Diltiazem Reduces Mortality and Breakdown of ATP in Red Blood Cell Induced by Isoproterenol in a Freely Moving Rat Model in Vivo †
Abstract
:1. Introduction
2. Results
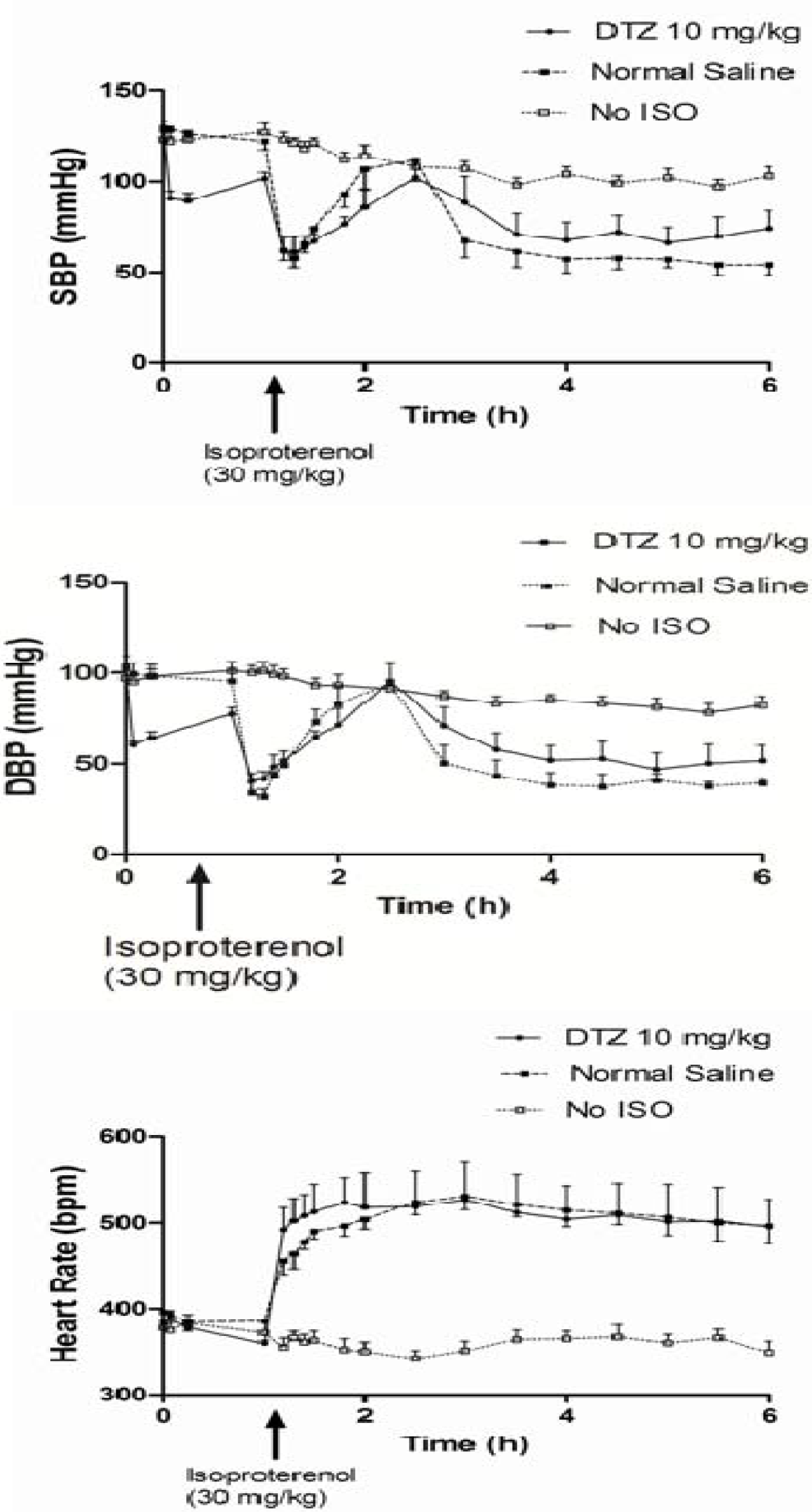
| Biomarkers/Treatment | A DTZ (5 mg/kg) (n = 6) | B DTZ (10 mg/kg) (n = 6) | C Control (No DTZ) (n = 10) | D Control (n = 11) No Iso and DTZ) |
|---|---|---|---|---|
| SBP (mmHg) before the last dose | 130 ± 8a | 130 ± 8 | 127 ± 14 | 123 ± 11 |
| DBP (mmHg) before the last dose | 105 ± 12 | 102 ± 7 | 104 ± 19 | 100 ± 15 |
| HR (bpm) before the last dose | 371 ± 22 | 385 ± 32 | 396 ± 40 | 378 ± 48 |
| SBP (mmHg) T0.25 | 98 ± 7* | 89 ± 10* | 126 ± 14 | 123 ± 15 |
| DBP (mmHg) T0.25 | 68 ± 4*,** | 60 ± 4* | 98 ± 21 | 98 ± 13 |
| HR (bpm) T1 | 351 ± 32 | 360 ± 9* | 386 ± 33 | 372 ± 47 |
| AUC of ATP in RBC from T0–T1 (mM*T) | 2.18 ± 0.53 | 1.92 ± 0.16 | 1.88 ± 0.25 | 1.64 ± 0.31 |
| AUC of ADP in RBC from T0–T1 (mM*T) | 0.33 ± 0.09* | 0.32 ± 0.02* | 0.46 ± 0.09 | 0.42 ± 0.15 |
| AUC of AMP in RBC from T0–T1 (mM*T) | 0.02 ± 0.01* | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.03 |
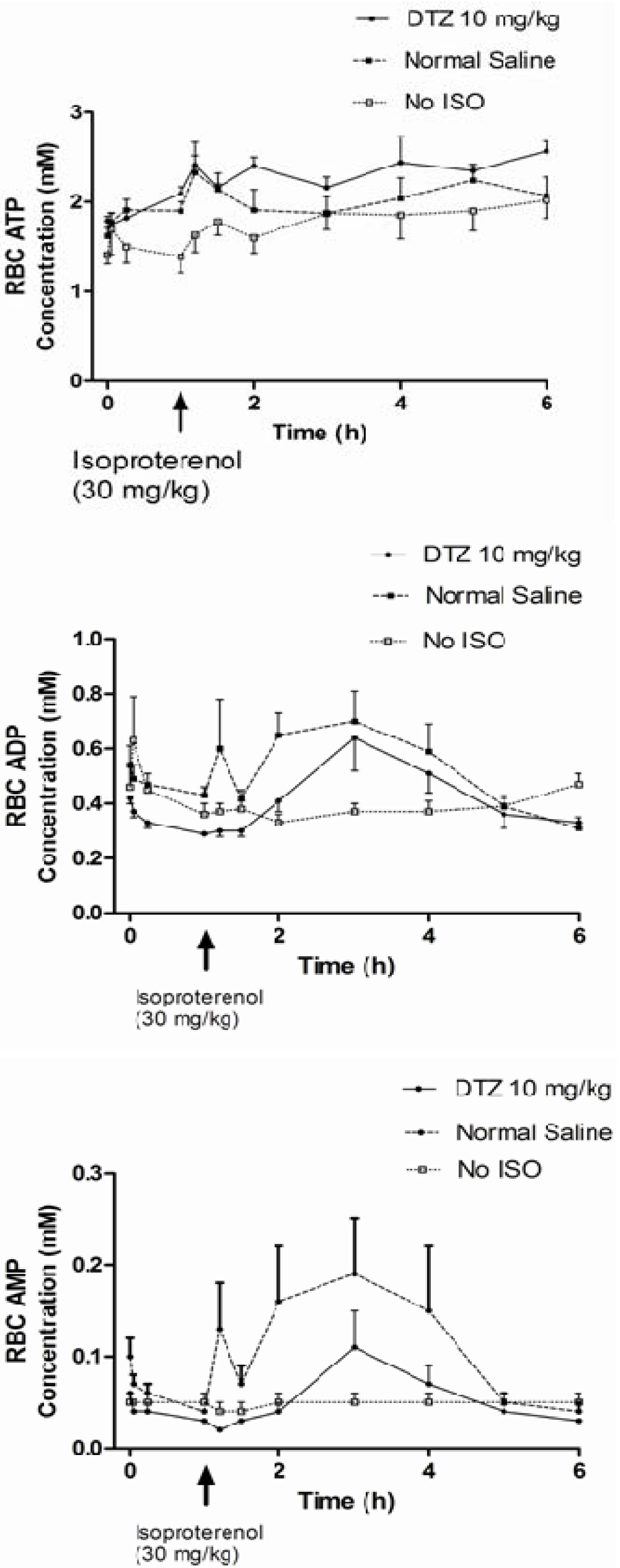
| Biomarkers/Treatment | A DTZ (5 mg/kg) (n = 6) | B DTZ (10 mg/kg) (n = 6) | C Control (No DTZ) (n = 10) | D Control (n = 11) No Iso and DTZ) |
|---|---|---|---|---|
| SBP (mmHg) immediately before Iso or at 1 hr | 110 ± 14a,** | 102 ± 8*,** | 126 ± 14 | 127 ± 15 |
| SBP (mmHg) 10 min after | 60 ± 10** | 62 ± 17** | 62 ± 17** | 123 ± 14 |
| Change in SBP (mmHg) | −50 ± 11** | −40 ± 17*,** | −64 ± 20** | −3 ± 10 |
| DBP (mmHg) immediately before Iso or at 1 hr | 83 ± 8** | 77 ± 9*,** | 95 ± 22 | 98 ± 13 |
| DBP (mmHg) 10 min after | 28 ± 15** | 41 ± 10** | 34 ± 17** | 100 ± 12 |
| Change in DBP (mmHg) | −49 ± 4** | −37 ± 15*,** | −61 ± 19** | 0 ± 10 |
| HR (bpm) immediately before Iso or at 1 hr | 351 ± 32 | 360 ± 9*,** | 386 ± 33 | 383 ± 32 |
| HR (bpm) 10 min after | 476 ± 19** | 492 ± 62** | 456 ± 50** | 372 ± 47 |
