Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine
Abstract
:1. Introduction
2. Results
2.1. Sensory Analysis
2.2. High Resolution Gas Chromatography-Olfactometry (HRGC-O) and Comparative Aroma Extract Dilution Analysis (cAEDA) of Urine before and after Garlic Consumption
2.3. Identification of Garlic-Derived Metabolites in Urine
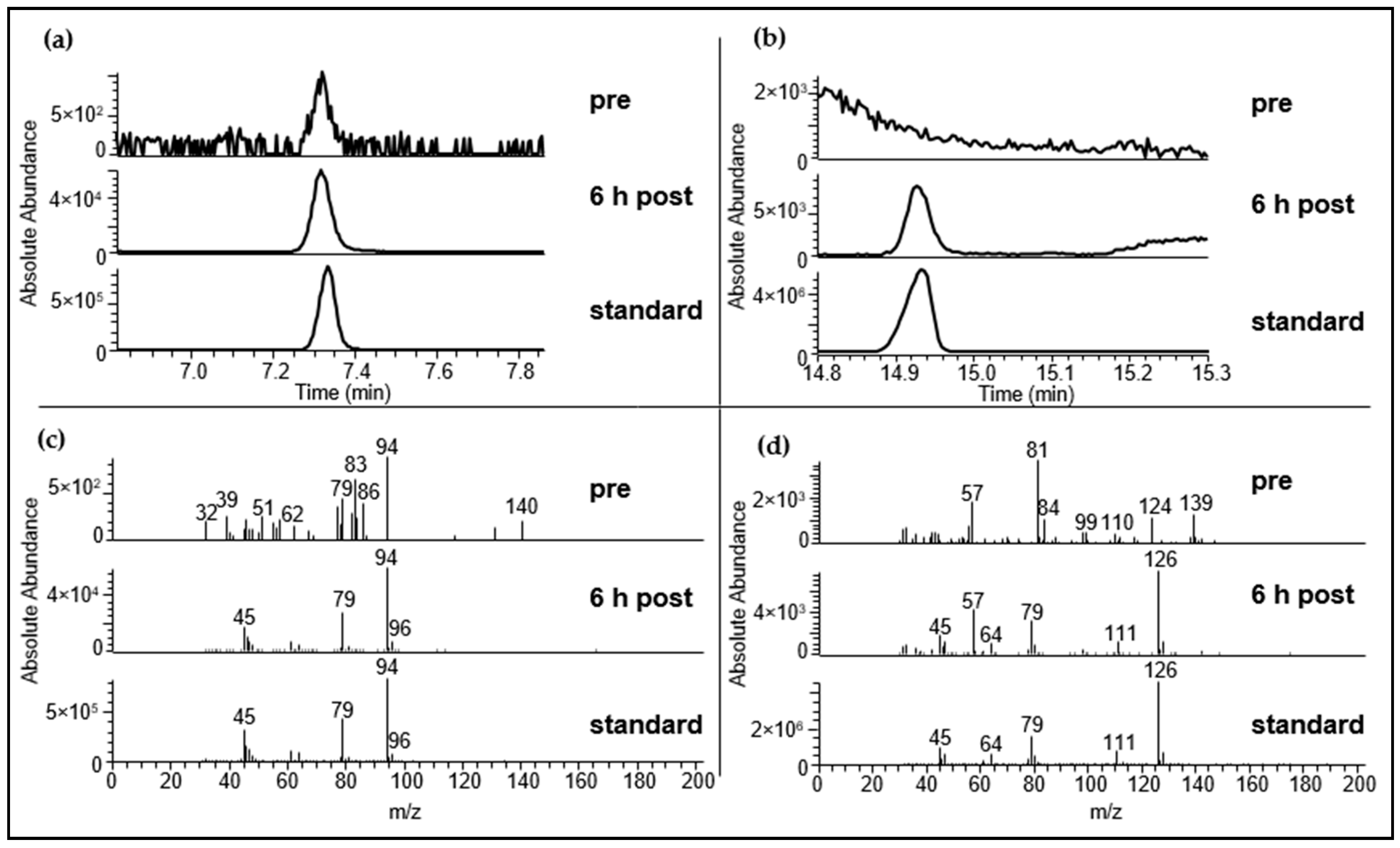
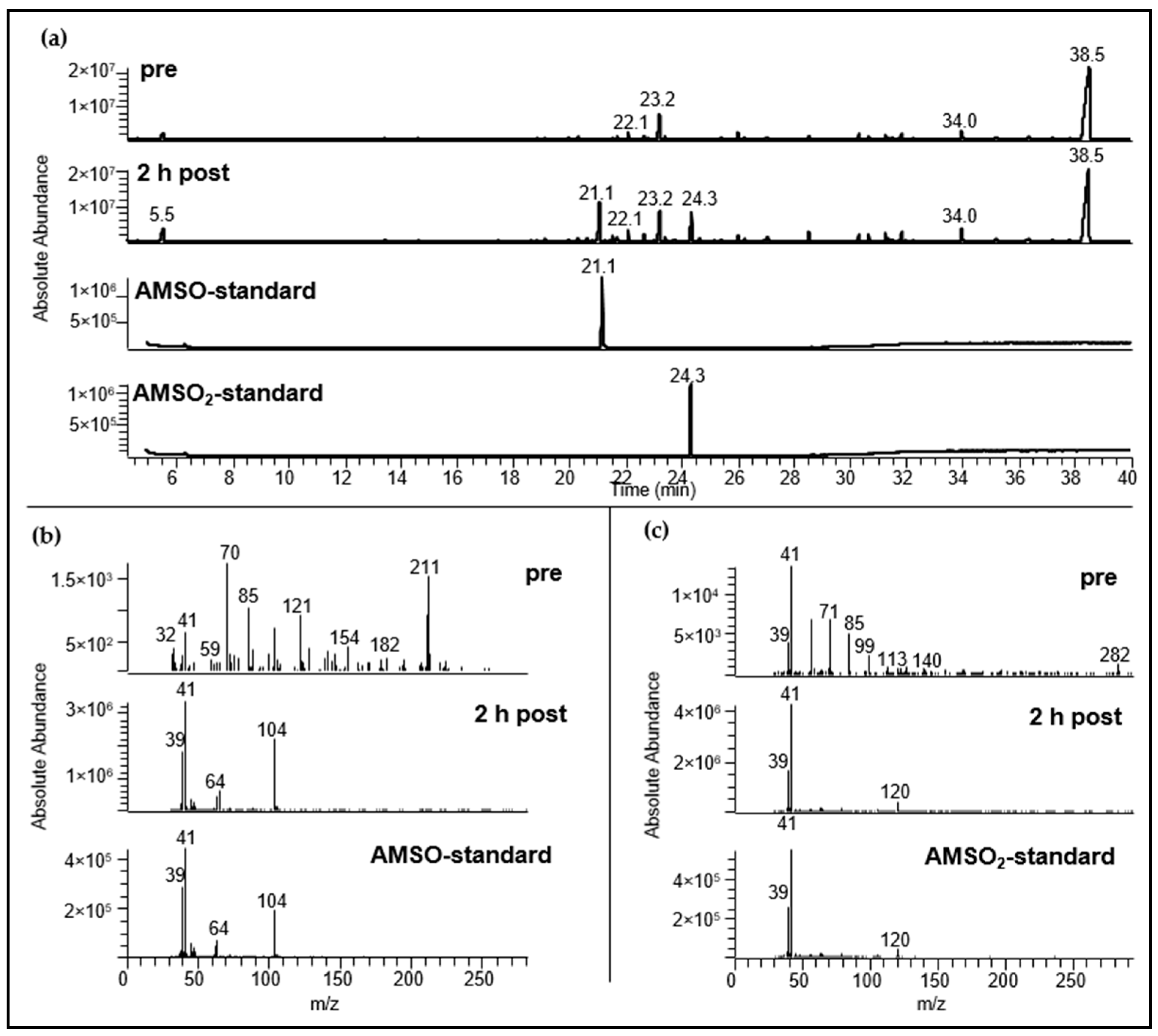
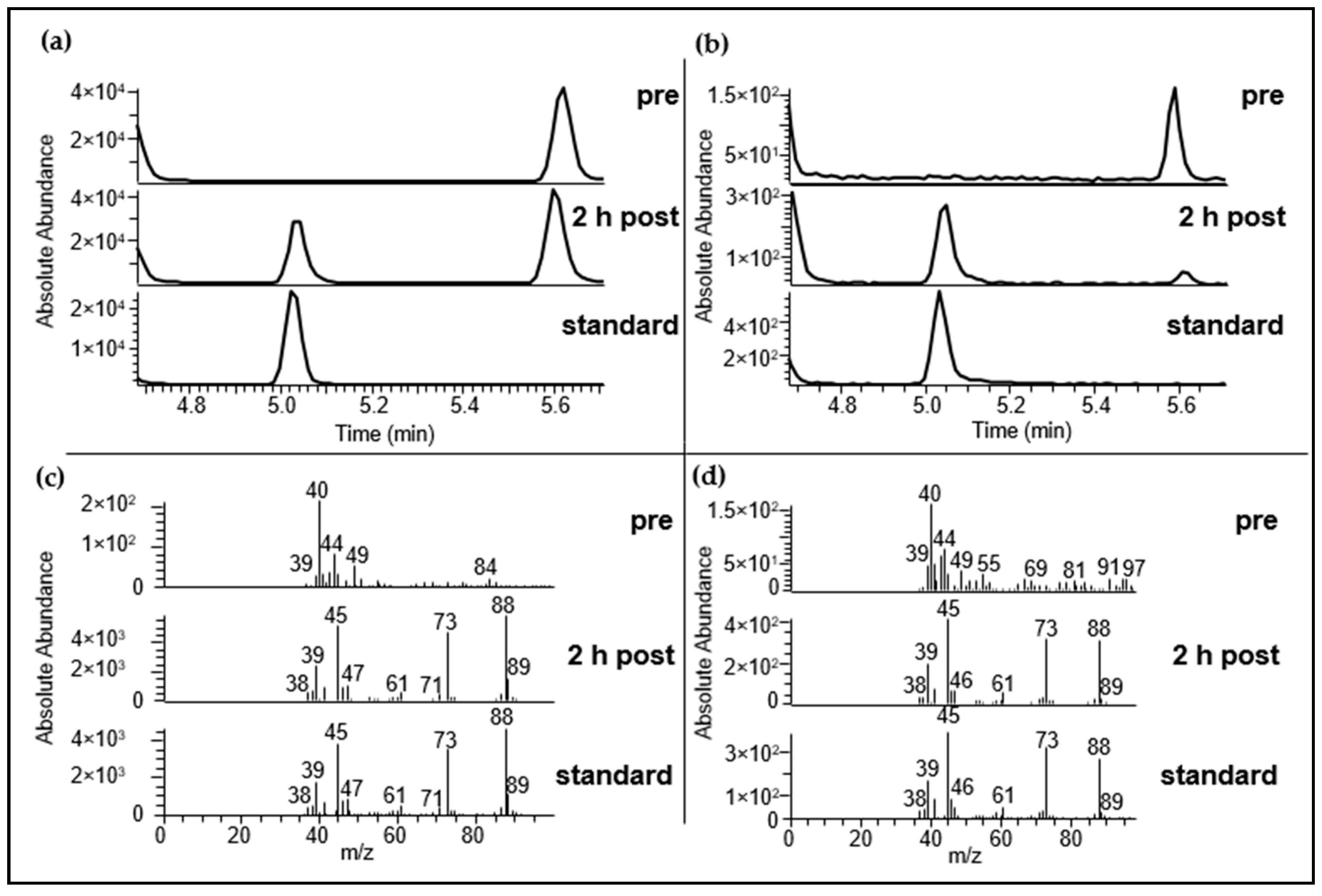
2.3.1. Identification Using HRGC-MS
2.3.2. Identification Using HRGC-GC-MS
2.4. Difference between Concentrations of the Garlic-Derived Metabolites Depending on Calculation Method
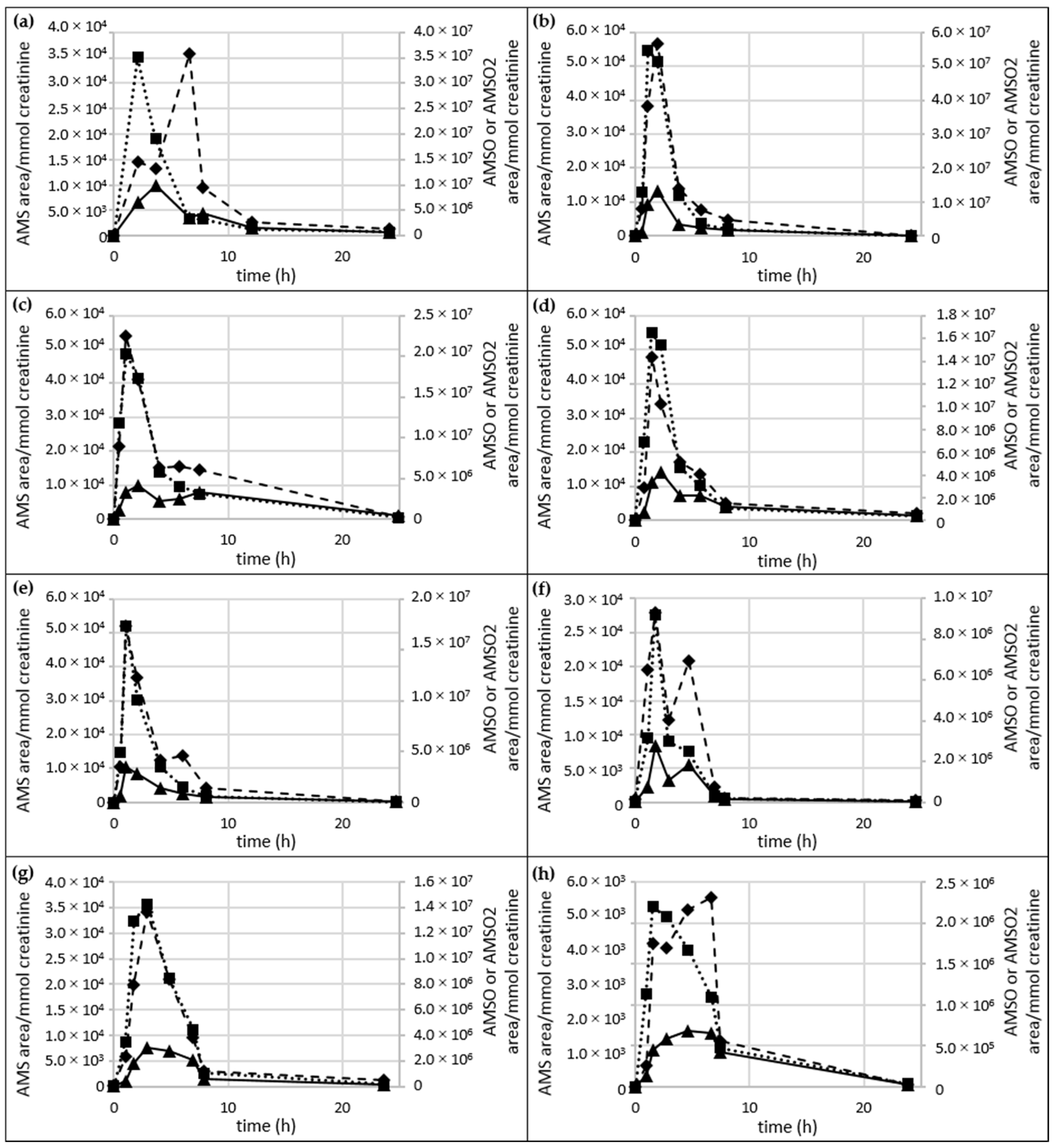
