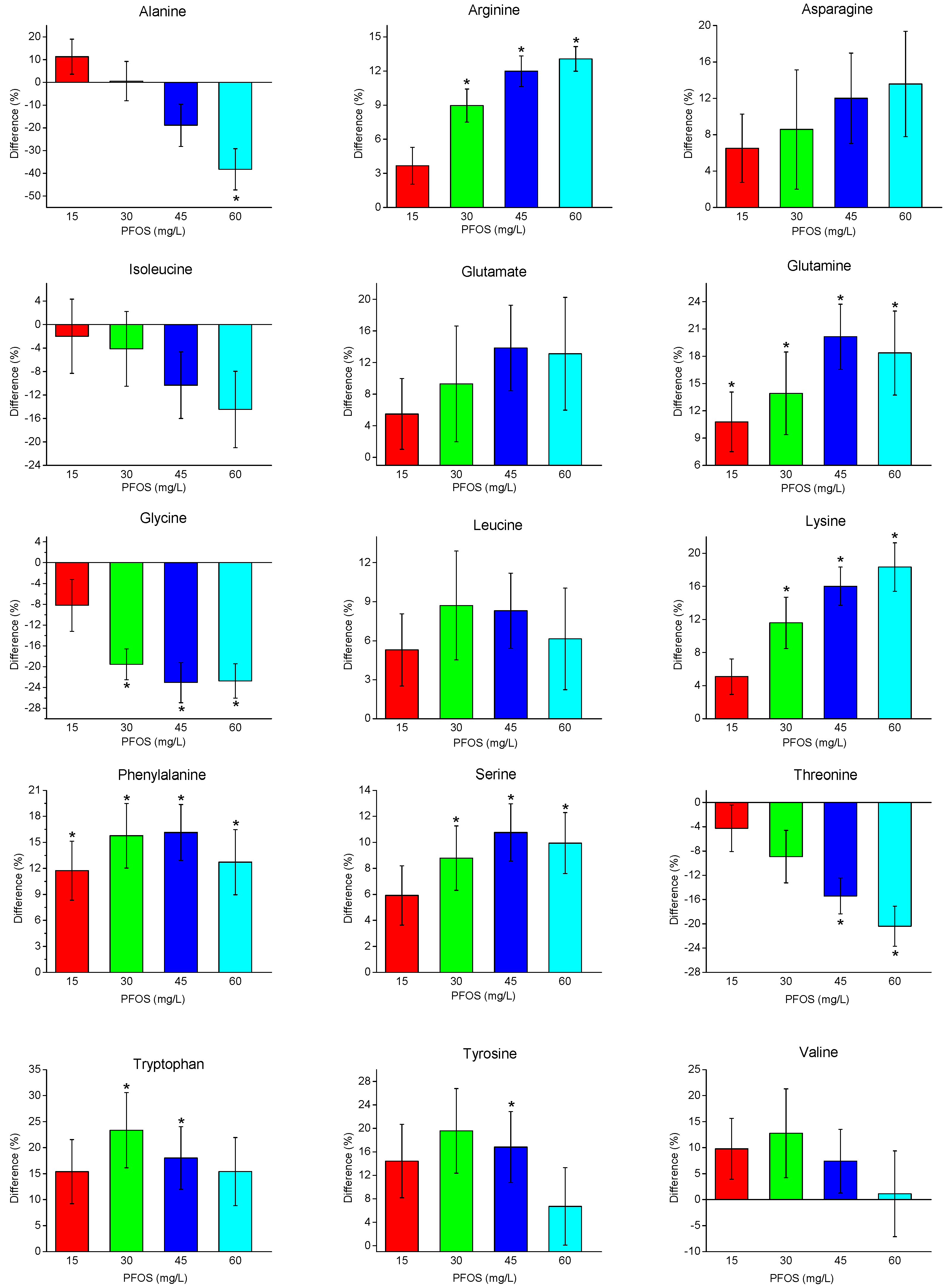
2. Results
2.1. Principal Component Analysis (PCA) and Partial Least Squares (PLS) Regression
2.2. Metabolite Changes with PFOS Exposure
3. Discussion
4. Materials and Methods
4.1. Culturing and Maintenance of Daphnia magna
4.2. Sub-Lethal Exposure Experiments
4.3. Metabolite Extraction and Analysis by 1H NMR Spectroscopy
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Hansen, K.J.; Wade, T.L.; Giesy, J.P. Perfluorooctane sulfonate in oysters, Crassostrea virginica, from the Gulf of Mexico and the Chesapeake Bay, USA. Arch. Environ. Contam. Toxicol. 2002, 42, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38–90. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, Z.; Feng, L.; Wang, Y. Perfluorooctane sulfonate (PFOS) induced embryotoxicity and disruption of cardiogenesis. Toxicol. In Vitro 2013, 27, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Johnson, H.O.; Eldridge, J.S.; Butenhoff, J.L.; Dick, L.A. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 2002, 36, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Lau, C. Perfluoroalkyl acids: Recent research highlights. Reprod. Toxicol. 2012, 33, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Ericson-Jogsten, I.; Gemma, P.; Nadal, M.; Van Bavel, B.; Kärrman, A. Human exposure to perfluorinated compounds in Catalonia, Spain: Contribution of drinking water and fish and shellfish. J. Agric. Food Chem. 2012, 60, 4408–4415. [Google Scholar] [CrossRef] [PubMed]
- Gewurtz, S.B.; Bhavsar, S.P.; Petro, S.; Mahon, C.G.; Zhao, X.; Morse, D.; Reiner, E.J.; Tittlemier, S.A.; Braekevelt, E.; Drouillard, K. High levels of perfluoroalkyl acids in sport fish species downstream of a firefighting training facility at Hamilton International Airport, Ontario, Canada. Environ. Int. 2014, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gewurtz, S.B.; De Silva, A.O.; Backus, S.M.; McGoldrick, D.J.; Keir, M.J.; Small, J.; Melymuk, L.; Muir, D.C.G. Perfluoroalkyl contaminants in Lake Ontario lake trout: Detailed examination of current status and long-term trends. Environ. Sci. Technol. 2012, 46, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Gulliver, J.S.; Simcik, M.F. Perfluorooctane sulfonate (PFOS) contamination of fish in urban lakes: A prioritization methodology for lake management. Water Res. 2013, 47, 7264–7272. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Rabearisoa, A.H.; Jiang, X.; Dai, Z. Bioaccumulation of perfluoroalkyl substances by Daphnia magna in water with different types and concentrations of protein. Environ. Sci. Technol. 2013, 47, 10955–10963. [Google Scholar] [CrossRef] [PubMed]
