Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress
Abstract
:1. Introduction
1.1. Metabolites in Cyanobacteria
1.2. Anhydrobiosis of Terrestrial Cyanobacteria
1.3. Reactive Oxygen Species (ROS) Induced Damages and Stress Responses
2. Photosynthetic Pigments
2.1. Chlorophylls and Their Derivatives
2.2. Antioxidant and Pro-Oxidant Activity of Chlorophylls and Phycobilins
2.3. Carotenoids and Their Derivatives
2.4. Antioxidant Activity of Carotenoids
| Radical species | IC50 [M] | |||
|---|---|---|---|---|
| Lutein | Zeaxanthin | Ascorbic acid | Trolox | |
| O2-• | 37 | 98 | >16,700 | − |
| OH• | 3.1 | 3.5 | 9,900 | − |
| DPPH• | 61 | 17.6 | 79.5 | 52 |
| ABTS• | >176 | 53 | 6.8 | 2.6 |
| Inhibition of lipid peroxidation | 3.9 | 3.2 | 2,800 | − |
3. Sunscreen Pigments
3.1. Mycosporine-Like Amino Acid (MAA)
3.1.1. Structural Characteristics of MAAs
3.1.2. Biosynthesis of MAAs
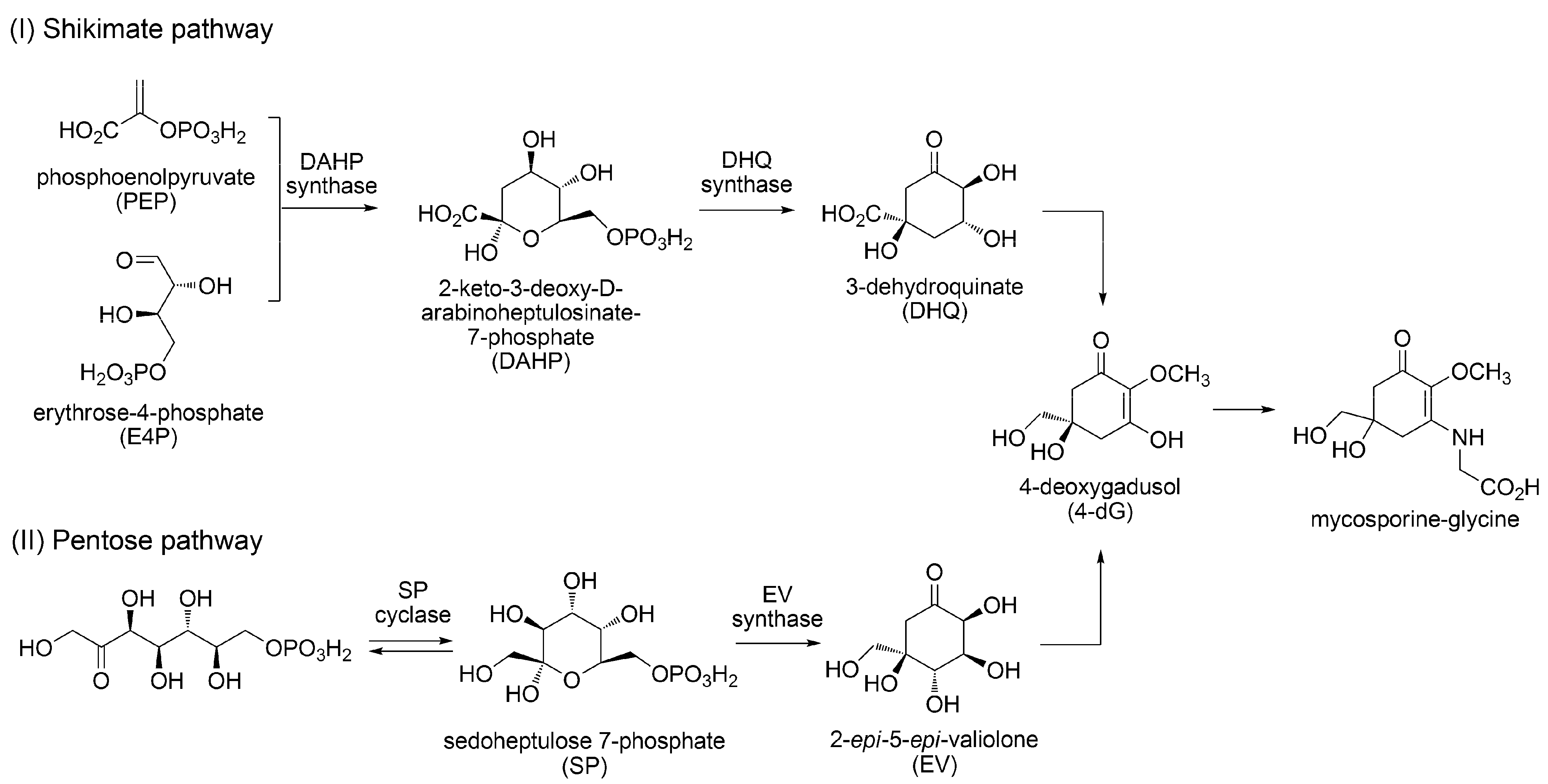
3.1.3. Sunscreen Properties of MAAs
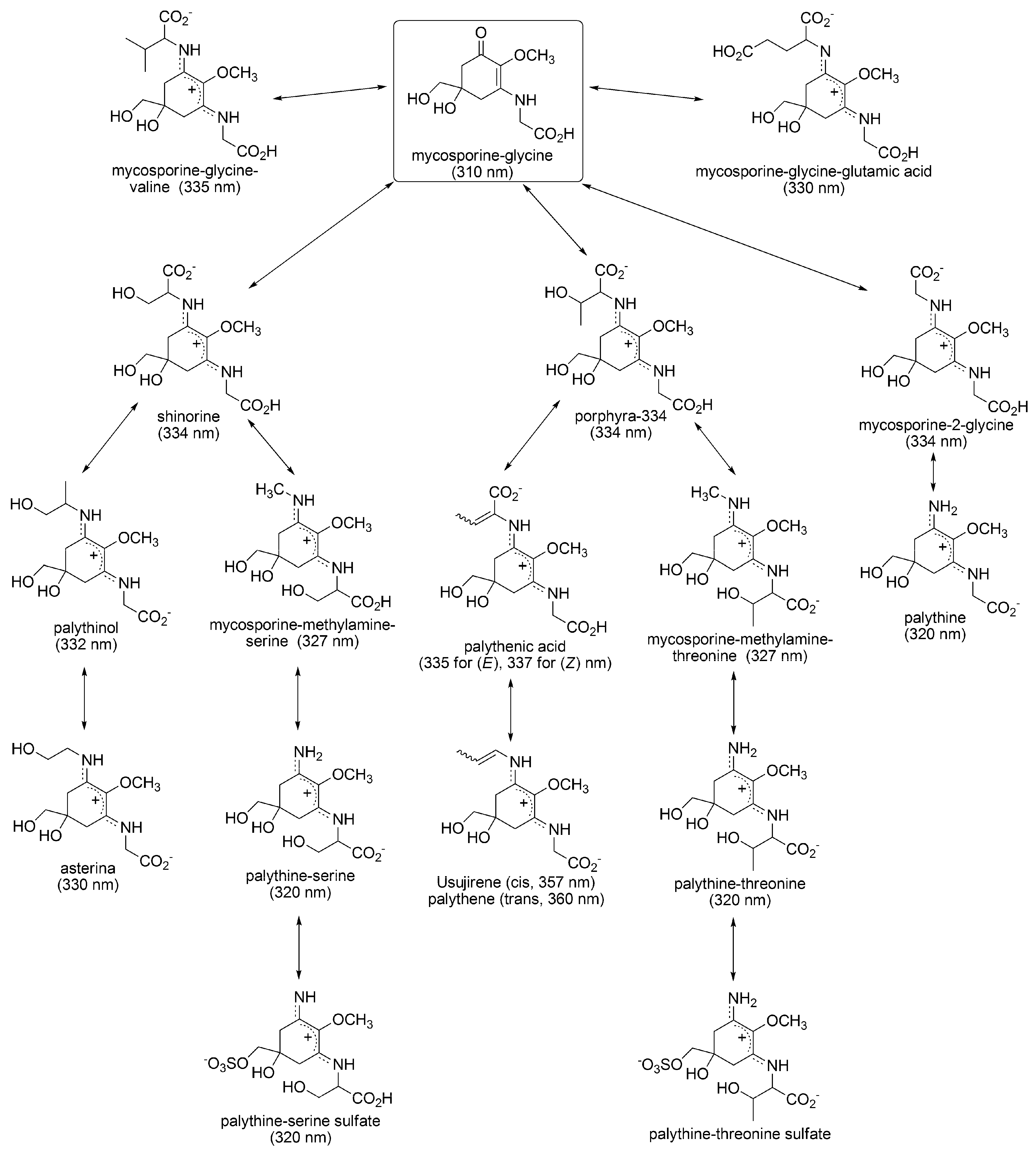
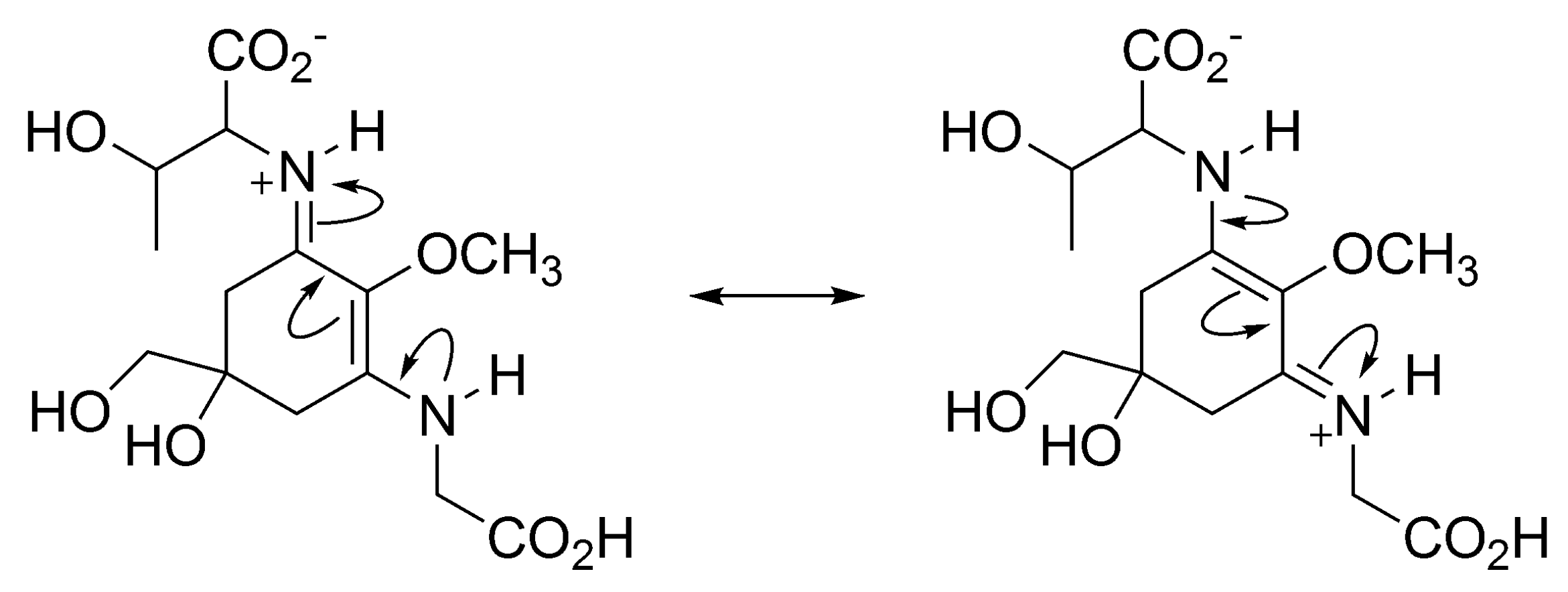
3.1.4. Radical Scavenging Activity of MAAs
| pH | ||||
| 6.0 | 7.5 | 8.5 | ||
| IC50 [μM] | Mycosporine-glycine | 20 | 4 | 3 |
| Porphyra-334 | 1000 | 400 | 80 | |
| Shinorine | − | − | 100 | |
| Asterina-330 | 100 | 60 | 10 | |
| Ascorbic acid | 11 | 4 | 26 | |
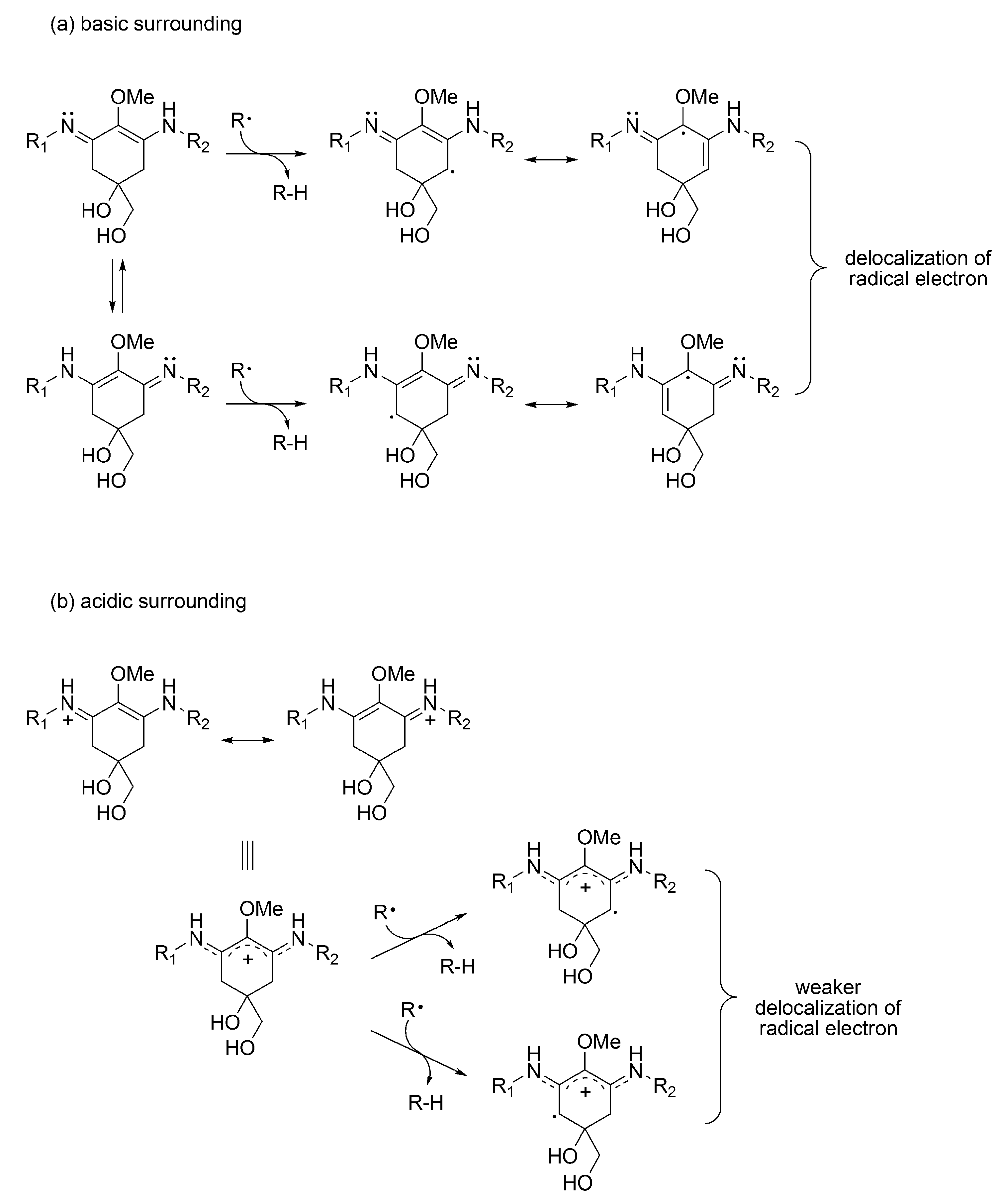
3.1.5. MAAs on Anhydrobiosis
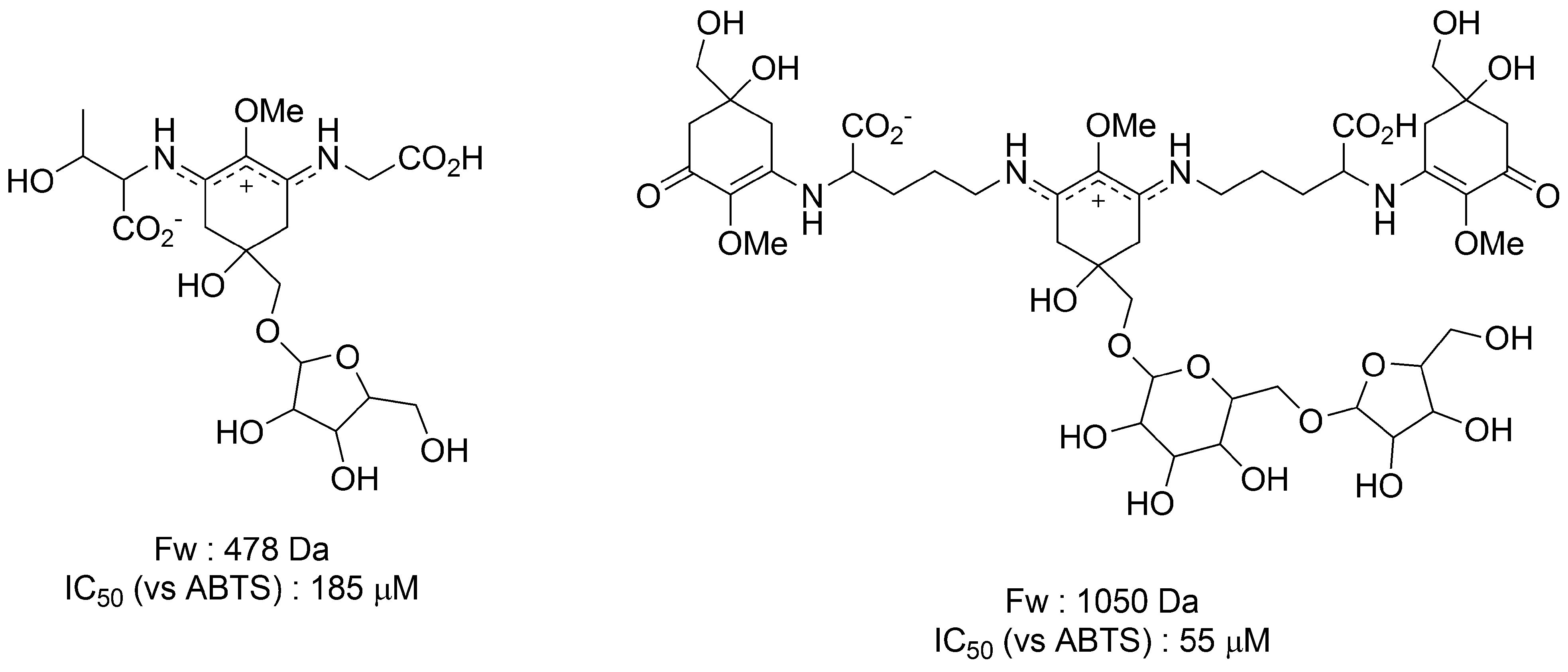
3.2. Scytonemin
3.2.1. Structural Characteristics of Scytonemin
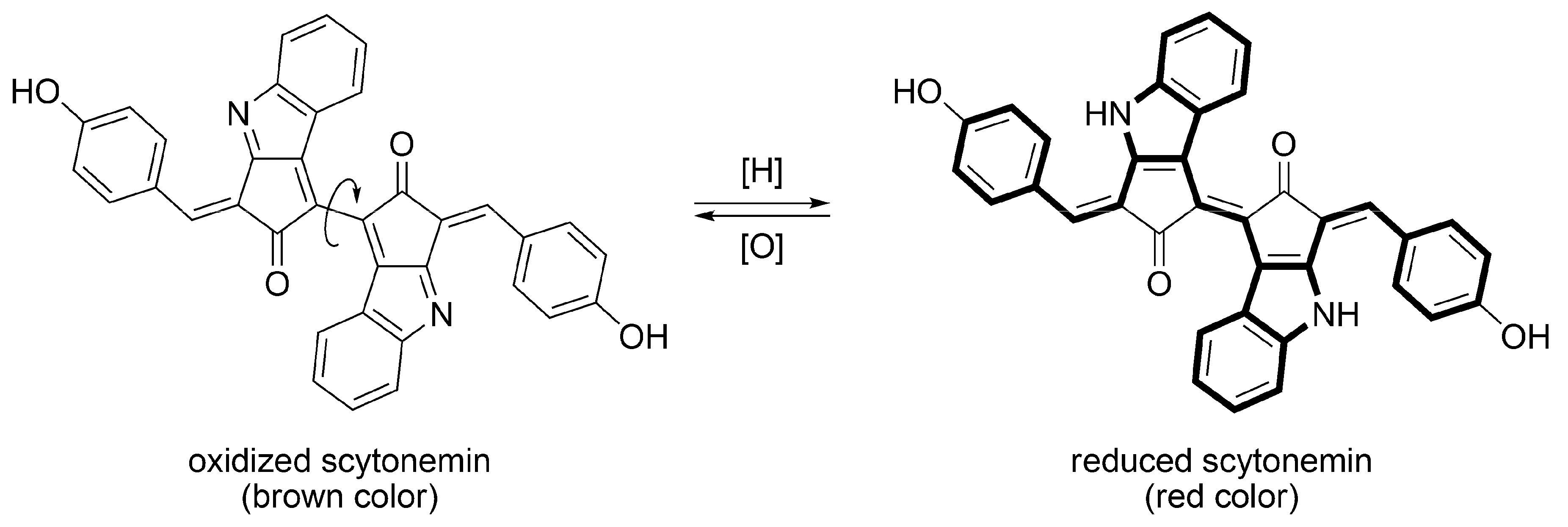
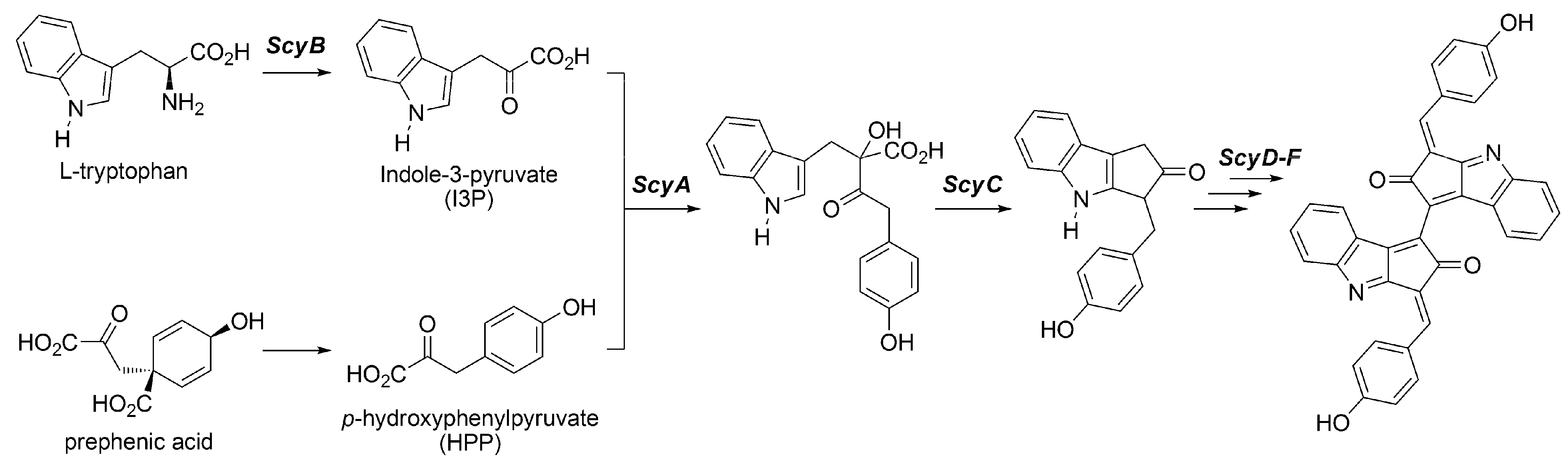
3.2.2. Biosynthesis of Scytonemin
3.2.3. Sunscreen Property and Radical Scavenging Activity of Scytonemin
3.2.4. Scytonemin on Anhydrobiosis
4. Future Prospective
References
