Impact of Salivary Amino Acid Concentrations on 8 km Running Performance in Male Undergraduate Students: A Prospective Observational Study Based on HPLC
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Reagents and Chemicals
2.3. Quantification of Salivary Amino Acids
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blagrove, R.C.; Howatson, G.; Hayes, P.R. Effects of Strength Training on the Physiological Determinants of Middle- and Long-Distance Running Performance: A Systematic Review. Sports Med. 2018, 48, 1117–1149. [Google Scholar] [CrossRef]
- di Prampero, P.E.; Atchou, G.; Brückner, J.C.; Moia, C. The energetics of endurance running. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J. Modeling: Optimal marathon performance on the basis of physiological factors. J. Appl. Physiol. 1991, 70, 683–687. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Howley, E.T.; Bassett, D.R., Jr.; Thompson, D.L.; Fitzhugh, E.C. Test of the classic model for predicting endurance running performance. Med. Sci Sports Exerc. 2010, 42, 991–997. [Google Scholar] [CrossRef]
- Morgan, D.W.; Craib, M. Physiological aspects of running economy. Med. Sci. Sports Exerc. 1992, 24, 456–461. [Google Scholar] [CrossRef]
- Lin, H.H.; Limesand, K.H.; Ann, D.K. Current State of Knowledge on Salivary Gland Cancers. Crit. Rev. Oncog. 2018, 23, 139–151. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Feng, Y.; Liu, X.; Wang, C.; Zhang, Y.; Wang, Z.; Zhang, D.; Guo, Y. Transcriptome analysis of salivary glands of rabies-virus-infected mice. Front. Microbiol. 2024, 15, 1354936. [Google Scholar] [CrossRef]
- Sezanova, K.; Gergulova, R.; Shestakova, P.; Rabadjieva, D. Thermodynamic and Kinetic Studies of the Precipitation of Double-Doped Amorphous Calcium Phosphate and Its Behaviour in Artificial Saliva. Biomimetics 2024, 9, 455. [Google Scholar] [CrossRef]
- Reinhard, D.A.; Konrath, S.H.; Lopez, W.D.; Cameron, H.G. Expensive egos: Narcissistic males have higher cortisol. PLoS ONE 2012, 7, e30858. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Xie, L.; Deng, G.; Kang, X. Common alterations in plasma free amino acid profiles and gut microbiota-derived tryptophan metabolites of five types of cancer patients. Amino Acids 2023, 55, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kang, X. Associations of Depression Score with Dialkyl Phosphate Metabolites in Urine: A Cross-Sectional Study. Brain Sci. 2024, 14, 1290. [Google Scholar] [CrossRef] [PubMed]