- Maki, A.; Bishop, W. Acute toxicity studies of surfactants to Daphnia magna and Daphnia pulex. Arch. Environ. Contam. Toxicol. 1979, 8, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Santojanni, A.; Gorbi, G.; Sartore, F. Prediction of mortality in chronic toxicity tests on Daphnia magna. Water Res. 1995, 29, 1453–1459. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. Iv. Cellular energy allocation: A new methodology to assess the energy budget of toxicant-stressed daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Vandenbrouck, T.; Jones, O.A.H.; Dom, N.; Griffin, J.L.; De Coen, W. Mixtures of similarly acting compounds in Daphnia magna: From gene to metabolite and beyond. Environ. Int. 2010, 36, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Perhar, G.; Kelly, N.E.; Ni, F.J.; Simpson, M.J.; Simpson, A.J.; Arhonditsis, G.B. Using daphnia physiology to drive food web dynamics: A theoretical revisit of Lotka-Volterra models. Ecol. Inform. 2016, 35, 29–42. [Google Scholar] [CrossRef]
- Kovacevic, V.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomics of Daphnia magna responses after sub-lethal exposure to triclosan, carbamazepine and ibuprofen. Comp. Biochem. Phys. D 2016, 19, 199–210. [Google Scholar]
- Li, L.; Wu, H.; Ji, C.; van Gestel, C.A.M.; Allen, H.E.; Peijnenburg, W.J.G.M. A metabolomic study on the responses of Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 119, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nagato, E.G.; D’eon, J.C.; Lankadurai, B.P.; Poirier, D.G.; Reiner, E.J.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomics investigation of Daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium. Chemosphere 2013, 93, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nagato, E.G.; Simpson, A.J.; Simpson, M.J. Metabolomics reveals energetic impairments in Daphnia magna exposed to diazinon, malathion and bisphenol-A. Aquat. Toxicol. 2016, 170, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Blalock, B.J.; Rogers, K.; Allen, H.J.; Loguinov, A.; Heckman, J.L.; Govindasmawy, S. Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.; Weber, R.; Southam, A.; Payne, T.; Hrydziuszko, O.; Arvanitis, T.; Viant, M. A new approach to toxicity testing in Daphnia magna: Application of high throughput FT-ICR mass spectrometry metabolomics. Metabolomics 2009, 5, 44–58. [Google Scholar] [CrossRef]
- Taylor, N.S.; Weber, R.J.M.; White, T.A.; Viant, M.R. Discriminating between different acute chemical toxicities via changes in the daphnid metabolome. Toxicol. Sci. 2010, 118, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.D.; Simpson, A.J.; Simpson, M.J. Metabolomic responses to sublethal contaminant exposure in neonate and adult Daphnia magna. Environ. Toxicol. Chem. 2017, 36, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Shi, Y.; Sarvajayakesavalu, S.; Baninla, Y.; Chen, J.; Lu, Y. Effects of perfluorooctane sulfonate on immobilization, heartbeat, reproductive and biochemical performance of Daphnia magna. Chemosphere 2017, 168, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.Y.; Yuk, M.S.; Jeon, J.; Kim, S.D. Multigenerational effect of perfluorooctane sulfonate (PFOS) on the individual fitness and population growth of Daphnia magna. Sci. Total Environ. 2016, 569–570, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.V.; García-Hortigüela, P.; Fernández, C. Acute and chronic toxicity of emerging contaminants, alone or in combination, in Chlorella vulgaris and Daphnia magna. Environ. Sci. Pollut. Res. 2015, 22, 5417–5424. [Google Scholar] [CrossRef] [PubMed]