- Castenholz, R.W.; Phylum, B.X. Cyanobacteria. Oxygenic photosynthetic bacteria. In Bergey’s Manual of Systematic Bacteriology. Volume 1: The Archaea and the Deeply Branching and Phototropic Bacteria; Garrity, G., Boone, D.R., Castenholz, R.W., Eds.; Springer-Verlag: New York, NY, USA, 2001; pp. 474–487. [Google Scholar]
- Namikoshi, M.; Rinehart, K.L. Bioactive compounds produced by cyanobacteria. J. Ind. Microbiol. 1996, 17, 373–384. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Nagarajan, M.; Maruthanayagam, V.; Sundararaman, M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 2012, 32, 153–185. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc. Commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef]
- Rebecchi, L.; Altiero, T.; Guidetti, R. Anhydrobiosis: The extreme limit of desiccation tolerance. Invertebr. Surv. J. 2007, 4, 65–81. [Google Scholar]
- Sakamoto, T.; Yoshida, T.; Arima, H.; Hatanaka, Y.; Takani, Y.; Tamaru, Y. Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc. Commune. Phycol. Res. 2009, 57, 66–73. [Google Scholar] [CrossRef]
- Crowe, J.; Carpenter, J.; Crowe, L. The role of vitrification on anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microb. 1995, 61, 3592–3597. [Google Scholar]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of microorganisms by drying: A review. J. Microbiol. Meth. 2006, 66, 183–193. [Google Scholar]
- Tunnacliffe, A.; Lapinski, J. Resurrecting Van Leeuwenhoek’s rotifers: A reappraisal of the role of disaccharides in anhydrobiosis. Phil. Trans. R. Soc. 2003, 358, 1755–1771. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kumihashi, K.; Kunita, S.; Masaura, T.; Inoue-Sakamoto, K.; Yamaguchi, M. The extracellular-matrix-retaining cyanobacterium Nostoc. verrucosum accumulates trehalose, but is sensitive to desiccation. FEMS Microbiol. Ecol. 2011, 77, 385–394. [Google Scholar] [CrossRef]