- Rustad, P.I.; Sailer, M.; Cumming, K.T.; Jeppesen, P.B.; Kolnes, K.J.; Sollie, O.; Franch, J.; Ivy, J.L.; Daniel, H.; Jensen, J. Intake of Protein Plus Carbohydrate during the First Two Hours after Exhaustive Cycling Improves Performance the following Day. PLoS ONE 2016, 11, e0153229. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Hattori, M.; Hatazawa, Y.; Kasahara, T.; Kanou, M.; Kanai, S.; Yuan, X.; Suganami, T.; Lamers, W.H.; Kitamura, T.; et al. FOXO1 activates glutamine synthetase gene in mouse skeletal muscles through a region downstream of 3′-UTR: Possible contribution to ammonia detoxification. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E485–E493. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Senoo, N.; Tadaishi, M.; Ogawa, Y.; Ezaki, O.; Kamei, Y.; Miura, S. Metabolomic Analysis of the Skeletal Muscle of Mice Overexpressing PGC-1α. PLoS ONE 2015, 10, e0129084. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Wolfe, R.R.; Hirsch, K.R.; Church, D.D.; Kviatkovsky, S.A.; Roberts, M.D.; Stout, J.R.; Gonzalez, D.E.; Sowinski, R.J.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Effects of essential amino acid supplementation on exercise and performance. J. Int. Soc. Sports Nutr. 2023, 20, 2263409. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Urban, R.J.; Sanford, A.P.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J. Clin. Endocrinol. Metab. 2004, 89, 4351–4358. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef]
- Kobayashi, H.; Børsheim, E.; Anthony, T.G.; Traber, D.L.; Badalamenti, J.; Kimball, S.R.; Jefferson, L.S.; Wolfe, R.R. Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E488–E498. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A.; Fogel, M.R.; Agrawal, R.M. Comparison of free amino acid and dipeptide absorption in the jejunum of sprue patients. Gastroenterology 1974, 67, 586–591. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552 Pt 1, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Hetenyi, G., Jr.; Anderson, P.J.; Kinson, G.A. Gluconeogenesis from threonine in normal and diabetic rats. Biochem. J. 1984, 224, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. A glutamine tug-of-war between cancer and immune cells: Recent advances in unraveling the ongoing battle. J. Exp. Clin. Cancer Res. 2024, 43, 74. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Anderson, P.M.; Lalla, R.V. Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients 2020, 12, 1675. [Google Scholar] [CrossRef]


| LLOD (µg/mL) | |
|---|---|
| Salivary Alanine | 0.04 |
| Salivary Arginine | 0.07 |
| Salivary Aspartic acid | 0.15 |
| Salivary Glutamate | 0.09 |
| Salivary Glutamine | 0.11 |
| Salivary Glycine | 0.04 |
| Salivary Histidine | 0.10 |
| Salivary Serine | 0.07 |
