High-Intensity Interval Training (HIIT): Impact of Duration on Body Composition, Cardiometabolic Health, and Aerobic Capacity in Adolescent Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
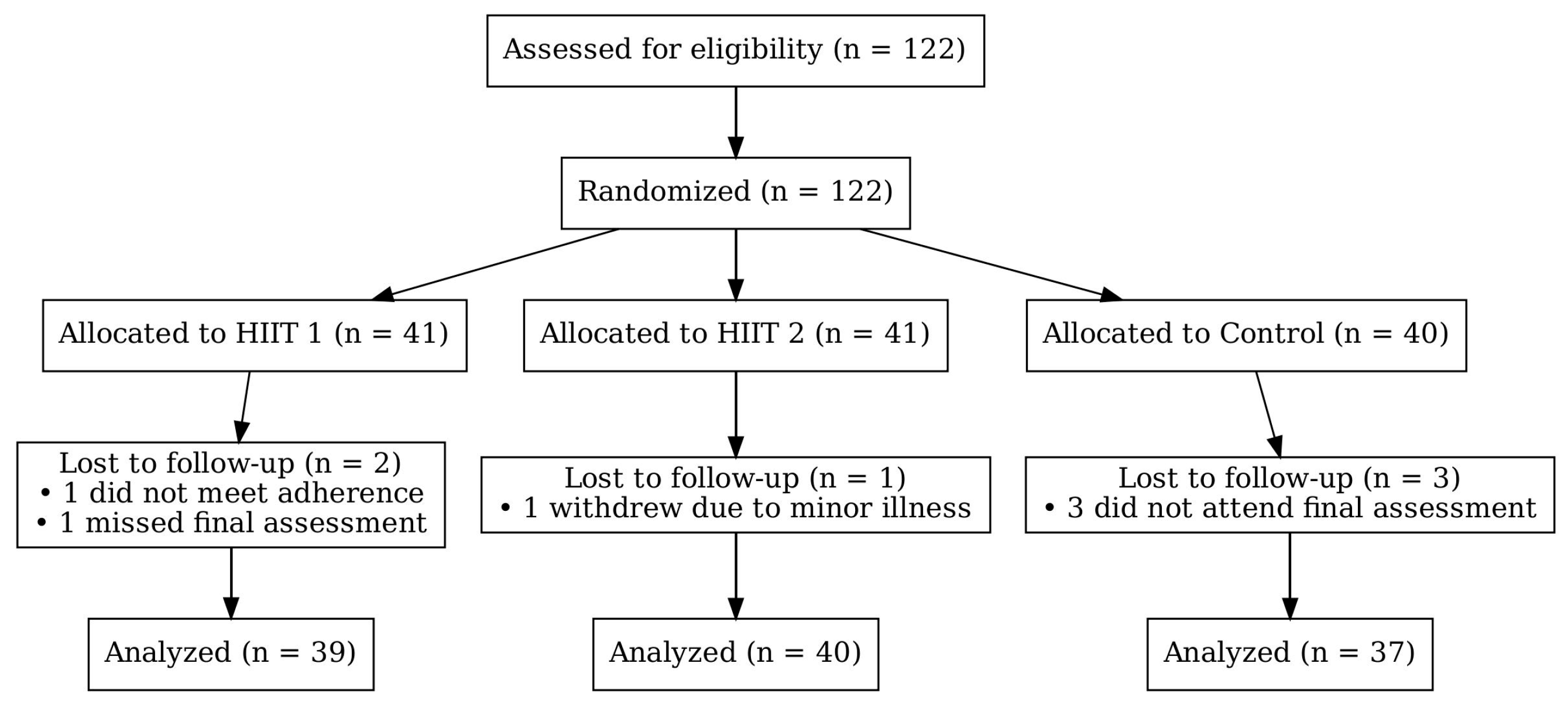
2.2. Participants
2.3. Testing Procedures
2.3.1. Body Composition
2.3.2. Cardiometabolic Health (GL, TG, SBP, DBP, TC, HDL, and LDL)
2.3.3. Aerobic Capacity (VO2max)
2.4. Experimental Program
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Xia, M.; Liu, J.; Liao, X.; Lei, T.; Liang, X.; Zhao, T.; Shi, Z.; Sun, L.; Chen, X. Development of functional connectome gradients during childhood and adolescence. Sci. Bull. 2022, 67, 1049–1061. [Google Scholar] [CrossRef]
- Gniewosz, G.; Gniewosz, B. Psychological adjustment during multiple transitions between childhood and adolescence. J. Early Adolesc. 2020, 40, 566–598. [Google Scholar] [CrossRef]
- Kljajević, V.; Stanković, M.; Đorđević, D.; Trkulja-Petković, D.; Jovanović, R.; Plazibat, K.; Oršolić, M.; Čurić, M.; Sporiš, G. Physical activity and physical fitness among university students—A systematic review. Int. J. Environ. Res. Public Health 2021, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, R.; Živković, M.; Stanković, M.; Zoretić, D.; Trajković, N. Effects of school-based high-intensity interval training on health-related fitness in adolescents. Front. Physiol. 2024, 15, 1487572. [Google Scholar] [CrossRef] [PubMed]
- van Sluijs, E.M.; Ekelund, U.; Crochemore-Silva, I.; Guthold, R.; Ha, A.; Lubans, D.; Oyeyemi, A.L.; Ding, D.; Katzmarzyk, P.T. Physical activity behaviours in adolescence: Current evidence and opportunities for intervention. Lancet 2021, 398, 429–442. [Google Scholar] [CrossRef]
- Edelmann, D.; Pfirrmann, D.; Heller, S.; Dietz, P.; Reichel, J.L.; Werner, A.M.; Schäfer, M.; Tibubos, A.N.; Deci, N.; Letzel, S. Physical activity and sedentary behavior in university students–the role of gender, age, field of study, targeted degree, and study semester. Front. Public Health 2022, 10, 821703. [Google Scholar] [CrossRef]
- Díaz-Quesada, G.; Jiménez-Jiménez, J.F.; Padial-Ruz, R.; Torres-Luque, G. An Analysis of Young Women University Students’s Physical Activity Levels. Sports 2025, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Shahid, W.; Noor, R.; Bashir, M.S. Effects of exercise on sex steroid hormones (estrogen, progesterone, testosterone) in eumenorrheic females: A systematic to review and meta-analysis. BMC Women’s Health 2024, 24, 354. [Google Scholar] [CrossRef]
- Edwards, J.J.; Griffiths, M.; Deenmamode, A.H.; O’Driscoll, J.M. High-intensity interval training and cardiometabolic health in the general population: A systematic review and meta-analysis of randomised controlled trials. Sports Med. 2023, 53, 1753–1763. [Google Scholar] [CrossRef]