- Dadheech, N. Desiccation tolerance in cyanobacteria. Afr. J. Microbiol. Res. 2010, 4, 1584–1593. [Google Scholar]
- Altiero, T.; Guidetti, R.; Boschini, D.; Rebecchi, L. Heat shock proteins in encysted and anhydrobiotic eutardigrades. J. Limnol. 2012, 71, 211–215. [Google Scholar]
- Shimizu, T.; Kanamori, Y.; Furuki, T.; Kikawada, H.; Okuda, T.; Takahashi, T.; Mihara, H.; Sakurai, M. Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry 2010, 49, 1092–1104. [Google Scholar]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Kranner, I.; Birtic, S. A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 2005, 45, 734–740. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Li, S.W.; Gaidamakova, E.K.; Matrosova, V.Y.; Zhai, M.; Sulloway, H.M.; Scholten, J.C.; Brown, M.G.; Balkwill, D.L.; Daly, M.J. Protein oxidation: key to bacterial desiccation resistance? ISME J. 2008, 2, 393–403. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar]
- Hoekstra, F.A.; Golovina, E.A. Membrane behavior during dehydration: Implications for desiccation tolerance. Russ. J. Plant. Physiol. 1999, 46, 295–306. [Google Scholar]
- Miyashita, H.; Adachi, K.; Kurano, N.; Ikemoto, H.; Chihara, M.; Miyachi, S. Chlorophyll d as a major pigment. Nature 1996, 383, 402. [Google Scholar] [CrossRef]
- Blankenship, R.E. Photosynthetic Pigments: Structure and Spectroscopy. In Molecular Mechanisms of Photosynthesis; Wiley-Blackwell: Hoboken, NJ, USA, 2002; pp. 42–60. [Google Scholar]
- Kehoe, D.M. Chromatic adaptation in cyanobacteria. PNAS 2010, 107, 9029–9030. [Google Scholar] [CrossRef]
- Richa, M.K.; Sinha, R.P. Antioxidants as natural arsenal against multiple stresses in cyanobacteria. Int. J. Pharma. Biosci. 2011, 2, B168–B187. [Google Scholar]