| Salivary Threonine | 0.07 |
| Characteristics | Total (n = 30) | Slow (n = 16) | Fast (n = 14) | p |
|---|---|---|---|---|
| Age (year) | 19 ± 1 | 19 ± 1 | 19 ± 1 | 0.387 |
| Distance (km) | 8.03 ± 0.12 | 7.99 ± 0.13 | 8.07 ± 0.09 | 0.084 |
| Running time (min) | 37.78 ± 2.47 | 39.64 ± 1.58 | 35.66 ± 1.30 | <0.001 |
| Average speed (km/h) | 12.80 ± 0.89 | 12.12 ± 0.53 | 13.59 ± 0.46 | <0.001 |
| Heart rate (bpm) | 161 ± 6 | 159 ± 4 | 163 ± 8 | 0.085 |
| Salivary AAs (µg/mL) | Total (n = 30) | Slow (n = 16) | Fast (n = 14) | p |
|---|---|---|---|---|
| Before | ||||
| Ala | 8.78 (4.28, 9.28) | 7.14 (3.13, 8.80) | 8.91 (4.81, 10.08) | 0.179 |
| Arg | 10.85 (2.95, 36.41) | 5.27 (1.34, 36.41) | 13.46 (7.33, 36.41) | 0.294 |
| Asp | 26.54 (10.74, 64.59) | 18.71 (7.46, 64.59) | 29.83 (19.03, 64.59) | 0.224 |
| Glu | 17.38 (11.49, 21.07) | 17.38 (11.75, 19.17) | 17.17 (10.76, 26.56) | 0.759 |
| Gln | 28.04 (12.46, 162.85) | 94.17 (14.69, 188.76) | 17.30 (4.18, 162.85) | 0.257 |
| Gly | 9.67 (2.07, 14.00) | 8.18 (0.11, 13.18) | 13.18 (2.75, 19.40) | 0.166 |
| His | 11.09 (4.43, 19.87) | 6.94 (2.65, 19.41) | 12.14 (6.31, 19.87) | 0.377 |
| Ser | 12.18 (5.89, 22.07) | 8.92 (3.32,19.25) | 20.19 (9.64, 29.99) | 0.013 |
| Thr | 9.58 (1.44, 36.60) | 9.58 (2.25, 36.60) | 9.77 (1.03, 36.60) | 0.667 |
| After | ||||
| Ala | 15.75 (8.89, 17.23) | 15.35 (4.65, 16.42) | 15.95 (10.30, 17.57) | 0.473 |
| Arg | 8.13 (2.22, 14.93) | 5.84 (1.65, 14.92) | 10.16 (4.13, 26.46) | 0.275 |
| Asp | 52.58 (20.60, 72.17) | 49.55 (18.96, 66.99) | 52.58 (24.01, 74.45) | 0.580 |
| Glu | 42.87 (18.12, 47.80) | 39.93 (21.58, 44.98) | 44.88 (17.46, 51.93) | 0.608 |
| Gln | 47.15 (11.89, 80.38) | 38.97 (9.80, 80.38) | 58.56 (21.39, 91.75) | 0.854 |
| Gly | 16.01 (5.22, 24.13) | 16.01 (6.39, 24.04) | 17.28 (2.10, 28.46) | 0.918 |
| His | 24.39 (9.64, 44.71) | 30.36 (6.65, 44.71) | 20.30 (12.28, 44.71) | 0.667 |
| Ser | 21.29 (7.26, 34.14) | 17.39 (4.69, 24.51) | 24.51 (10.58, 41.23) | 0.313 |
| Thr | 17.16 (6.20, 27.76) | 17.16 (1.57, 27.71) | 20.90 (8.71, 40.89) | 0.154 |
| 24 h later | ||||
| Ala | 7.51 (2.80, 19.04) | 9.43 (4.48, 19.62) | 4.44 (1.87, 9.37) | 0.058 |
| Arg | 18.53 (6.29, 31.86) | 16.75 (5.40, 26.07) | 22.43 (7.33, 40.55) | 0.400 |
| Asp | 8.39 (2.51, 22.66) | 30.08 (14.28, 198.67) | 63.70 (15.86, 106.06) | 0.951 |
| Glu | 17.16 (8.69, 33.69) | 29.84 (11.56, 34.61) | 11.79 (4.56, 25.66) | 0.101 |
| Gln | 11.51 (3.04, 31.04) | 24.52 (4.37, 31.04) | 6.65 (0.11, 18.68) | 0.047 |
| Gly | 9.66 (2.96, 35.13) | 24.83 (3.85, 41.69) | 9.10 (2.29, 35.13) | 0.525 |
