Reducing False Positives in Newborn Screening: The Role of Perinatal Factors in the Dutch NBS Program
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Data Selection and Inclusion
2.3. Data Analysis
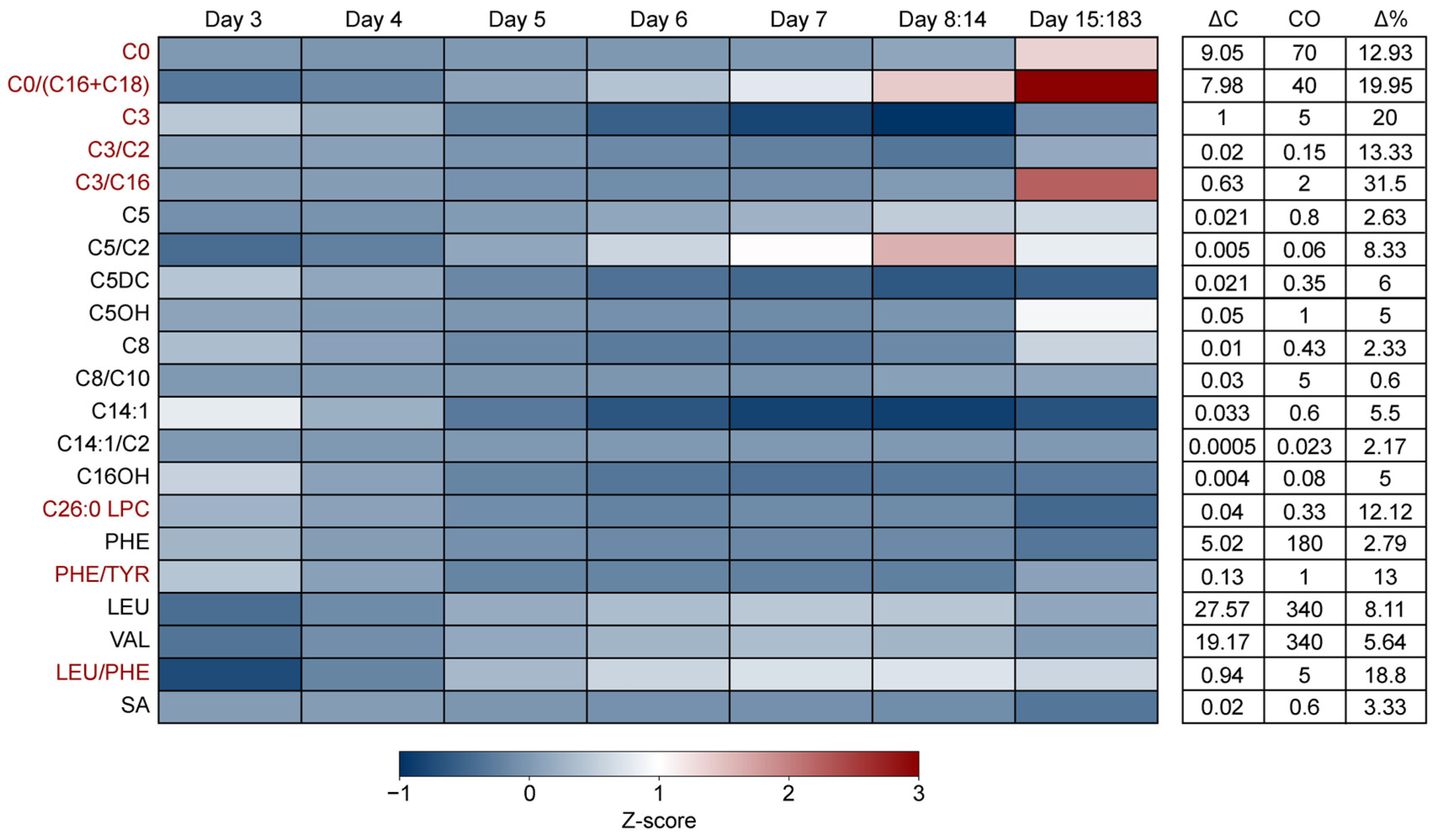
3. Results
3.1. Characteristics of the Study Population
3.2. The Combined Impact of Gestational Age and Birthweight on Dutch NBS Markers
3.3. The Impact of Age of Sampling on NBS Marker Levels
4. Discussion
5. Conclusions
6. Limitations and Benefits of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NBS | Newborn screening |
| PPV | Positive Predictive Value |
| MS/MS | Tandem mass spectrometry |
| IMD | Inborn metabolic disorder |
| CLIR | Collaborative Laboratory Integrated Reports |
| EP | Extreme premature |
| P | Premature |
| LP | Late premature |
| T | Term |
| POS | Post term |
| SGA | Small for gestational age |
| AGA | Average for gestational age |
| LGA | Large for gestational age |
| ΔC | Largest observed deviation |
| CO | Cut-off value |
| Δ% | The relevance of the observed change in concentration |
| CPT1 | Carnitine Palmitoyltransferase 1 |
| IVA | Isovaleric acidemia |
| PKU | Phenylketonuria |
| MMA | Methylmalonic acidaemia |
| ALD | Adrenoleukodystrophy |
| MSUD | Maple syrup urine disease |
| MAAI-D | Maleylacetoacetate isomerase deficiency |
| MCADD | Medium-chain acyl-CoA dehydrogenase deficiency |
| PCD | Primary carnitine deficiency |
| PA | Propionic acidemia |
References
- van der Ploeg, K.; van der Mast, O.; Verkerk, P.H. The Newborn Blood Spot Screening in The Netherlands-Monitor 2023; TNO–Child Health: Leiden, The Netherlands, 2023. [Google Scholar]
- Gezondheidsraad. Neonatale Screening op OCTN2-Deficiëntie. Available online: https://www.gezondheidsraad.nl/onderwerpen/p/preventie/alle-adviezen-over-preventie/neonatale-screening-op-octn2-deficientie (accessed on 17 June 2025).