- Nagato, E.G.; Lankadurai, B.P.; Soong, R.; Simpson, A.J.; Simpson, M.J. Development of an nmr microprobe procedure for high-throughput environmental metabolomics of Daphnia magna. Magn. Reson. Chem. 2015, 53, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.; Davey, M.; Viant, M. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3. [Google Scholar] [CrossRef]
- Lankadurai, B.P.; Nagato, E.G.; Simpson, M.J. Environmental metabolomics: An emerging approach to study organism responses to environmental stressors. Env. Rev. 2013, 21, 180–205. [Google Scholar] [CrossRef]
- Viant, M. Applications of metabolomics to the environmental sciences. Metabolomics 2009, 5, 1–2. [Google Scholar] [CrossRef]
- Fasulo, S.; Iacono, F.; Cappello, T.; Corsaro, C.; Maisano, M.; D’Agata, A.; Giannetto, A.; De Domenico, E.; Parrino, V.; Lo Paro, G.; et al. Metabolomic investigation of mytilus galloprovincialis (lamarck 1819) caged in aquatic environments. Ecotoxicol. Environ. Saf. 2012, 84, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Brandao, F.; Cappello, T.; Raimundo, J.; Santos, M.A.; Maisano, M.; Mauceri, A.; Pacheco, M.; Pereira, P. Unravelling the mechanisms of mercury hepatotoxicity in wild fish (Liza aurata) through a triad approach: Bioaccumulation, metabolomic profiles and oxidative stress. Metallomics 2015, 7, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Brandão, F.; Guilherme, S.; Santos, M.A.; Maisano, M.; Mauceri, A.; Canário, J.; Pacheco, M.; Pereira, P. Insights into the mechanisms underlying mercury-induced oxidative stress in gills of wild fish (Liza aurata) combining 1H NMR metabolomics and conventional biochemical assays. Sci. Tot. Environ. 2016, 548, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Mauceri, A.; Corsaro, C.; Maisano, M.; Parrino, V.; Lo Paro, G.; Messina, G.; Fasulo, S. Impact of environmental pollution on caged mussels Mytilus galloprovincialis using nmr-based metabolomics. Mar. Pollut. Bull. 2013, 77, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Pereira, P.; Maisano, M.; Mauceri, A.; Pacheco, M.; Fasulo, S. Advances in understanding the mechanisms of mercury toxicity in wild golden grey mullet (Liza aurata) by 1H NMR-based metabolomics. Environ. Pollut. 2016, 219, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R. Environmental metabolomics using 1H-NMR spectroscopy. Methods Mol. Biol. 2008, 410, 137–150. [Google Scholar] [PubMed]
- Simpson, M.J.; Bearden, D.W. Environmental metabolomics: NMR techniques. eMagRes 2013, 2, 549–560. [Google Scholar]
- Poynton, H.C.; Taylor, N.S.; Hicks, J.; Colson, K.; Chan, S.; Clark, C.; Scanlan, L.; Loguinov, A.V.; Vulpe, C.; Viant, M.R. Metabolomics of microliter hemolymph samples enables an improved understanding of the combined metabolic and transcriptional responses of Daphnia magna to cadmium. Environ. Sci. Technol. 2011, 45, 3710–3717. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, T.M.; Sibley, P.K.; Mabury, S.A.; Muir, D.G.C.; Solomon, K.R. Laboratory evaluation of the toxicity of perfluorooctane sulfonate (PFOS) on Selenastrum capricornutum, Chlorella vulgaris, Lemna gibba, Daphnia magna, and Daphnia pulicaria. Arch. Environ. Contam. Toxicol. 2003, 44, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.H.; Spurgeon, D.J.; Svendsen, C.; Griffin, J.L. A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere 2008, 71, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wang, Y.; Mu, J.; Wang, J. Aquatic predicted no-effect-concentration derivation for perfluorooctane sulfonic acid. Environ. Toxicol. Chem. 2011, 30, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Lewis, I.A.; Hegeman, A.D.; Anderson, M.E.; Li, J.; Schulte, C.F.; Westler, W.M.; Eghbalnia, H.R.; Sussman, M.R.; Markley, J.L. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008, 26, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Lankadurai, B.P.; Furdui, V.I.; Reiner, E.J.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomic analysis of sub-lethal perfluorooctane sulfonate exposure to the earthworm, Eisenia fetida, in soil. Metabolites 2013, 3, 718–740. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Gao, G.; Xue, H.; Zhang, X.; Wang, H. Exposure of perfluorononanoic acid suppresses the hepatic insulin signal pathway and increases serum glucose in rats. Toxicology 2012, 294, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.B.; Thibodeaux, J.R.; Wood, C.R.; Zehr, R.D.; Schmid, J.E.; Lau, C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicol. Appl. Pharmacol. 2007, 239, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Gorrochategui, E.; Pérez-Albaladejo, E.; Casas, J.; Lacorte, S.; Porte, C. Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol. 2014, 277, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ronnett, G.V.; Kim, E.-K.; Landree, L.E.; Tu, Y. Fatty acid metabolism as a target for obesity treatment. Phys. Behav. 2005, 85, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, J.S.; Keung, W.; Wang, W.; Ussher, J.R.; Lopaschuk, G.D. Targeting fatty acid and carbohydrate oxidation—A novel therapeutic intervention in the ischemic and failing heart. Biochimica et Biophysica Acta-Mol. Cell Res. 2011, 1813, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, A.; Knapen, D.; Meyer, I.J.; van der Ven, K.; Hoff, P.; de Coen, W. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio). Aquat. Toxicol. 2008, 88, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, Y.; Wu, D.; Zhu, Z.; Lü, C. Immunotoxicity and hepatotoxicity of PFOS and PFOA in tilapia (Oreochromis niloticus). Chin. J. Geochem. 2012, 31, 424–430. [Google Scholar] [CrossRef]
- Sato, Y.; Ashihara, H. Long-term effect of nacl on the activity of uridine and uracil salvage for nucleotide synthesis in cultured mangrove (Bruguiera sexangula) cells. Plant Sci. 2009, 176, 383–389. [Google Scholar] [CrossRef]
- Merényi, G.; Kónya, E.; Vértessy, B.G. Drosophila proteins involved in metabolism of uracil-DNA possess different types of nuclear localization signals. FEBS J. 2010, 277, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Pane, E.F.; McGeer, J.C.; Wood, C.M. Effects of chronic waterborne nickel exposure on two successive generations of Daphnia magna. Eniviron. Toxicol. Chem. 2004, 23, 1051–1056. [Google Scholar] [CrossRef]