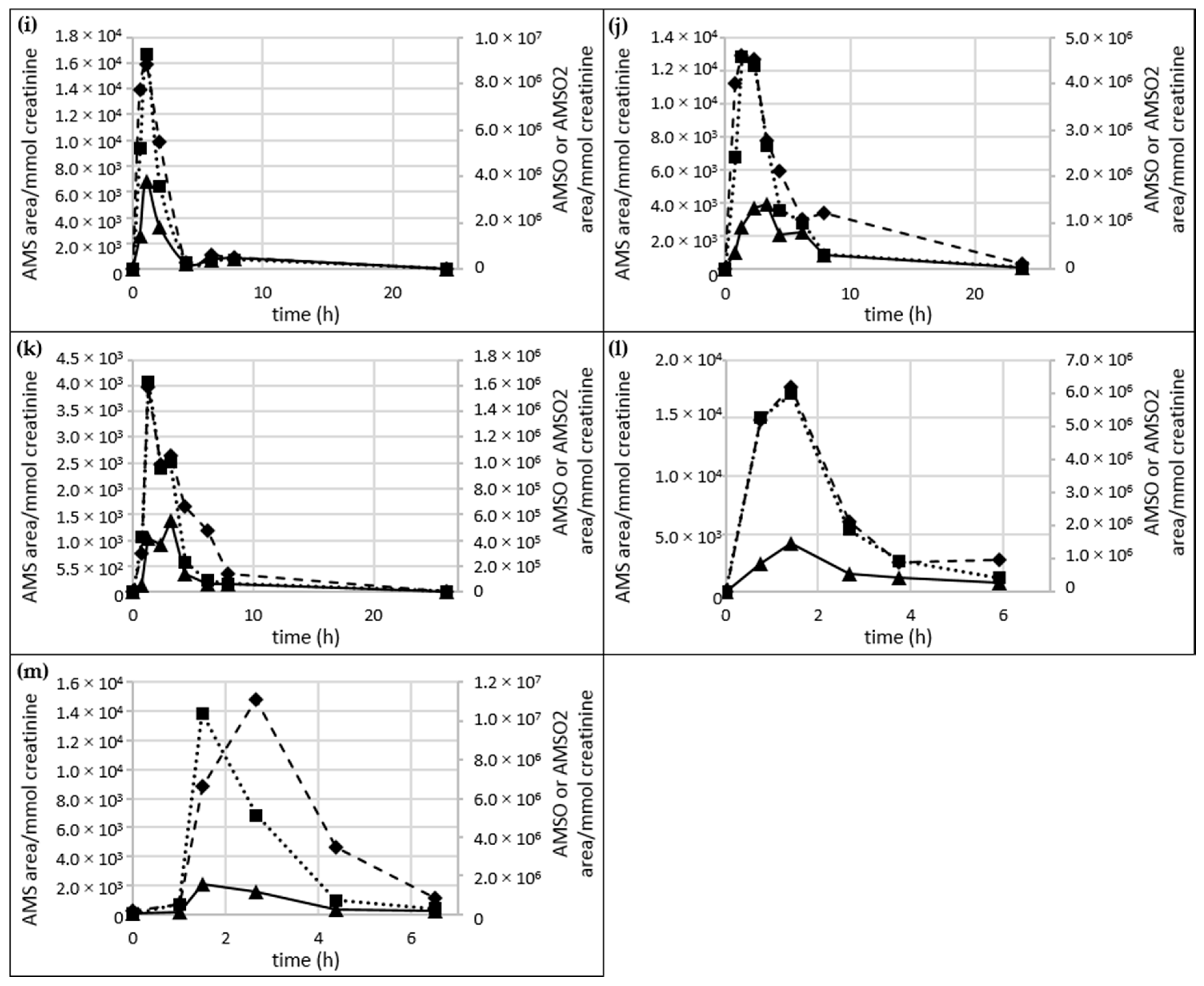
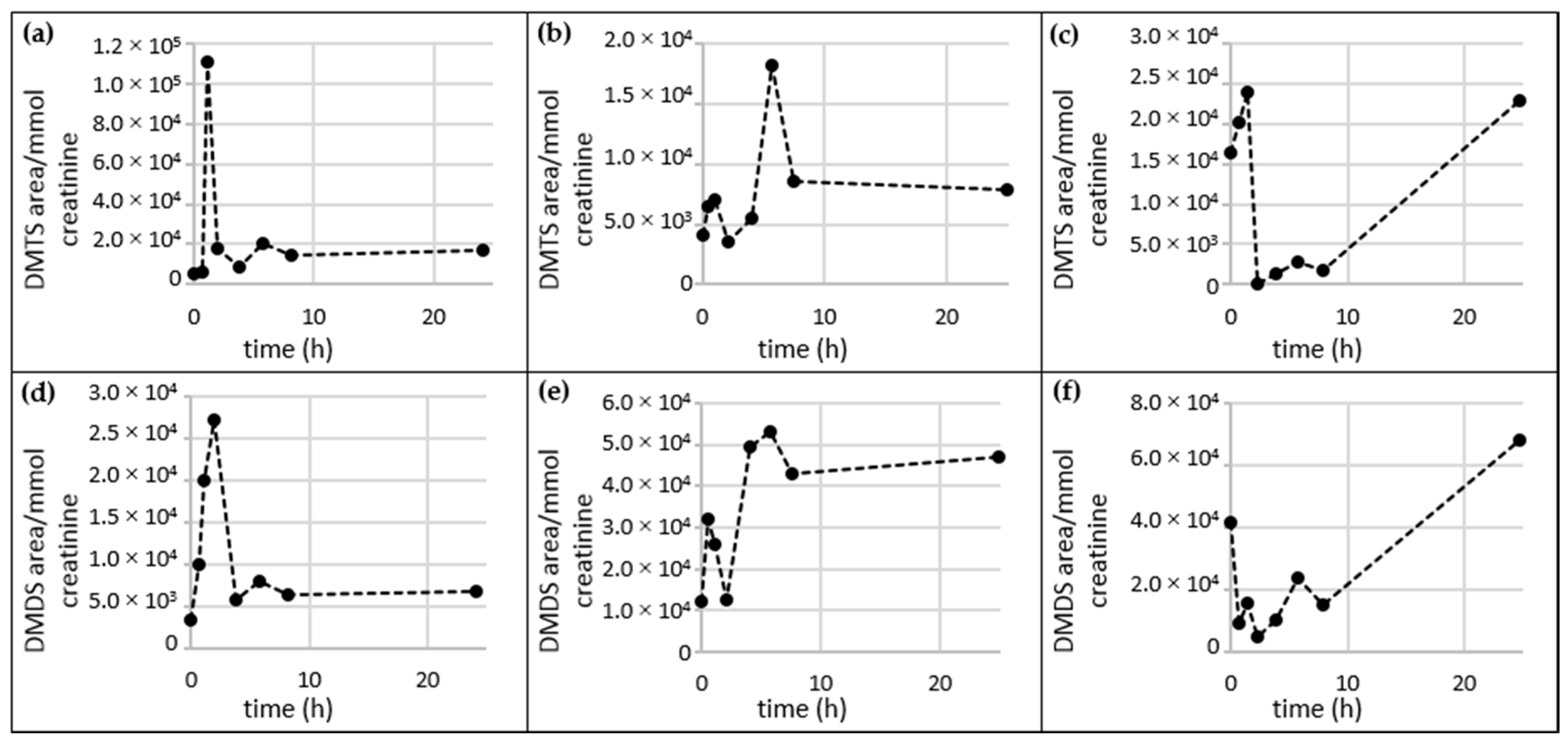
2.5. Time Dependency of Appearance of Specific Garlic-Derived Metabolites in Urine after Consumption of Garlic
2.5.1. AMS, AMSO and AMSO2
- Urine series b–l:For most of the urine sets (Urine f, g, h, j, l) the maximum concentration of all three garlic-derived metabolites, AMS, AMSO and AMSO2, approximately coincided, namely at about 1 h after garlic consumption. This maximum was observed at about 2.4 h after garlic consumption in sample set h only. The maxima in AMS and AMSO for sets b, d, e, i and k were reached before AMSO2. AMS and AMSO showed the highest values at about 1 