- Gibala, M.J. High-intensity interval training: A time-efficient strategy for health promotion? Curr. Sports Med. Rep. 2007, 6, 211–213. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cerrillo-Urbina, A.; Herrera-Valenzuela, T.; Cristi-Montero, C.; Saavedra, J.; Martínez-Vizcaíno, V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes. Rev. 2016, 17, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.; Taaffe, D.R.; Lubans, D.R. High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Xie, Y.; Li, K.; Yuan, R.; Zhang, L. The global burden and risk factors of cardiovascular diseases in adolescent and young adults, 1990–2019. BMC Public Health 2024, 24, 1017. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Bourne, J.E.; Little, J.P. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate-and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS ONE 2014, 9, e114541. [Google Scholar] [CrossRef]
- Foster, C.; Farland, C.V.; Guidotti, F.; Harbin, M.; Roberts, B.; Schuette, J.; Tuuri, A.; Doberstein, S.T.; Porcari, J.P. The effects of high intensity interval training vs steady state training on aerobic and anaerobic capacity. J. Sports Sci. Med. 2015, 14, 747. [Google Scholar]
- Frimpong, E.; Dafkin, C.; Donaldson, J.; Millen, A.M.E.; Meiring, R.M. The effect of home-based low-volume, high-intensity interval training on cardiorespiratory fitness, body composition and cardiometabolic health in women of normal body mass and those with overweight or obesity: Protocol for a randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2019, 11, 39. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. The effects of running compared with functional high-intensity interval training on body composition and aerobic fitness in female university students. Int. J. Environ. Res. Public Health 2021, 18, 11312. [Google Scholar] [CrossRef]
- Song, Y.; Lan, H. The Effects of High-Intensity Interval Training on Cardiometabolic Health in Children and Adolescents: A Systematic Review and Meta-Analysis. J. Sports Sci. Med. 2024, 23, 690. [Google Scholar] [CrossRef]
- Batterham, A.M.; Atkinson, G. How big does my sample need to be? A primer on the murky world of sample size estimation. Phys. Ther. Sport 2005, 6, 153–163. [Google Scholar] [CrossRef]
- Djurdjevic, D.; Todorović, J.; Sipetic-Grujičić, S. Validity and reliability of the International Physical Activity Questionnaire-Short Form in adolescents. Kinesiology 2024, 56, 304–311. [Google Scholar] [CrossRef]
- Chinapaw, M.J.; Slootmaker, S.M.; Schuit, A.J.; van Zuidam, M.; van Mechelen, W. Reliability and validity of the Activity Questionnaire for Adults and Adolescents (AQuAA). BMC Med. Res. Methodol. 2009, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Giustino, V.; Hirose, N.; Messina, G.; Cataldi, S.; Grigoli, G.; Marchese, A.; Mulè, G.; Drid, P.; Palma, A. Effects of an experimental short-time high-intensity warm-up on explosive muscle strength performance in soccer players: A pilot study. Front. Physiol. 2022, 13, 984305. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.A.; Sandercock, G.R.; Voss, C.; Eisenmann, J.C.; Reed, K. Prevalence of elevated mean arterial pressure and how fitness moderates its association with BMI in youth. Public Health Nutr. 2013, 16, 2046–2054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Chukwuemeka, U.M.; Nnalue, A.D.; Obiekwe, S.J.; Maruf, F.A.; Anakor, A.C.; Moses, M.O.; Amaechi, C.; Okonkwo, U.P.; Amaechi, I.A. Comparative validity assessment of three android step counter applications; a semi-structured laboratory-based study. BMC Digit. Health 2025, 3, 20. [Google Scholar] [CrossRef]
- Pozuelo-Carrascosa, D.P.; Sanchez-Lopez, M.; Cavero-Redondo, I.; Torres-Costoso, A.; Bermejo-Cantarero, A.; Martinez-Vizcaino, V. Obesity as a mediator between cardiorespiratory fitness and blood pressure in preschoolers. J. Pediatr. 2017, 182, 114–119.e112. [Google Scholar] [CrossRef]
- Vischer, A.S.; Burkard, T. Principles of blood pressure measurement-current techniques, office vs ambulatory blood pressure measurement. Adv. Exp. Med. Biol. 2017, 956, 85–96. [Google Scholar]
- Matsuzaka, A.; Takahashi, Y.; Yamazoe, M.; Kumakura, N.; Ikeda, A.; Wilk, B.; Bar-Or, O. Validity of the multistage 20-m shuttle-run test for Japanese children, adolescents, and adults. Pediatr. Exerc. Sci. 2004, 16, 113–125. [Google Scholar] [CrossRef]
- da Silva Bento, A.F.P.; Páez, L.C.; de Mendonça Raimundo, A.M. School-based high-intensity interval training programs for promoting physical activity and fitness in adolescents: A systematic review. J. Teach. Phys. Educ. 2021, 41, 288–300. [Google Scholar] [CrossRef]
- Duncombe, S.L.; Barker, A.R.; Bond, B.; Earle, R.; Varley-Campbell, J.; Vlachopoulos, D.; Walker, J.L.; Weston, K.L.; Stylianou, M. School-based high-intensity interval training programs in children and adolescents: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0266427. [Google Scholar] [CrossRef]
- da Silva Bento, A.F.P.; Páez, L.C.; de Mendonça Raimundo, A.M. High-intensity interval training in high-school physical education classes: Study protocol for a randomized controlled trial. Contemp. Clin. Trials Commun. 2021, 24, 100867. [Google Scholar] [CrossRef]
- Bossmann, T.; Woll, A.; Wagner, I. Effects of different types of high-intensity interval training (HIIT) on endurance and strength parameters in children and adolescents. Int. J. Environ. Res. Public Health 2022, 19, 6855. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Latorre-Román, P.; Jerez-Mayorga, D.; Caamano-Navarrete, F.; García-Pinillos, F. Feasibility of incorporating high-intensity interval training into physical education programs to improve body composition and cardiorespiratory capacity of overweight and obese children: A systematic review. J. Exerc. Sci. Fit. 2019, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Sperlich, B.; Holmberg, H.-C.; Engel, F.A. Effects of high-intensity interval training in school on the physical performance and health of children and adolescents: A systematic review with meta-analysis. Sports Med.-Open 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Ricci, J.M.; Currie, K.D.; Astorino, T.A.; Erickson, K.; Pfeiffer, K.A. Program Evaluation of Fitness-And Skill-based High-intensity Interval Training (hiit) In Elementary School Physical Education: 706. Med. Sci. Sports Exerc. 2021, 53, 238–239. [Google Scholar] [CrossRef]