| Change in HR (bpm) | +120 ± 37*,** | +132 ± 66** | +70 ± 53** | −10 ± 29 |
| Change in HR (bpm) at the end of experiment | +149 ± 62** | +175 ± 73** | +157 ± 56** | −14 ± 43 |
| AUC of ATP in RBC from T1 – T last (mM*T) | NDb | 9.83 ± 4.75 | 7.74 ± 3.76 | 9.08 ± 3.03 |
| AUC of ADP in RBC from T1 – T last (mM*T) | ND | 2.21 ± 0.45 | 2.15 ± 0.73 | 1.85 ± 0.49 |
| AUC of AMP in RBC from T1 – T last (mM*T) | ND | 0.29 ± 0.13 | 0.48 ± 0.37 | 0.23 ± 0.14 |
| AUC AMP / AUC ATP in RBC from T1 – Tlast | ND | 0.02 ± 0.01* | 0.09 ± 0.09** | 0.02 ± 0.02 |
| Cmax of ATP in RBC (mM) after Iso or 1 hr | 2.56 ± 0.53 | 2.76 ± 0.41 | 2.42 ± 0.65 | 2.34 ± 0.70 |
| Cmax of ADP in RBC (mM) after Iso or 1 hr | 0.49 ± 0.23* | 0.60 ± 0.28 | 0.88 ± 0.37** | 0.50 ± 0.12 |
| Cmax of AMP in RBC (mM) after Iso or 1 hr | 0.11 ± 0.13 | 0.10 ± 0.086* | 0.29 ± 0.21** | 0.059 ± 0.030 |
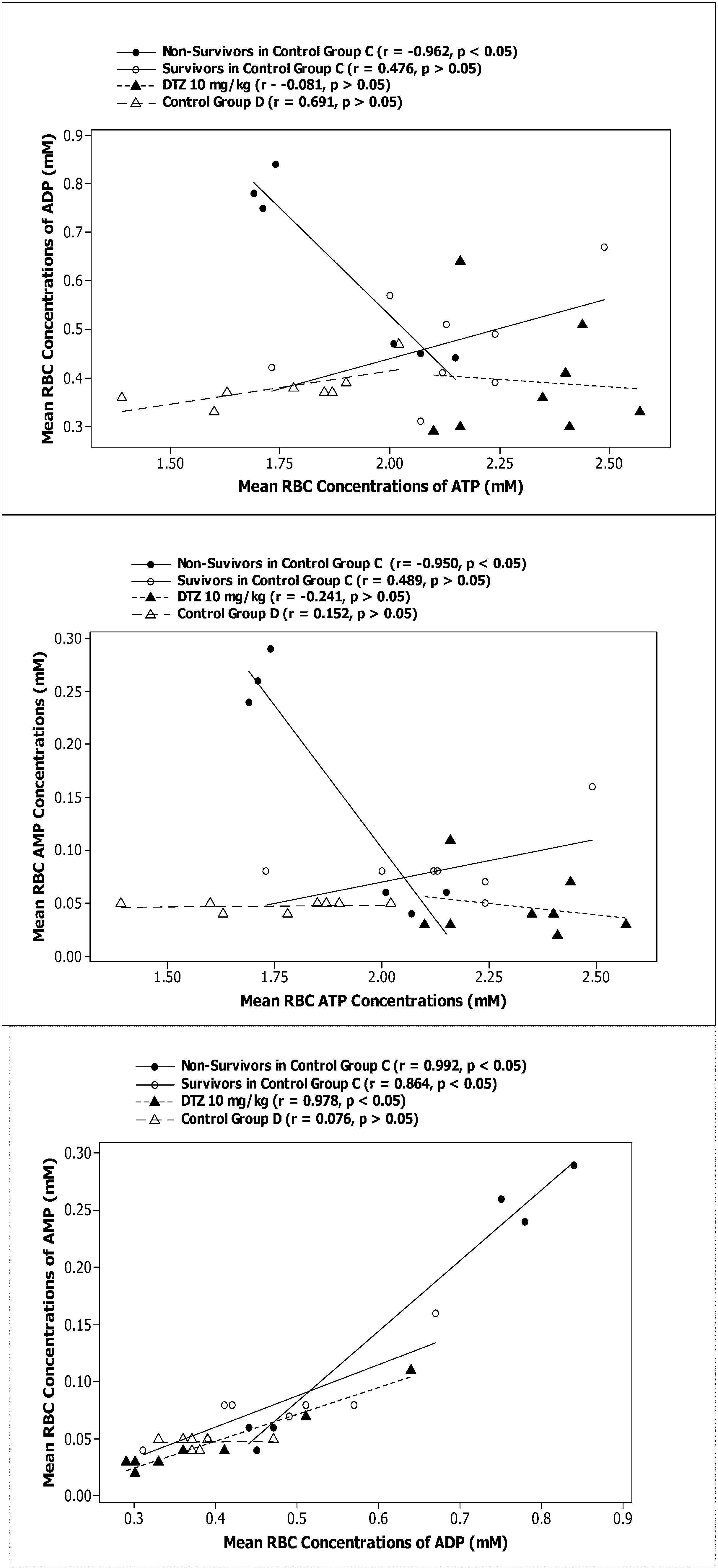
| Biomarkers/Treatment | A DTZ (5 mg/kg) (n = 6) | B DTZ (10 mg/kg) (n = 6)c | C Control No DTZ (n = 5) Survivors | C Control No DTZ (n = 5) Victims |
|---|---|---|---|---|
| ATP vs. AMP r | NDb | −0.308 ± 0.326a | 0.056 ± 0.409* | −0.721 ± 0.352 |
| ATP vs. AMP β | ND | −0.097 ± 0.177 | 0.023 ± 0.063* | −0.317 ± 0.190 |
| ATP vs. ADP r | ND | −0.038 ± 0.571 | 0.016 ± 0.460 | −0.426 ± 0.656 |
| ATP vs. ADP β | ND | −0.260 ± 0.534 | 0.039 ± 0.198 | −0.521 ± 0.643 |
| ADP vs. AMP r | ND | 0.744 ± 0.501 | 0.708 ± 0.384 | 0.763 ± 0.290 |
| ADP vs. AMP β | ND | −0.014 ± 0.527 | 0.102 ± 0.104* | 0.470 ± 0.282 |
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Animal Study
4.3. Data Analysis
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Neutel, J.M. Long-term blood pressure control: What can we do? Postgrad. Med. 2011, 123, 88–93. [Google Scholar]
- Mallat, S.G. What is a preferred angiotensin II receptor blocker-based combination therapy for blood pressure control in hypertensive patients with diabetic and non-diabetic renal impairment? Cardiovasc. Diabetol. 2012, 11. [Google Scholar] [CrossRef]