to 1.5 h after garlic consumption (at 2 h for sample set b), whereas AMSO2 was observed to peak at about 2 to 3 h (4.5 h for sample set i). AMSO reached its maximum before AMS and AMSO2 only in sample set c. Whereas AMSO was excreted mostly at about 0.9 h after garlic consumption, AMS and AMSO2 reached their highest values at about 1.8 h after garlic consumption. Apart from the first maximum, a second increase in the metabolites was observed in several cases, which differed in time as well as intensity between the sample sets, indicating large inter-individual differences in the metabolism and excretion rate of garlic constituents. In most cases the second increase was smaller than the first; this was the case for AMS in sets d, f, g, i, j, k and l, for AMSO in sets b, j and l, and for AMSO2 in sets d, e, g, j, k and l. On the other hand, the second increase was more distinct than the first for AMS in two sample sets (b, i) and for AMSO2 in set l. No second increase was observed for all three garlic-derived metabolites in set c, for AMS in sets e and h, for AMSO in sets d, e, f, g, h and k, and for AMSO2 in sets b, f and h. In some cases the formation of a “shoulder” was observed for AMS (sets e and h), AMSO (sets g, h and k), and AMSO2 (sets b and h) at time intervals of 3 h (set l) to 7.8 h (set k) after garlic consumption.