- Martin-Smith, R.; Cox, A.; Buchan, D.S.; Baker, J.S.; Grace, F.; Sculthorpe, N. High intensity interval training (HIIT) improves cardiorespiratory fitness (CRF) in healthy, overweight and obese adolescents: A systematic review and meta-analysis of controlled studies. Int. J. Environ. Res. Public Health 2020, 17, 2955. [Google Scholar] [CrossRef]
- Mitić, P.; Jovanović, R.; Stojiljković, N.; Trajković, N.; Olanescu, M.; Suciu, A.; Popa, D.; Peris, M. Implementing High-Intensity Interval Training in Physical Education: Effects on Adolescents’ Exercise Motivation. Behav. Sci. 2025, 15, 501. [Google Scholar] [CrossRef]
- Ricci, J.M.; Currie, K.D.; Astorino, T.A.; Erickson, K.; Pfeiffer, K.A. Program evaluation and preliminary efficacy of fitness and skill-based high-intensity interval training in physical education. Res. Q. Exerc. Sport 2023, 94, 1042–1052. [Google Scholar] [CrossRef]
- Huh, Y.; Park, H.S. Associations of overweight and obesity with cardiometabolic risk factor clusters among Korean adolescents. Sci. Rep. 2024, 14, 3581. [Google Scholar] [CrossRef]
- Jung, M.K.; Yoo, E.-G. Hypertriglyceridemia in obese children and adolescents. J. Obes. Metab. Syndr. 2018, 27, 143. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Kang, Y.S.; Kwon, E.B.; Yoo, E.-G. The triglyceride-to-high density lipoprotein cholesterol ratio in overweight Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Mieyer, P.A.; Nieto-Martinez, R.; Velasquez, A.E.; Mou, X.; Young-Moss, S.; Mechanick, J.I.; Grant, C.C.; Neira, C.P. Disparities in the cardiometabolic impact of adiposity among African American and Hispanic adolescents. Nutrients 2024, 16, 3143. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-W.; Lai, Y.-W.; Chin, Y.-T.; Tsai, S.; Yang, T.-M.; Lin, W.-T.; Lee, C.-Y.; Tsai, W.-C.; Huang, H.-L.; Seal, D.W. Stability and transformation of metabolic syndrome in adolescents: A prospective assessment in relation to the change of cardiometabolic risk factors. Nutrients 2022, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Higgins, V.; Adeli, K. Postprandial dyslipidemia in insulin resistant states in adolescent populations. J. Biomed. Res. 2020, 34, 328. [Google Scholar] [CrossRef]
- Cai, B.; Luo, X.; Zhang, P.; Luan, Y.; Cai, X.; He, X. Effect of vitamin D supplementation on markers of cardiometabolic risk in children and adolescents: A meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2800–2814. [Google Scholar] [CrossRef]