| His | 12.02 (5.67, 15.56) | 12.02 (7.08, 17.61) | 10.53 (4.64, 14.58) | 0.377 |
| Ser | 8.35 (4.01, 12.30) | 9.67 (5.29, 13.55) | 5.15 (2.17, 12.04) | 0.179 |
| Thr | 8.39 (2.51, 22.66) | 7.64 (2.95, 22.66) | 9.39 (0.11, 22.66) | 0.886 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Speed | ||||
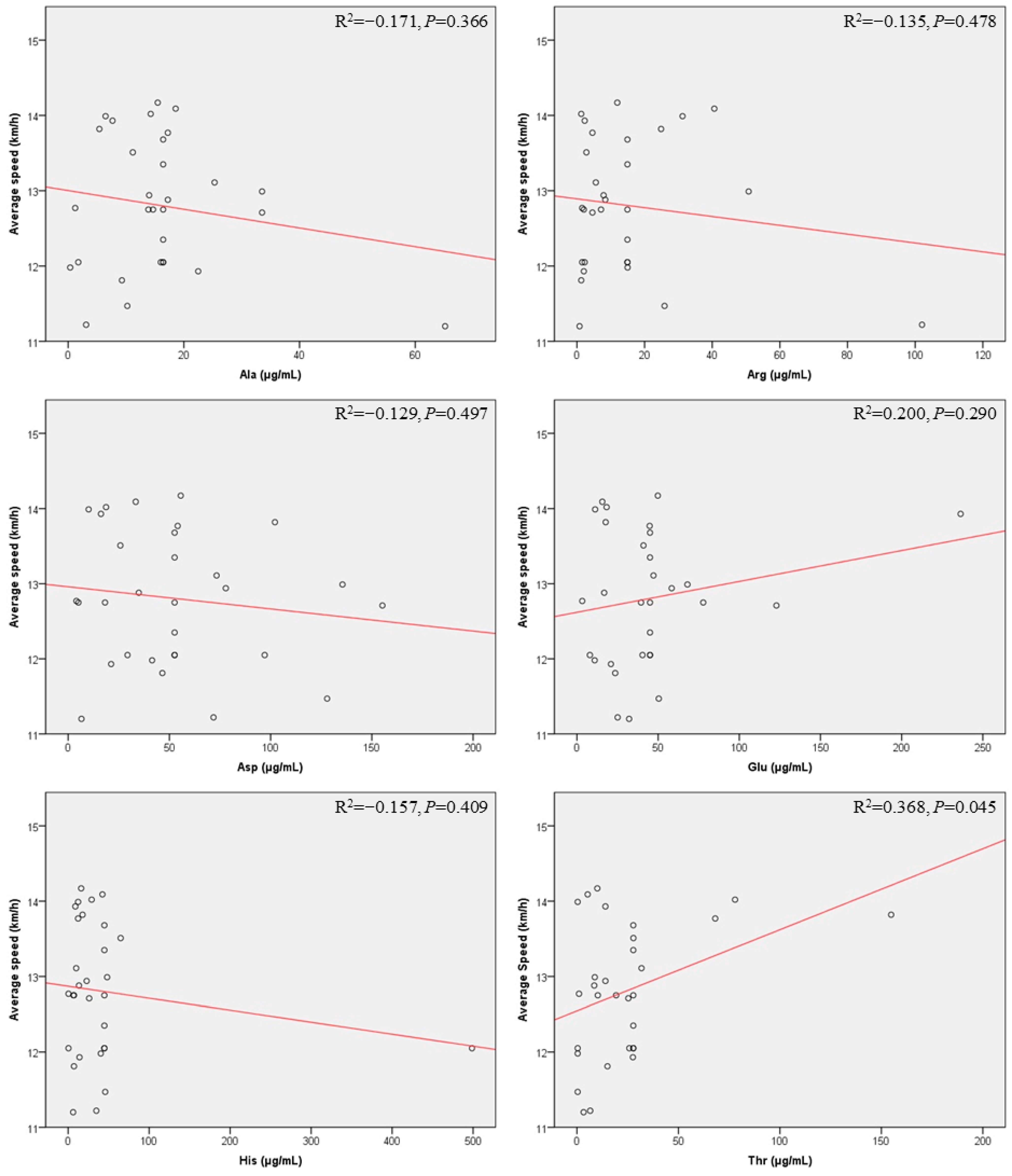
| Ala | 0.99 (0.96–1.02) | 0.366 | 1.00 (0.97–1.03) | 0.721 |
| Arg | 0.99 (0.98–1.01) | 0.478 | 1.00 (0.98–1.01) | 0.713 |
| Asp | 1.00 (0.99–1.01) | 0.497 | 1.00 (0.99–1.01) | 0.682 |
| Glu | 1.00 (1.00–1.01) | 0.290 | 1.00 (0.99–1.01) | 0.735 |
| Gln | 1.00 (1.00–1.00) | 0.738 | 1.00 (0.99–1.00) | 0.566 |
| Gly | 1.00 (0.98–1.02) | 0.993 | 1.00 (0.99–1.02) | 0.772 |
| His | 1.00 (0.99–1.00) | 0.409 | 1.00 (0.99–1.00) | 0.421 |
| Ser | 1.00 (0.99–1.02) | 0.974 | 1.00 (0.99–1.02) | 0.799 |
| Thr | 1.01 (1.00–1.02) | 0.045 | 1.01 (1.00–1.02) | 0.049 |
| Performance | ||||
| Ala | 1.00 (0.94–1.06) | 0.923 | 1.01 (0.95–1.08) | 0.734 |
| Arg | 1.00 (0.97–1.04) | 0.813 | 1.02 (0.98–1.07) | 0.311 |
| Asp | 1.00 (0.98–1.02) | 0.950 | 1.00 (0.98–1.02) | 0.716 |
| Glu | 1.01 (0.99–1.03) | 0.485 | 1.00 (0.97–1.03) | 0.851 |
| Gln | 1.00 (0.99–1.01) | 0.658 | 1.00 (0.99–1.01) | 0.495 |
| Gly | 1.00 (0.97–1.04) | 0.853 | 1.01 (0.97–1.05) | 0.648 |
| His | 0.99 (0.98–1.01) | 0.494 | 1.00 (0.98–1.01) | 0.496 |
| Ser | 1.01 (0.98–1.04) | 0.558 | 1.01 (0.98–1.05) | 0.442 |
| Thr | 1.03 (0.99–1.08) | 0.152 | 1.04 (0.99–1.09) | 0.142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Shen, K.; Fan, W.; Li, M.; Kang, X. Impact of Salivary Amino Acid Concentrations on 8 km Running Performance in Male Undergraduate Students: A Prospective Observational Study Based on HPLC. Metabolites 2025, 15, 625. https://doi.org/10.3390/metabo15090625
Zhao H, Shen K, Fan W, Li M, Kang X. Impact of Salivary Amino Acid Concentrations on 8 km Running Performance in Male Undergraduate Students: A Prospective Observational Study Based on HPLC. Metabolites. 2025; 15(9):625. https://doi.org/10.3390/metabo15090625
Chicago/Turabian StyleZhao, Hai, Kangwei Shen, Wei Fan, Mengjie Li, and Xuejun Kang. 2025. "Impact of Salivary Amino Acid Concentrations on 8 km Running Performance in Male Undergraduate Students: A Prospective Observational Study Based on HPLC" Metabolites 15, no. 9: 625. https://doi.org/10.3390/metabo15090625
APA StyleZhao, H., Shen, K., Fan, W., Li, M., & Kang, X. (2025). Impact of Salivary Amino Acid Concentrations on 8 km Running Performance in Male Undergraduate Students: A Prospective Observational Study Based on HPLC. Metabolites, 15(9), 625. https://doi.org/10.3390/metabo15090625