- Urine series m–n:Urine sample sets m and n were investigated over a period of about 6 h, comprising six different samples. Only one maximum was measured for each metabolite during the sampling period. For sample set m all three metabolites reached their maximum at about 1.5 h after garlic ingestion, whereas for sample set n maximum concentrations of both AMSO and AMSO2 were excreted at about 1.5 h but AMS reached its maximum at about 2.5 h after garlic ingestion.
2.5.2. DMTS and DMDS
3. Discussion
3.1. Sensory Analysis
3.2. Identification of Garlic-Derived Metabolites in Human Urine
3.3. Metabolism of Garlic
4. Materials and Methods
4.1. Chemicals/Materials
4.2. Human Urine Samples
- High dosage test: One urine sample was provided 4 min prior to garlic consumption (30 g) and three samples were collected afterwards at approx. 2 h intervals. The urine samples were immediately evaluated and analyzed. This sample set was termed Urine a.
- Pre-trials: One urine sample was provided 5 min prior to garlic consumption (3 g) and seven samples were collected afterwards, at 2.1 h, 3.7 h, 6.6 h, 7.8 h, 12.1 h, and 24.1 h after garlic consumption. The urine samples were immediately evaluated and analyzed. This sample set was termed Urine b.
- Main tests: In each test set, one urine sample was provided immediately prior to garlic consumption (3 g) and seven samples were collected afterwards, at 0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h and 24 h after garlic consumption. Two volunteers did not provide a sample at 24 h as they were nursing mothers. Another two volunteers each provided an additional sample between 2 h and 4 h after garlic consumption. The samples were immediately evaluated and analyzed or kept frozen at −80 °C until further analysis. This protocol was applied to a set of urine samples from 12 different volunteers that were termed Urine c to Urine n.
- -
- Pre: prior garlic consumption
- -
- 0.5 h post: 0.3 h to 0.74 h after garlic consumption
- -
- 1 h post: 0.75 h to 1.49 h after garlic consumption
- -
- 2 h post: 1.5 h to 2.49 h after garlic consumption
- -
- 3 h post: 2.5 h to 3.49 h after garlic consumption
- -
- 4 h post: 3.5 h to 5 h after garlic consumption
- -
- 6 h post: 5 h to 7 h after garlic consumption
- -
- 8 h post: 7 h to 9 h after garlic consumption
- -
- 24 h post: 23 h to 26 h after garlic consumption
4.3. Study Design and Ethics Approval
4.4. Sensory Analysis
4.5. Determination of the Creatinine Concentration in Urine Samples
4.6. Solvent-Assisted Flavor Evaporation (SAFE) of Volatiles from Human Urine
4.7. High Resolution Gas Chromatography-Olfactometry (HRGC-O)
4.8. Comparative Aroma Extract Dilution Analysis (cAEDA)
4.9. High Resolution Gas Chromatography-Mass Spectrometry (HRGC-MS)
4.10. Two-Dimensional High Resolution Gas Chromatography-Mass Spectrometry/Olfactometry (HRGC-GC-MS/O) (Heart-Cut)
4.11. Identification of Metabolites and Calculation of Metabolite Profiles
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMDS: | Allyl methyl disulfide |
| AMS: | Allyl methyl sulfide |
| AMSO: | Allyl methyl sulfoxide |
| AMSO2: | Allyl methyl sulfone |
| cAEDA: | Comparative aroma extract dilution analysis |
| CVD: | Cardiovascular disease |
| DADS: | Diallyl disulfide |
| DAS: | Diallyl sulfide |
| DASO: | Diallyl sulfoxide |
| DASO2: | Diallyl sulfone |
| DATS: | Diallyl trisulfide |
| DMDS: | Dimethyl disulfide |
| DMTS: | Dimethyl trisulfide |
| FD factor: | Flavor dilution factor |
| FID: | Flame ionization detector |
| HDL: | High density lipoprotein |
| HRGC-GC-MS: | Two dimensional high-resolution gas chromatography-mass spectrometry |
| HRGC-MS: | High-resolution gas chromatography-mass spectrometry |
| HRGC-O: | High-resolution gas chromatography-olfactometry |
| LDL: | Low density lipoprotein |
| RI: | (Linear) retention indices |
| SAFE: | Solvent-assisted flavor evaporation |
| TC: | Total cholesterol |
| TIC: | Total ion chromatogram |
References
- Lucier, G.; Lin, B.-H. Garlic: Flavor of the ages. Agric. Outlook 2000, 272, 7–10. [Google Scholar]
- Lehmann, F.A. Investigation of the pharmacology of allium sativum (garlic). Arch. Exp. Pathol. Pharmakol. 1930, 147, 245–264. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Andrianova, I.V.; Fomchenkov, I.V.; Gorchakova, T.V.; Orekhov, A.N. Time-released garlic powder tablets lower systolic and diastolic blood pressure in men with mild and moderate arterial hypertension. Hypertens. Res. 2009, 32, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract reduces blood pressure in hypertensives: A dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 64–70. [Google Scholar] [CrossRef] [PubMed]
- De A Santos, O.S.; Griinwald, J. Effect of garlic powder tablets on blood lipids and blood pressure—A six month placebo controlled, double blind study. Br. J. Clin. Pract. Suppl. 1993, 4, 37–44. [Google Scholar]
- Adler, A.J.; Holub, B.J. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am. J. Clin. Nutr. 1997, 65, 445–450. [Google Scholar] [PubMed]
- Sobenin, I.A.; Pryanishnikov, V.V.; Kunnova, L.M.; Rabinovich, Y.A.; Martirosyan, D.M.; Orekhov, A.N. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001, 131, 1000S–1005S. [Google Scholar] [PubMed]
- Lawson, L.D. Garlic: A review of its medicinal effects and indicated active compounds. ACS Publ. 1998, 691, 176–209. [Google Scholar]