- Garcia, R.F.; Gazola, V.A.F.G.; Barrena, H.C.; Hartmann, E.M.; Berti, J.; Toyama, M.H.; Boschero, A.C.; Carneiro, E.M.; Manso, F.C.; Bazotte, R.B. Blood amino acids concentration during insulin induced hypoglycemia in rats: The role of alanine and glutamine in glucose recovery. Amino Acids 2007, 33, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ekman, D.R.; Teng, Q.; Villeneuve, D.L.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Ankley, G.T.; Collette, T.W. Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17α-ethynylestradiol with metabolite profiling. Environ. Sci. Technol. 2008, 42, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Easton, J.M.; Stentiford, G.D.; Ludwig, C.; Arvanitis, T.N.; Viant, M.R. Metabolic changes in flatfish hepatic tumours revealed by NMR-based metabolomics and metabolic correlation networks. J. Proteome Res. 2008, 7, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Sibley, P.K.; Martin, J.W.; Maclean, D.D.; Solomon, K.R.; Mabury, S.A.; Van Der Kraak, G.J. Short-term exposures of fish to perfluorooctane sulfonate: Acute effects on fatty acyl-coa oxidase activity, oxidative stress, and circulating sex steroids. Environ. Toxicol. Chem. 2005, 24, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.A.; Lau, C.; Chang, S.C.; Butenhoff, J.L.; Wallace, K.B. Perfluorooctane sulfonate-induced changes in fetal rat liver gene expression. Toxicology 2008, 251, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A.; Mortensen, A.S. Lipid peroxidation and oxidative stress responses of salmon fed a diet containing perfluorooctane sulfonic- or perfluorooctane carboxylic acids. Comp. Biochem. Phys. C 2011, 154, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Sohlenius, A.K.; Lundgren, B.; Depierre, J.W. Perfluorooctanoic acid has persistent effects on peroxisome proliferation and related parameters in mouse-liver. J. Biochem. Toxicol. 1992, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Nordén, M.; Westman, O.; Venizelos, N.; Engwall, M. Perfluorooctane sulfonate increases β-oxidation of palmitic acid in chicken liver. Environ. Sci. Pollut. Res. 2012, 19, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Aiba, K.; Fukuda, K.; Tanaka, M. The induction of peroxisome proliferation in rat-liver by perfluorinated fatty-acids, metabolically inert derivatives of fatty-acids. J. Biochem 1985, 98, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.; Conceição, L.E.C.; Barr, Y.; Helland, S.; Pousão-Ferreira, P.; Yúfera, M.; Dinis, M.T. Tyrosine and phenylalanine supplementation on diplodus sargus larvae: Effect on growth and quality. Aquac. Res. 2010, 41, 1523–1532. [Google Scholar]