- Mourad, J.J.; Le Jeune, S.; Pirollo, A.; Mourad, C.; Gaudouen, Y.; Lopez-Sublet, M. Combinations of inhibitors of the renin-angiotensin system with calcium channel blockers for the treatment of hypertension: Focus on perindopril/amlodipine. Curr. Med. Res. Opin. 2010, 26, 2263–2276. [Google Scholar]
- Nishiyama, A.; Nakano, D.; Hitomi, H. [Calcium antagonists: Current and future applications based on new evidence. Effects of calcium channel blockers on oxidative stress]. Clin. Calcium. 2010, 20, 38–44. [Google Scholar]
- Morimoto, Y.; Kureishi-Bando, Y.; Murohara, T. Calcium antagonists: Current and future applications based on new evidence. Pleiotropic effects of calcium channel blockers on vascular endothelial function. Clin. Calcium. 2010, 20, 69–75. [Google Scholar]
- Basile, J. The Role of Existing and Newer Calcium Channel Blockers in the Treatment of Hypertension. J. Clin. Hypertens. 2004, 6, 621–631. [Google Scholar] [CrossRef]
- Basile, J.N.; Chrysant, S. The importance of early antihypertensive efficacy: The role of angiotensin II receptor blocker therapy. J. Hum. Hypertens. 2006, 20, 169–175. [Google Scholar] [CrossRef]
- Scheen, A.J. Dipeptidylpeptidase-4 inhibitors (gliptins): Focus on drug-drug interactions. Clin. Pharmacokinet. 2010, 49, 573–588. [Google Scholar] [CrossRef]
- Boddu, S.P.; Yamsani, M.R.; Potharaju, S.; Veeraraghavan, S.; Rajak, S.; Kuma, S.V.; Avery, B.A.; Repka, M.A.; Varanasi, V.S. Influence of grapefruit juice on the pharmacokinetics of diltiazem in Wistar rats upon single and multiple dosage regimens. Pharmazie 2009, 64, 525–531. [Google Scholar]
- Hong, S.P.; Yang, J.S.; Han, J.Y.; Ha, S.I.; Chung, J.W.; Koh, Y.Y.; Chang, K.S.; Choi, D.H. Effects of lovastatin on the pharmacokinetics of diltiazem and its main metabolite, desacetyldiltiazem, in rats: Possible role of cytochrome P450 3A4 and P-glycoprotein inhibition by lovastatin. J. Pharm. Pharmacol. 2011, 63, 129–135. [Google Scholar] [CrossRef]