- Musunuru, K.; Grandinette, S.A.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; Silverstein, R.A.; Hille, L.T.; et al. Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, M.; Chung, W.K. DNA Sequencing in Newborn Screening: Opportunities, Challenges, and Future Directions. Clin. Chem. 2025, 71, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Koval-Burt, C.; Kay, D.M.; Suchy, S.F.; Begtrup, A.; Langley, K.G.; Hernan, R.; Amendola, L.M.; Boyd, B.M.; Bradley, J.; et al. Expanded Newborn Screening Using Genome Sequencing for Early Actionable Conditions. JAMA 2025, 333, 232–240. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, L.M.; van der Pal, S.M.; Verschoof-Puite, R.K.; Klapwijk, J.E.; Elsinghorst, E.; Dekkers, E.; van der Ploeg, C.P.B.; Henneman, L. Psychosocial Impact of a True-Positive, False-Positive, or Inconclusive Newborn Bloodspot Screening Result: A Questionnaire Study among Parents. Int. J. Neonatal Screen. 2024, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Martens, R.C.; Boelen, A.; van der Kemp, M.H.; Bosch, A.M.; Berghout, E.M.; Weijman, G.; Zwaveling-Soonawala, N.; Verschoof-Puite, R.K.; de Jonge, R.; Hannema, S.E.; et al. The Value of Reducing Inconclusive and False-Positive Newborn Screening Results for Congenital Hypothyroidism, Congenital Adrenal Hyperplasia and Maple Syrup Urine Disease in The Netherlands. Int. J. Neonatal Screen. 2024, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. Biosyst. 2025, 11, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.L.; Meinzen-Derr, J.; Rose, S.R.; Leslie, N.D.; Chandrasekar, R.; Linard, S.M.; Akinbi, H.T. The effects of gestational age and birth weight on false-positive newborn-screening rates. Pediatrics 2010, 126, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Crefcoeur, L.L.; de Sain-van der Velden, M.G.M.; Ferdinandusse, S.; Langeveld, M.; Maase, R.; Vaz, F.M.; Visser, G.; Wanders, R.J.A.; Wijburg, F.A.; Verschoof-Puite, R.K.; et al. Neonatal carnitine concentrations in relation to gestational age and weight. JIMD Rep. 2020, 56, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; de Fontnouvelle, C.A.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Elevated methylmalonic acidemia (MMA) screening markers in Hispanic and preterm newborns. Mol. Genet. Metab. 2019, 126, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, R.; Roschinger, W.; Muntau, A.C.; Dame, T.; Kreischer, J.; Arnecke, R.; Superti-Furga, A.; Troxler, H.; Liebl, B.; Olgemöller, B.; et al. Hepatic carnitine palmitoyltransferase I deficiency: Acylcarnitine profiles in blood spots are highly specific. Clin. Chem. 2001, 47, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Odell, W.D. Acute release of thyrotropin in the newborn. J. Clin. Invest. 1969, 48, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Gruneiro de Papendieck, L.; Prieto, L.; Chiesa, A.; Bengolea, S.; Bergada, C. Congenital adrenal hyperplasia and early newborn screening: 17 alpha-hydroxyprogesterone (17 alpha-OHP) during the first days of life. J. Med. Screen. 1998, 5, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.; Fisher, D.A.; Wang, C.C. Serum thyrotropin, prolactin, and growth hormone levels during the early neonatal period in the human infant. J. Pediatr. 1976, 89, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.L.; Derraik, J.G.B.; Webster, D.; Hofman, P.L. The impact of demographic factors on newborn TSH levels and congenital hypothyroidism screening. Clin. Endocrinol. 2019, 91, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.D.; Stoway, S.D.; Ahlman, H.; Arora, V.; Caggana, M.; Fornari, A.; Hagar, A.; Hall, P.L.; Marquardt, G.C.; Miller, B.J.; et al. A Novel Approach to Improve Newborn Screening for Congenital Hypothyroidism by Integrating Covariate-Adjusted Results of Different Tests into CLIR Customized Interpretive Tools. Int. J. Neonatal. Screen. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Bouva, M.J.; Voorhoeve, E. Afkapgrenzen en Beslissingscriteria Neonatale Screening. Available online: https://draaiboekhielprikscreening.rivm.nl/proces/de-uitslag/afkapgrenzen (accessed on 17 June 2025).