- McCoole, M.D.; Atkinson, N.J.; Graham, D.I.; Grasser, E.B.; Joselow, A.L.; McCall, N.M.; Welker, A.M.; Wilsterman, E.J., Jr.; Baer, K.N.; Tilden, A.R.; et al. Genomic analyses of aminergic signaling systems (dopamine, octopamine and serotonin) in Daphnia pulex. Comp. Biochem. Physol. D 2012, 7, D35–58. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Kutchko, K.; Siltberg-Liberles, J. Metazoan innovation: From aromatic amino acids to extracellular signaling. Amino Acids 2013, 45, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Combaret, L.; Dardevet, D.; Rieu, I.; Pouch, M.-N.; Béchet, D.; Taillandier, D.; Grizard, J.; Attaix, D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J. Phys.-Lond. 2005, 569, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Schriever, S.C.; Deutsch, M.J.; Adamski, J.; Roscher, A.A.; Ensenauer, R. Cellular signaling of amino acids towards mtorc1 activation in impaired human leucine catabolism. J. Nutr. Biochem. 2013, 24, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Chipman, D.; Barak, Z.; Schloss, J.V. Biosynthesis of 2-aceto-2-hydroxy acids: Acetolactate synthases and acetohydroxyacid synthases. Biochimica et Biophysica Acta-Protein Struct. Mol. Enzymol. 1998, 1385, 401–419. [Google Scholar] [CrossRef]
- Andersen, S.M.; Holen, E.; Aksnes, A.; Rønnestad, I.; Zerrahn, J.-E.; Espe, M. Dietary arginine affects energy metabolism through polyamine turnover in juvenile atlantic salmon (Salmo salar). Br. J. Nutr. 2013, 110, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D.I. Arginine. Biomed. Pharmacother. 2002, 56, 439–445. [Google Scholar] [CrossRef]
- Environmenta Canada. Biological Test Method: Reference Method for Determining Acute Lethality of Effluents to Daphnia magna; Environmental Technology Centre: Ottawa, ON, Canada, 2000. [Google Scholar]
- Simpson, A.J.; Brown, S.A. Purge NMR: Effective and easy solvent suppression. J. Magn. Reson. 2005, 175, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.T. Recent advances in solvent suppression for solution NMR: A practical reference. Annu. Rep. NMR Spectrogr. 2009, 66, 33–76. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, M.N.; Nagato, E.G.; Lankadurai, B.P.; Simpson, A.J.; Simpson, M.J. Analysis of Sub-Lethal Toxicity of Perfluorooctane Sulfonate (PFOS) to Daphnia magna Using 1H Nuclear Magnetic Resonance-Based Metabolomics. Metabolites 2017, 7, 15. https://doi.org/10.3390/metabo7020015
Kariuki MN, Nagato EG, Lankadurai BP, Simpson AJ, Simpson MJ. Analysis of Sub-Lethal Toxicity of Perfluorooctane Sulfonate (PFOS) to Daphnia magna Using 1H Nuclear Magnetic Resonance-Based Metabolomics. Metabolites. 2017; 7(2):15. https://doi.org/10.3390/metabo7020015
Chicago/Turabian StyleKariuki, Martha N., Edward G. Nagato, Brian P. Lankadurai, André J. Simpson, and Myrna J. Simpson. 2017. "Analysis of Sub-Lethal Toxicity of Perfluorooctane Sulfonate (PFOS) to Daphnia magna Using 1H Nuclear Magnetic Resonance-Based Metabolomics" Metabolites 7, no. 2: 15. https://doi.org/10.3390/metabo7020015
APA StyleKariuki, M. N., Nagato, E. G., Lankadurai, B. P., Simpson, A. J., & Simpson, M. J. (2017). Analysis of Sub-Lethal Toxicity of Perfluorooctane Sulfonate (PFOS) to Daphnia magna Using 1H Nuclear Magnetic Resonance-Based Metabolomics. Metabolites, 7(2), 15. https://doi.org/10.3390/metabo7020015