- Skelly, L.E.; Barbour-Tuck, E.N.; Kurgan, N.; Calleja, M.; Klentrou, P.; Falk, B.; Josse, A.R. Neutral effect of increased dairy product intake, as part of a lifestyle modification program, on cardiometabolic health in adolescent girls with overweight/obesity: A secondary analysis from a randomized controlled trial. Front. Nutr. 2021, 8, 673589. [Google Scholar] [CrossRef]
- Nogueira, M.D.; Braga, R.A.; Manios, Y.; Androutsos, O.; Molnár, D.; Polito, A.; Gómez-Martínez, S.; Béghin, L.; Widhalm, K.; Bueno, G. New indices in predicting cardiometabolic risk and its relation to endothelial dysfunction in adolescents: The HELENA study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1037–1048. [Google Scholar] [CrossRef]
- Furdela, V.; Pavlyshyn, H.; Shulhai, A.-M.; Kozak, K.; Furdela, M. Triglyceride glucose index, pediatric NAFLD fibrosis index, and triglyceride-to-high-density lipoprotein cholesterol ratio are the most predictive markers of the metabolically unhealthy phenotype in overweight/obese adolescent boys. Front. Endocrinol. 2023, 14, 1124019. [Google Scholar] [CrossRef]
- Blüher, S.; Käpplinger, J.; Herget, S.; Reichardt, S.; Böttcher, Y.; Grimm, A.; Kratzsch, J.; Petroff, D. Cardiometabolic risk markers, adipocyte fatty acid binding protein (aFABP) and the impact of high-intensity interval training (HIIT) in obese adolescents. Metabolism 2017, 68, 77–87. [Google Scholar] [CrossRef]
- Bond, B.; Cockcroft, E.J.; Williams, C.A.; Harris, S.; Gates, P.E.; Jackman, S.R.; Armstrong, N.; Barker, A.R. Two weeks of high-intensity interval training improves novel but not traditional cardiovascular disease risk factors in adolescents. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1039–H1047. [Google Scholar] [CrossRef]
- Racil, G.; Coquart, J.; Elmontassar, W.; Haddad, M.; Goebel, R.; Chaouachi, A.; Amri, M.; Chamari, K. Greater effects of high-compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol. Sport 2016, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Stølen, T.O.; Bye, A.; Volden, M.; Slørdahl, S.A.; Ødegård, R.; Skogvoll, E.; Wisløff, U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin. Sci. 2009, 116, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, J.; Yuan, W. The effect of HIIT on body composition, cardiovascular fitness, psychological well-being, and executive function of overweight/obese female young adults. Front. Psychol. 2023, 13, 1095328. [Google Scholar] [CrossRef]
- Sun, F.; Williams, C.A.; Sun, Q.; Hu, F.; Zhang, T. Effect of eight-week high-intensity interval training versus moderate-intensity continuous training programme on body composition, cardiometabolic risk factors in sedentary adolescents. Front. Physiol. 2024, 15, 1450341. [Google Scholar] [CrossRef] [PubMed]
- Khammassi, M.; Ouerghi, N.; Hadj-Taieb, S.; Feki, M.; Thivel, D.; Bouassida, A. Impact of a 12-week high-intensity interval training without caloric restriction on body composition and lipid profile in sedentary healthy overweight/obese youth. J. Exerc. Rehabil. 2018, 14, 118. [Google Scholar] [CrossRef]