- Ferruzzi, M.G.; Bohm, V.; Courtney, P.D.; Schwarts, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J. Food Sci. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2004, 56, 337–346. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina. platensis. J. App. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Liu, L.N.; Chen, X.L.; Wang, J.X.; Chen, M.; Zhang, Y.Z.; Zhou, B.C. Factors that effect antioxidant activity of C-phycocyanins from Spirulina. platensis. J. Food Biochem. 2005, 29, 313–322. [Google Scholar]
- Takaichi, S.; Maoka, T.; Mochimaru, M. Unique carotenoids in the terrestrial cyanobacterium Nostoc. Commune NIES-24: 2-Hydroxymyxol 20-fucoside, nostoxanthin and canthaxanthin. Curr. Microbiol. 2009, 59, 413–419. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Sawaya, M.R.; Brahmandam, V.; Cascio, D.; Ho, K.K.; Trevithick-Sutton, C.C.; Krogmann, D.W.; Yeates, T.O. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure 2003, 11, 55–65. [Google Scholar] [CrossRef]
- Boulay, C.; Abasova, L.; Six, C.; Vass, I.; Kirilovsky, D. Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. Biochim. Biophy. Acta 2008, 1777, 1344–1354. [Google Scholar] [CrossRef]
- Wilson, A.; Gall, A.; Bonetti, C.; Alexandre, M.; Routaboul, J.M.; Kerfeld, C.A.; van Grondelle, R.; Robert, B.; Kennis, J.T.M.; Kirilovsky, D. A photoactive carotenoid protein acting as light intensity sensor. Proc. Natl. Acad. Sci. USA 2008, 105, 12075–12080. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc. commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar]
- Wachi, Y.; Burgess, J.G.; Iwamoto, K.; Yamada, N.; Nakamura, N.; Matsunaga, T. Effect of ultraviolet-A (UV-A) light on growth, photosynthetic activity and production of biopterin glucoside by the marine UV-A resistant cyanobacterium Oscillatoria. sp. Biochim. Biophys. Acta 1995, 1244, 165–168. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Preethi, K.C.; Kuttan, R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Ind. J. Exp. Biol. 2010, 48, 843–848. [Google Scholar]