- Yeung, P.; Alcos, A.; Marcoux, T.; Tang, J. Comparing Pharmacokinetics and Metabolism of Diltiazem in Normotensive Sprague Dawley and Wistar Kyoto Rats vs. Spontaneously Hypertensive Rats in vivo. Drug Metab. Drug Interact. 2011, 26, 119–125. [Google Scholar]
- Elliott, W.J.; Ram, C.V. Calcium channel blockers. J. Clin. Hypertens. (Greenwich) 2011, 13, 687–689. [Google Scholar] [CrossRef]
- Quimby, S.; Fern, R. Novel morphological features of developing white matter pericytes and rapid scavenging of reactive oxygen species in the neighbouring endothelia. J. Anat. 2011, 219, 65–77. [Google Scholar] [CrossRef]
- Liu, W.; Matsumori, A. Calcium channel blockers and modulation of innate immunity. Curr. Opin. Infect. Dis. 2011, 24, 254–258. [Google Scholar] [CrossRef]
- Yeung, P.; Alcos, A.; Tang, J. Pharmacokinetics and hemodynamic effects of diltiazem in rats following single vs. multiple doses in vivo. Open Drug Metab. J. 2009, 3, 55–62. [Google Scholar]
- Yeung, P.; Alcos, A.; Tang, J.; Casley, W. Hemodynamic effects of diltiazem in spontaneously hypertensive rats vs. normotensive rats following multiple doses in vivo. Curr. Top. Pharmacol. 2008, 12, 39–44. [Google Scholar]
- Yeung, P.K.F.; Mosher, S.J.; MacRae, D.A.; Klassen, G.A. Effect of diltiazem and its metabolites on the uptake of adenosine in blood: An in-vitro investigation. J. Pharm. Pharmacol. 1991, 43, 685–689. [Google Scholar] [CrossRef]
- Cohen, M.V.; Downey, J.M. Adenosine: Trigger and mediator of cardioprotection. Basic Res. Cardiol. 2008, 103, 203–215. [Google Scholar] [CrossRef]
- Westermann, D.; Knollmann, B.C.; Steendijk, P.; Rutschow, S.; Riad, A.; Pauschinger, M.; Potter, J.D.; Schultheiss, H.P.; Tschope, C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur. J. Heart Fail 2006, 8, 115–121. [Google Scholar] [CrossRef]