- Ilic, D.; Nikolic, V.; Nikolic, L.; Stankovic, M.; Stanojevic, L.; Cakic, M. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. Facta Universitatis Series 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Egen-Schwind, C.; Eckard, R.; Jekat, F.W.; Winterhoff, H. Pharmacokinetics of vinyldithiins, transformation products of allicin. Planta Medica 1992, 58, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.H.; Wu, C.M. Stability of allicin in garlic juice. J. Food Sci. 1989, 54, 977–981. [Google Scholar] [CrossRef]
- Freeman, F.; Kodera, Y. Garlic chemistry: Stability of s-(2-propenyl) 2-propene-1-sulfinothioate (allicin) in blood, solvents, and simulated physiological fluids. J. Agric. Food Chem. 1995, 43, 2332–2338. [Google Scholar] [CrossRef]
- Iberl, B.; Winkler, G.; Knobloch, K. Products of allicin transformation: Ajoenes and dithiins, characterization and their determination by HPLC. Planta Medica 1990, 56, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Villmann, C.; Sahin-Nadeem, H.; Pischetsrieder, M.; Buettner, A. Gabaa receptor modulation by the volatile fractions of sideritis species used as ‘greek’ or ‘turkish’ mountain tea. Flavour. Frag. J. 2012, 27, 297–303. [Google Scholar] [CrossRef]
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.C.R.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. Gabaa receptor modulation by terpenoids from sideritis extracts. Mol. Nutr. Food Res. 2014, 58, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAa receptors and a homo-oligomeric GABA receptor from drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, A.; Buettner, A. Monitoring of biotransformation of hop aroma compounds in an in vitro digestion model. Food Funct. 2012, 3, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, A.; Metzger, M.; Walles, H.; Buettner, A. Transport of hop aroma compounds across caco-2 monolayers. Food Funct. 2014, 5, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, L.; Sauermann, Y.; Zeh, G.; Hauf, K.; Heinlein, A.; Sharapa, C.; Buettner, A. Detection of volatile metabolites of garlic in human breast milk. Metabolites 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, F.; Horst, K.; Röhrig, W.; Rychlik, M.; Buettner, A. Tracing metabolite profiles in human milk: Studies on the odorant 1,8-cineole transferred into breast milk after oral intake. Metabolomics 2012, 9, 483–496. [Google Scholar] [CrossRef]
- Horst, K.; Rychlik, M. Quantification of 1,8-cineole and of its metabolites in humans using stable isotope dilution assays. Mol. Nutr. Food Res. 2010, 54, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Schaffarczyk, M.; Balaban, T.S.; Rychlik, M.; Buettner, A. Syntheses of chiral 1,8-cineole metabolites and determination of their enantiomeric composition in human urine after ingestion of 1,8-cineole-containing capsules. ChemPlusChem 2013, 78, 77–85. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bloavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.F.; Ishizaki, H.; Fukuto, J.M.; Lin, M.C.; Fadel, A.; Gapac, J.M.; Yang, C.S. Inhibition of cytochrome p-450 2e1 by diallyl sulfide and its metabolites. Chem. Res. Toxicol. 1991, 4, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Baillie, T.A. Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chem. Res. Toxicol. 1997, 10, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Germain, E.; Auger, J.; Ginies, C.; Siess, M.H.; Teyssier, C. In vivo metabolism of diallyl disulphide in the rat: Identification of two new metabolites. Xenobiotica 2002, 32, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Egen-Schwind, C.; Eckard, R.; Kemper, F.H. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Medica 1992, 58, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Sheen, L.Y.; Wu, C.C.; Lii, C.K.; Tsai, S.J. Metabolites of diallyl disulfide and diallyl sulfide in primary rat hepatocytes. Food Chem. Toxicol. 1999, 37, 1139–1146. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Wagenstaller, M.; Buettner, A. Characterization of odorants in human urine using a combined chemo-analytical and human-sensory approach: A potential diagnostic strategy. Metabolomics 2013, 9, 9–20. [Google Scholar] [CrossRef]
- Wagenstaller, M.; Buettner, A. Quantitative determination of common urinary odorants and their glucuronide conjugates in human urine. Metabolites 2013, 3, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Wagenstaller, M.; Buettner, A. Coffee aroma constituents and odorant metabolites in human urine. Metabolomics 2013, 10. [Google Scholar] [CrossRef]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.Y.; Lech, J.; Rosen, S.L.; Hartman, T.G. The determination of metabolites of garlic preparations in breath and human plasma. Biofactors 2000, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Hartman, T.G.; Karmas, K.; Lech, J.; Rosen, R.T. Breath analysis of garlic-borne phytochemicals in human-subjects: Combined adsorbent trapping and short-path thermal-desorption gas chromatography-mass spectrometry. In Food Phytochemicals for Cancer Prevention I: Fruits and Vegetables; Huang, M.T., Osawa, T., Ho, C.T., Rosen, R.T., Eds.; American Chemical Society: Washington, DC, USA, 1994; Volume 546, pp. 102–119. [Google Scholar]
- Suarez, F.; Springfield, J.; Furne, J.; Levitt, M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am. J. Physiol. Gastroint. Liver Physiol. 1999, 276, G425–G430. [Google Scholar]
- Mazza, G.; Ciaravolo, S.; Chiricosta, G.; Celli, S. Volatile flavour components from ripening and mature garlic bulbs. Flavour. Frag. J. 1992, 7, 111–116. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Dembicki, J.W.; Goldshlag, P.; Hanus, L.O.; Dembitsky, V.M. The use of the ‘Cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the allium sativum. J. Food Compos. Anal. 2004, 17, 235–245. [Google Scholar] [CrossRef]