- Shimizu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fisheries Sci. 1996, 62, 134–137. [Google Scholar]
- Starcevic, A.; Akthar, S.; Dunlap, W.C.; Shick, J.M.; Hranueli, D.; Cullum, J. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella. vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar]
- Sinha, R.P.; Häder, D.P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Wu, J.J.; Chalker, B.E.; Rideout, J.A. Two new UV-absorbing compounds from Stylophora. pistillata: Sulfate esters of mycosporine-like amino acids. Tetrahedron Lett. 1997, 38, 2525–2526. [Google Scholar] [CrossRef]
- Böhm, G.A.; Pfleiderer, W.; Böger, P.; Scherer, S. Structure of a novel oligosaccharide- mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc. commune. J. Biol. Chem. 1995, 270, 8536–8539. [Google Scholar]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Spence, E.; Dunlap, W.C.; Shick, J.M.; Long, P.F. Redundant pathways of sunscreen biosynthesis in a cyanobacterium. Chem. BioChem. 2012, 13, 531–533. [Google Scholar]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis. sp. strain PCC 6912. Phycologia. 2003, 42, 384–392. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. An ATP-grasp ligase involved in the last biosynthetic step of the iminomycosporine shinorine in Nostoc. punctiforme ATCC 29133. J. Bacteriol. 2011, 193, 5923–5928. [Google Scholar] [CrossRef]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.P. Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem. Photobiol. 2008, 84, 1500–1505. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV-radiation. In The Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B.A., Potts, M., Eds.; Springer: Heidelberg, Germany, 2002; pp. 591–611. [Google Scholar]