- Yeung, P.; Seeto, D. A study of the effect of isoproterenol on red blood cell concentrations of adenine nucleotides in a freely moving rat model in vivo. Cardiovas. Pharmacol. 2013, 2, 102. [Google Scholar]
- Yeung, P.; Dauphinee, J.; Gouzoules, T.; Simonson, K.; Schindler, C. Exercise improves hemodynamic profiles and increases red blood cell concentrations of purine nucleotides in a rodent model. Ther. Adv. Cardiovasc. Dis. 2010, 4, 341–347. [Google Scholar] [CrossRef]
- Bertera, F.M.; Mayer, M.A.; Opezzo, J.A.; Taira, C.A.; Hocht, C. Increased sensitivity to diltiazem hypotensive effect in an experimental model of high-renin hypertension. J. Pharm. Pharmacol. 2009, 61, 79–87. [Google Scholar] [CrossRef]
- Klein, L.C.; Yeung, P.K.; Berman, J.N. Cladribine inhibits a diltiazem-induced increase in red blood cell purine nucleotide concentrations in a zebrafish model. Biomarkers 2009, 14, 554–559. [Google Scholar] [CrossRef]
- Yeung, P.; Ding, L.; Casley, W. HPLC assay with UV detection for determination of RBC purine nucleotides concentrations and application for biomarker study in vivo. J. Pharm. Biomed. Anal. 2008, 47, 377–382. [Google Scholar] [CrossRef]
- Yeung, P.; Dauphinee, J.; Simonson, K.; Gouzoules, T. Anti-Ischemia Drugs have no Effect on the In Vivo Metabolism of ATP by RBC in Normotensive Restrained Rats. Open Drug Metab. J. 2011, 5, 1–6. [Google Scholar] [CrossRef]
- Ford, D.A.; Sharp, J.A.; Rovetto, M.J. Erythrocyte adenosine transport: Effects of Ca2+ channel antagonists and ions. Am. J. Physiol. 1985, 248, H593–H598. [Google Scholar]
- Yeung, P.; Mosher, S.; Li, R.; Farmer, P.; Klassen, G.; Pollak, P.; McMullen, M.; Ferrier, G. Erythrocyte adenosine transport: A rapid screening test for cardiovascular drugs. J. Pharmacol. Meth. 1993, 30, 163–167. [Google Scholar] [CrossRef]
- Takeo, S.; Tanonaka, K.; Tazuma, Y.; Fukao, N.; Yoshikawa, C.; Fukumoto, T.; Tanaka, T. Diltiazem and verapamil reduce the loss of adenine nucleotide metabolites from hypoxic hearts. J. Mol. Cell Cardiol. 1988, 20, 443–456. [Google Scholar] [CrossRef]