- Tokarska, B.; Karwowska, K. The role of sulfur-compounds in evaluation of flavoring value of some plant raw-materials. Nahrung-Food 1983, 27, 443–447. [Google Scholar] [CrossRef]
- Ma, Y.; Song, D.; Wang, Z.; Jiang, J.; Jiang, T.; Cui, F.; Fan, X. Effect of ultrahigh pressure treatment on volatile compounds in garlic. J. Food Process. Eng. 2011, 34, 1915–1930. [Google Scholar] [CrossRef]
- Wahl, H.G.; Hoffmann, A.; Luft, D.; Liebich, H.M. Analysis of volatile organic compounds in human urine by headspace gas chromatography-mass spectrometry with a multipurpose sampler. J. Chromatogr. A 1999, 847, 117–125. [Google Scholar] [CrossRef]
- Mills, G.A.; Walker, V. Headspace solid-phase microextraction profiling of volatile compounds in urine: Application to metabolic investigations. J. Chromatogr. B: Biomed. Sci. Appl. 2001, 753, 259–268. [Google Scholar] [CrossRef]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; Costello, B.d.L.; Ratcliffe, N.M.; Probert, C.S. A comparative study of the analysis of human urine headspace using gas chromatography-mass spectrometry. J. Breath Res. 2008, 2, 037022. [Google Scholar] [CrossRef] [PubMed]
- Taucher, J.; Hansel, A.; Jordan, A.; Lindinger, W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996, 44, 3778–3782. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Metzger, J.; Fraisse, D.; Scharff, C. GC-MS (EI, PCI, NCI) computer-analysis of volatile sulfur-compounds in garlic essential oils. Application of the mass fragmentometry sim technique. Planta Medica 1986, 96–101. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Liou, Y.C. Volatile compounds from garlic. J. Agric. Food Chem. 1989, 37, 725–730. [Google Scholar] [CrossRef]
- Fleming, H.P.; Etchells, J.L.; Bell, T.A. Vapor analysis of fermented spanish-type green olives by gas chromatography. J. Food Sci. 1969, 34, 419–422. [Google Scholar] [CrossRef]
- Collin, S.; Nizet, S.; Muls, S.; Iraqi, R.; Bouseta, A. Characterization of odor-active compounds in extracts obtained by simultaneous extraction/distillation from moroccan black olives. J. Agric. Food Chem 2008, 56, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Kim, N.S.; Lee, D.S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003, 377, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Mondy, N.; Naudin, A.; Christides, J.P.; Mandon, N.; Auger, J. Comparison of GC-MS and HPLC for the analysis of allium volatiles. Chromatographia 2001, 53, S356–S360. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium Macrocarpon Ait.) Using Gas Chromatography–Olfactometry (GC-O) and odor activity value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Buhr, K.; Eisgruber, K.; Kiefl, J.; Schieberle, P. Garlic breath sampling and monitoring by proton transfer reaction-mass spectrometry. In Proceedings of the 4th PTR-MS Conference, Innsbruck, Austria, 16–21 February 2009; pp. 203–207.
- Hansanugrum, A.; Barringer, S.A. Effect of milk on the deodorization of malodorous breath after garlic ingestion. J. Food Sci. 2010, 75, C549–C558. [Google Scholar] [CrossRef] [PubMed]
- Munch, R.; Barringer, S.A. Deodorization of garlic breath volatiles by food and food components. J. Food Sci. 2014, 79, C526–C533. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.Y.; Lech, J.; Rosen, S.L.; Hartman, T.G. Determination of allicin, S-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001, 131, 968S–971S. [Google Scholar] [PubMed]
- Laakso, I.; Seppanen-Laakso, T.; Hiltunen, R.; Muller, B.; Jansen, H.; Knobloch, K. Volatile garlic odor components: Gas phases and adsorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Medica 1989, 55, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Boku, T.; Inada, K.; Morita, M.; Okazaki, Y. Odor components of human breath after the ingestion of grated raw garlic. J. Food Sci. 1989, 54. [Google Scholar] [CrossRef]
- Cai, X.J.; Block, E.; Uden, P.C.; Quimby, B.D.; Sullivan, J.J. Allium chemistry: Identification of natural-abundance organoselenium compounds in human breath after ingestion of garlic using gas-chromatography with atomic-emission detection. J. Agric. Food Chem. 1995, 43, 1751–1753. [Google Scholar] [CrossRef]
- David, S.; Patrik, Š.; Jens, H.; Jonathan, B. Mass spectrometry for real-time quantitative breath analysis. J. Breath Res. 2014, 8, 027101. [Google Scholar]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-allylmercaptoglutathione: The reaction product of allicin with glutathione possesses sh-modifying and antioxidant properties. Biochimica et Biophysica Acta 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- De Rooij, B.M.; Boogaard, P.J.; Rijksen, D.A.; Commandeur, J.N.M.; Vermeulen, N.P.E. Urinary excretion of N-acetyl-S-allyl-l-cysteine upon garlic consumption by human volunteers. Arch. Toxicol 1996, 70, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Jandke, J.; Spiteller, G. Unusual conjugates in biological profiles originating from consumption of onions and garlic. J. Chromatogr. B 1987, 421, 1–8. [Google Scholar] [CrossRef]
- Jaffe, M. Ueber den niederschlag, welchen pikrinsäure im normalen harn erzeugt, und über einen neue reaction des kreatinins. Zeitschrift für Physiologische Chemie 1886, 10, 391–400. (In German) [Google Scholar]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Bemelmans, J.M.H. Review of isolation and concentration techniques. In Proceedings of the 2nd Weurman Flavour Research Symposium, Norwich, UK, 2–6 April 1978; pp. 79–98.