- Shick, J.M. The continuity and intensity of ultraviolet irradiation affect the kinetics of biosynthesis, accumulation, and conversion of mycosporine-like amino acids (MAAs) in the coral Stylophora. pistillata. Limnol. Oceanogr. 2004, 49, 442–458. [Google Scholar]
- Klisch, M.; Richter, P.; Puchta, R.; Häder, D.P; Bauer, W. The stereostructure of porphyra-334: An experimental and calculational NMR investigation. evidence for an efficient “proton sponge”. Helvetica Chim. Acta. 2007, 90, 488–511. [Google Scholar]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B 2000, 56, 139–144. [Google Scholar]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of excited-state properties and photostability of the mycosporine-like amino acid palythine in water solution. Photochem. Photobiol. Sci. 2007, 6, 669–674. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Nakayama, R.; Tamura, Y.; Kikuzaki, H.; Nakatani, N. Antioxidant effect of the constituents of susabinori (Porphyra yezoensis). J. Am. Oil Chem. Soc. 1999, 76, 649–653. [Google Scholar] [CrossRef]
- Furusaki, A.; Matsumoto, T.; Tsujino, I.; Sekikawa, I. The crystal and molecular structure of palythine, trihydrate. Bull. Chem. Soc. Jpn. 1980, 53, 319–323. [Google Scholar]
- Matsui, K.; Nazifi, E.; Kunita, S.; Wada, N.; Matsugo, S.; Sakamoto, T. Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc. commune. J. Photochem. Photobiol. B: Biol. 2011, 105, 81–89. [Google Scholar]
- Wright, D.J.; Smith, S.C.; Joardar, V.; Scherer, S.; Jervis, J.; Warren, A.; Helm, R.F.; Potts, M. UV irradiation and desiccation modulate the three-dimensional extracellular matrix of Nostoc. commune (Cyanobacteria). J. Biol. Chem. 2005, 280, 40271–40281. [Google Scholar]