- Van Belle, H. Nucleoside transport inhibition: A therapeutic approach to cardioprotection via adenosine ? Cardiovas. Res 1993, 27, 68–76. [Google Scholar]
- Kalsi, K.K.; Smolenski, R.T.; Yacoub, M.H. Effects of nucleoside transport inhibitors and adenine/ribose on ATP concentration and adenosine production in cardiac myocytes. Adv. Exp. Med. Biol. 1998, 431, 95–98. [Google Scholar]
- Ellsworth, M.L. The red blood cell as an oxygen sensor: What is the evidence? Acta Physiol. Scand. 2000, 168, 551–559. [Google Scholar]
- Jensen, F.B. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009, 212, 3387–3393. [Google Scholar]
- Bergfeld, G.R.; Forrester, T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc. Res. 1992, 26, 40–47. [Google Scholar] [CrossRef]
- Lopez-Barneo, J.; Nurse, C.A.; Nilsson, G.E.; Buck, L.T.; Gassmann, M.; Bogdanova, A.Y. First aid kit for hypoxic survival: Sensors and strategies. Physiol. Biochem. Zool. 2010, 83, 753–763. [Google Scholar]
- eCPS. Compendium of Pharmaceuticals and Speccialties; Canadian Pharmaceutical Association: Ottawa, ON, Canada, 2014. [Google Scholar]
- Yeung, P.K.F.; Mosher, S.J.; Quilliam, M.A.; Montague, T.J. Species comparison of pharmacokinetics and metabolism of diltiazem in humans, dogs, rabbits, and rats. Drug Met. Disp. 1990, 18, 1055–1059. [Google Scholar]
- Conti, V.; Russomanno, G.; Corbi, G.; Izzo, V.; Vecchione, C.; Filippelli, A. Adrenoreceptors and nitric oxide in the cardiovascular system. Front. Physiol. 2013, 4, 321. [Google Scholar] [CrossRef]
- Huang, C.J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Kinnamon, D.D.; Lipshultz, S.E.; Rusconi, P. Associations Between Neurohormonal and Inflammatory Activation and Heart Failure in Children. Am. Heart J. 2008, 155, 527–533. [Google Scholar] [CrossRef]