- Sandgruber, S.; Much, D.; Amann-Gassner, U.; Hauner, H.; Buettner, A. Sensory and molecular characterisation of the protective effect of storage at −80 °C on the odour profiles of human milk. Food Chem. 2012, 130, 236–242. [Google Scholar] [CrossRef]
- Schomburg, G.; Behlau, H.; Dielmann, R.; Weeke, F.; Husmann, H. Sampling techniques in capillary gas chromatography. J. Chromatogr. A 1977, 142, 87–102. [Google Scholar] [CrossRef]
- Schieberle, P. New developments in methods for analysis of volatile flavor compounds and their precursors. In Characterization of Food: Emerging Methods; Gaonkar, A.G., Ed.; Elsevier: Amsterdam, Nederland, 1995; pp. 403–431. [Google Scholar]
- Buettner, A.; Schieberle, P. Application of a comparative aroma extract dilution analysis to monitor changes in orange juice aroma compounds during processing. In Gas Chromatography-Olfactometry; American Chemical Society: Washington, DC, USA, 2001; Volume 782, pp. 33–45. [Google Scholar]

| Sample | Urine b | Urine c | Urine d | Urine e | Urine f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FD | FD | FD | FD | FD | ||||||
| AMS | DMTS | AMS | DMTS | AMS | DMTS | AMS | DMTS | AMS | DMTS | |
| pre | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4 | n.d. | 8 |
| 0.5 h post | - | - | n.d. | n.d. | 32 | n.d. | 1 | 1 | 16 | 2 |
| 1 h post | - | - | n.d. | 1 | 16 | 1 | 8 | 8 | 8 | 1 |
| 2 h post | 2 | 1 | 4 | 1 | 128 | 1 | 16 | n.d. | 4 | 4 |
| 4 h post | 8 | 2 | 8 | 2 | 4 | 2 | 1 | 1 | 4 | 16 |
| 6 h post | 32 | 32 | 16 | 16 | 8 | 4 | 4 | 2 | 64 | 32 |
| 8 h post | 8 | 32 | 8 | 4 | 4 | 1 | 4 | 2 | 16 | 4 |
| 12 h post | 2 | 4 | - | - | - | - | - | - | - | - |
| 24 h post | n.d. | 4 | n.d. | 32 | n.d. | 1 | 2 | 16 | n.d. | 4 |
| Substance (Abbreviation) | Structure | RI | m/z | Odor Quality | Detected in | ||
|---|---|---|---|---|---|---|---|
| FFAP | DB-5 | U a 4 h post | U f 2 h post | ||||
| Allyl methyl sulfide (AMS) |  | <1000 | 715 | 73 + 88 | Garlic-like a,b | Yes | Yes |
| Allyl methyl sulfoxide (AMSO) |  | 1742 | 1018 | 104 | Odorless c | Yes | Yes d |
| Allyl methyl sulfone (AMSO2) | 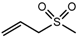 | 1983 | 1061 | 107 | Odorless c | Yes | Yes d |
| Diallyl sulfoxide (DASO) | 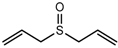 | 1889 | 1163 | 81 | Garlic-like c | n.d. | n.a. |
| Diallyl sulfone (DASO2) | 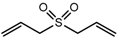 | 2079 | 1289 | 67 | Odorless c | n.d. | n.a. |
| Diallyl disulfide (DADS) | 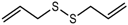 | 1462 | 1083 | 146 | Garlic-like a | n.d. | n.a. |
| Garlic-like, pungent b | |||||||
| Allyl methyl disulfide (AMDS) | 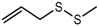 | 1265 | 921 | 120 | Garlic-like a | Yes | n.d. |
| Cooked garlic-like b | |||||||
| Dimethyl disulfide (DMDS) | 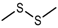 | 1071 | 751 | 94 | Cabbage-like a | Yes | n.d. e |
| Cooked garlic-like, onion-like, rubber-like b | |||||||
| Dimethyl trisulfide (DMTS) | 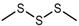 | 1362 | 973 | 126 | Garlic-like a | Yes | Yes d |
| Burnt garlic-like, diffusive, penetrating, sulfury b | |||||||
| Diallyl trisulfide (DATS) | 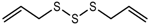 | 1771 | 1308 | 113 | Garlic-like a | n.d. | n.a. |
| Garlic-like, onion-like b | |||||||
| Diallyl sulfide (DAS) | 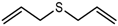 | 1138 | 868 | 114 | Garlic-like a | n.d. | n.a. |
| 2-Vinyl-4H-1,3-dithiin |  | 1827 | 1222 | 144 | Garlic-like a | n.d. | n.a. |
| 3-Vinyl-4H-1,2-dithiin | 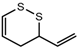 | 1720 | 1194 | 144 | Pungent-garlic-like a | n.d. | n.a. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffler, L.; Sauermann, Y.; Heinlein, A.; Sharapa, C.; Buettner, A. Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine. Metabolites 2016, 6, 43. https://doi.org/10.3390/metabo6040043
Scheffler L, Sauermann Y, Heinlein A, Sharapa C, Buettner A. Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine. Metabolites. 2016; 6(4):43. https://doi.org/10.3390/metabo6040043
Chicago/Turabian StyleScheffler, Laura, Yvonne Sauermann, Anja Heinlein, Constanze Sharapa, and Andrea Buettner. 2016. "Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine" Metabolites 6, no. 4: 43. https://doi.org/10.3390/metabo6040043
APA StyleScheffler, L., Sauermann, Y., Heinlein, A., Sharapa, C., & Buettner, A. (2016). Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine. Metabolites, 6(4), 43. https://doi.org/10.3390/metabo6040043