- Hill, D.R.; Peat, A.; Potts, M. Biochemistry and structure of the glycan secreted by desiccation-tolerant Nostoc. commune (Cyanobacteria). Protoplasma. 1994, 182, 126–148. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia. 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Bultel-Poncé, V.; Felix-Theodore, F.; Sarthon, C.; Ponge, J.F.; Bodo, B. New pigments from the terrestrial cyanobacterium Scytonema. sp. collected on the Mitaraka Inselberg, French Guyana. J. Nat. Prod. 2004, 67, 678–681. [Google Scholar] [CrossRef]
- Soule, T.; Stout, V.; Swingley, W.D.; Meeks, J.C.; Garcia-Pichel, F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc. punctiforme ATCC 29133. J. Bacteriol. 2007, 189, 4465–4472. [Google Scholar]
- Ohshima, T.; Nishida, N.; Bakthavatsalam, S.; Kataoka, K.; Takada, H.; Yoshimura, T.; Esaki, N.; Soda, K. The purification, characterization, cloning and sequencing of the gene for a halostable and thermostable leucine dehydrogenase from Thermoactinomyces. intermedius. Eur. J. Biochem. 1994, 222, 305–312. [Google Scholar] [CrossRef]
- Chipman, D.; Barak, Z.A.; Schloss, J.V. Biosynthesis of 2-aceto-2-hydroxy acids: Acetolactate synthases and acetohydroxyacid synthases. Biochim. Biophys. Acta 1998, 1385, 401–419. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. An enzymatic cyclopentyl[b]indole formation involved in scytonemin biosynthesis. J. Am. Chem. Soc. 2009, 131, 14648–14649. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.P. Responses of aquatic algae and cyanobacteria to solar UV-B. Plant. Ecol. 2001, 154, 219–236. [Google Scholar]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis. sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef]
- Fleming, E.D.; Castenholz, R.W. Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ. Microbiol. 2007, 9, 1448–1455. [Google Scholar] [CrossRef]