- De Champlain, J.; Karas, M.; Nguyen, P.; Cartier, P.; Wistaff, R.; Toal, C.; Nadeau, R.; Larochelle, P. Different effects of nifedipine and amlodipine on circulating catecholamine levels in essential hypertensive patients. J. Hypertens. 1998, 16, 1357–1369. [Google Scholar]
- Grossman, E.; Messerli, F. Effect of calcium antagonists on plasma norepinephrine levels, heart rate, and blood pressure. Am. J. Cardiol. 1997, 80, 1453–1458. [Google Scholar] [CrossRef]
- Fogari, R.; Zoppi, A.; Corradi, L.; Preti, P.; Malalamani, G.D.; Mugellini, A. Effects of different dihydropyridine calcium antagonists on plasma norepinephrine in essential hypertension. J. Hypertens. 2000, 18, 1871–1875. [Google Scholar] [CrossRef]
- Yeung, P.K.; Dauphinee, J.; Gouzoules, T.; Simonson, K.; Schindler, C. Exercise improves hemodynamic profiles and increases red blood cell concentrations of purine nucleotides in a rodent model. Ther. Adv. Cardiovasc. Dis. 2010, 4, 341–347. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yeung, P.K.F.; Xu, Z.; Seeto, D. Diltiazem Reduces Mortality and Breakdown of ATP in Red Blood Cell Induced by Isoproterenol in a Freely Moving Rat Model in Vivo . Metabolites 2014, 4, 775-789. https://doi.org/10.3390/metabo4030775
Yeung PKF, Xu Z, Seeto D. Diltiazem Reduces Mortality and Breakdown of ATP in Red Blood Cell Induced by Isoproterenol in a Freely Moving Rat Model in Vivo . Metabolites. 2014; 4(3):775-789. https://doi.org/10.3390/metabo4030775
Chicago/Turabian StyleYeung, Pollen K.F., Zhaolin Xu, and Dena Seeto. 2014. "Diltiazem Reduces Mortality and Breakdown of ATP in Red Blood Cell Induced by Isoproterenol in a Freely Moving Rat Model in Vivo " Metabolites 4, no. 3: 775-789. https://doi.org/10.3390/metabo4030775
APA StyleYeung, P. K. F., Xu, Z., & Seeto, D. (2014). Diltiazem Reduces Mortality and Breakdown of ATP in Red Blood Cell Induced by Isoproterenol in a Freely Moving Rat Model in Vivo . Metabolites, 4(3), 775-789. https://doi.org/10.3390/metabo4030775