- Yang, H.; Al-Hertani, W.; Cyr, D.; Laframboise, R.; Parizeault, G.; Wang, S.P.; Rossignol, F.; Berthier, M.-T.; Giguère, Y.; Waters, P.J.; et al. Hypersuccinylacetonaemia and normal liver function in maleylacetoacetate isomerase deficiency. J. Med. Genet. 2017, 54, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yahyaoui, R.; Blasco-Alonso, J.; Benito, C.; Rodriguez-Garcia, E.; Andrade, F.; Aldámiz-Echevarría, L.; Muñoz-Hernández, M.C.; Vega, A.I.; Pérez-Cerdá, C.; García-Martín, M.L.; et al. A new metabolic disorder in human cationic amino acid transporter-2 that mimics arginase 1 deficiency in newborn screening. J. Inherit. Metab. Dis. 2019, 42, 407–413. [Google Scholar] [CrossRef] [PubMed]

| Category | Count (Percentage) |
|---|---|
| Biological sex | |
| Male | 505,322 (51.27%) |
| Female | 480,307 (48.73%) |
| Total | 985,629 (100%) |
| Birthweight in grams | |
| <1000 | 2691 (0.27%) |
| 1000–1499 | 5273 (0.53%) |
| 1500–1999 | 11,024 (1.12%) |
| 2000–2499 | 35,032 (3.55%) |
| 2500–2999 | 131,040 (13.30%) |
| 3000–3499 | 339,973 (34.49%) |
| 3500–4000 | 333,044 (33.79%) |
| >4000 | 127,552 (12.94%) |
| Total | 985,629 (100%) |
| Age at heel prick in days | |
| 3 | 107,297 (10.89%) |
| 4 | 474,684 (48.16%) |
| 5 | 221,651 (22.49%) |
| 6 | 147,899 (15.01%) |
| 7 | 24,200 (2.46%) |
| 8–14 | 4678 (0.47%) |
| >14 | 5220 (0.53%) |
| Total | 985,629 (100%) |
| Gestational age in weeks | |
| <28 | 2595 (0.26%) |
| 28–31 | 6588 (0.67%) |
| 32–37 | 53,731 (5.45%) |
| 37–42 | 918,919 (93.23%) |
| >42 | 3796 (0.39%) |
| Total | 985,629 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijer, N.W.F.; Maase, R.E.; Hall, P.L.; Visser, W.F.; Koop, K.; Bosch, A.M.; Heiner-Fokkema, M.R.; de Sain‐van der Velden, M.G.M.; the CLIR-NBS Group. Reducing False Positives in Newborn Screening: The Role of Perinatal Factors in the Dutch NBS Program. Metabolites 2025, 15, 634. https://doi.org/10.3390/metabo15090634
Meijer NWF, Maase RE, Hall PL, Visser WF, Koop K, Bosch AM, Heiner-Fokkema MR, de Sain‐van der Velden MGM, the CLIR-NBS Group. Reducing False Positives in Newborn Screening: The Role of Perinatal Factors in the Dutch NBS Program. Metabolites. 2025; 15(9):634. https://doi.org/10.3390/metabo15090634
Chicago/Turabian StyleMeijer, Nils W. F., Rose E. Maase, Patricia L. Hall, Wouter F. Visser, Klaas Koop, Annet M. Bosch, M. Rebecca Heiner-Fokkema, Monique G. M. de Sain‐van der Velden, and the CLIR-NBS Group. 2025. "Reducing False Positives in Newborn Screening: The Role of Perinatal Factors in the Dutch NBS Program" Metabolites 15, no. 9: 634. https://doi.org/10.3390/metabo15090634
APA StyleMeijer, N. W. F., Maase, R. E., Hall, P. L., Visser, W. F., Koop, K., Bosch, A. M., Heiner-Fokkema, M. R., de Sain‐van der Velden, M. G. M., & the CLIR-NBS Group. (2025). Reducing False Positives in Newborn Screening: The Role of Perinatal Factors in the Dutch NBS Program. Metabolites, 15(9), 634. https://doi.org/10.3390/metabo15090634