- Fleming, E.D.; Castenholz, R.W. Effects of nitrogen source on the synthesis of the UV-screening compound, scytonemin, in the cyanobacterium Nostoc. punctiforme PCC 73102. FEMS Microbiol. Ecol. 2008, 63, 301–308. [Google Scholar] [CrossRef]
- Vincent, W.F.; Downes, M.T.; Castenholz, R.W.; Howard-Williams, C. Community structure and pigment organisation of cyanobacteria-dominated microbial mats in Antarctica. Eur. J. Phycol. 1993, 28, 213–221. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for an ultra-violet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis. sp. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial UV-absorbing pigment scytonemin displays radical scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef]
- Hunsucker, S.W.; Tissue, B.A.; Potts, M.; Helm, R.F. Screening protocol for the ultraviolet-photoprotective pigment scytonemin. Anal. Biochem. 2001, 288, 227–230. [Google Scholar] [CrossRef]
- Richter, P.R.; Sinha, R.P.; Häder, D.P. Scytonemin-rich epilithic cyanobacteria survive acetone treatment. Curr. Trends Microbiol. 2006, 2, 13–19. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wada, N.; Sakamoto, T.; Matsugo, S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites 2013, 3, 463-483. https://doi.org/10.3390/metabo3020463
Wada N, Sakamoto T, Matsugo S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites. 2013; 3(2):463-483. https://doi.org/10.3390/metabo3020463
Chicago/Turabian StyleWada, Naoki, Toshio Sakamoto, and Seiichi Matsugo. 2013. "Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress" Metabolites 3, no. 2: 463-483. https://doi.org/10.3390/metabo3020463
APA StyleWada, N., Sakamoto, T., & Matsugo, S. (2013). Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites, 3(2), 463-483. https://doi.org/10.3390/metabo3020463