- Baquet, G.; Berthoin, S.; Gerbeaux, M.; Van Praagh, E. High-intensity aerobic training during a 10 week one-hour physical education cycle: Effects on physical fitness of adolescents aged 11 to 16. Int. J. Sports Med. 2001, 22, 295–300. [Google Scholar] [CrossRef]
- González-Gálvez, N.; Soler-Marín, A.; Abelleira-Lamela, T.; Abenza-Cano, L.; Mateo-Orcajada, A.; Vaquero-Cristóbal, R. Eight weeks of high-intensity interval vs. sprint interval training effects on overweight and obese adolescents carried out during the cool-down period of physical education classes: Randomized controlled trial. Front. Public Health 2024, 12, 1394328. [Google Scholar] [CrossRef]
- Poon, E.T.-C.; Sum, W.M.-K.; Lubans, D.; Wong, S.H.-S.; Ho, R.S.-T. High-intensity interval training for improving cardiometabolic health in children and adolescents: An umbrella review of systematic reviews. J. Sports Sci. 2024, 42, 2199–2215. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Li, S.; Zou, Y. Effects of high-intensity interval training and moderate-intensity continuous training on cardiometabolic risk factors in overweight and obesity children and adolescents: A meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2021, 18, 11905. [Google Scholar] [CrossRef]
- Cao, M.; Quan, M.; Zhuang, J. Effect of high-intensity interval training versus moderate-intensity continuous training on cardiorespiratory fitness in children and adolescents: A meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 1533. [Google Scholar] [CrossRef]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Aleixo, A.A.; Guimarães, E.L.; Walsh, I.A.P.d.; Pereira, K. Influence of overweight and obesity on posture, overall praxis and balance in schoolchildren. J. Hum. Growth Dev. 2012, 22, 239–245. [Google Scholar] [CrossRef]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef]
- Plavsic, L.; Knezevic, O.M.; Sovtic, A.; Minic, P.; Vukovic, R.; Mazibrada, I.; Stanojlovic, O.; Hrncic, D.; Rasic-Markovic, A.; Macut, D. Effects of high-intensity interval training and nutrition advice on cardiometabolic markers and aerobic fitness in adolescent girls with obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 294–300. [Google Scholar] [CrossRef]
| Outcome | Initial | ||
|---|---|---|---|
| HIIT 1 | HIIT 2 | C | |
| Age | 18.65 ± 0.87 | 18.99 ± 0.74 | 18.74 ± 0.42 |
| BH [cm] | 173.42 ± 9.91 | 174.15 ± 8.47 | 174.33 ± 9.54 |
| BM [kg] | 78.49 ± 2.23 | 78.71 ± 2.26 | 78.52 ± 2.24 |
| Initial Measurement | Final Measurement | |||||
|---|---|---|---|---|---|---|
| HIIT 1 | HIIT 2 | C | HIIT 1 | HIIT 2 | C | |
| M ± SD 95% CI | M ± SD 95% CI | M ± SD 95% CI | M ± SD 95% CI | M ± SD 95% CI | M ± SD 95% CI | |
| BM | 78.49 ± 2.23 [77.80, 79.18] | 78.71 ± 2.26 [78.01, 79.41] | 78.52 ± 2.24 [77.83, 79.21] | 74.73 ± 1.95 [74.13, 75.33] | 73.88 ± 2.44 [73.12, 74.64] | 78.56 ± 2.25 [77.86, 79.26] |
| BMI | 24.02 ± 1.06 [23.69, 24.35] | 23.64 ± 0.99 [23.33, 23.95] | 24.49 ± 1.02 [24.17, 24.81] | 23.11 ± 1.32 [22.70, 23.52] | 22.96 ± 1.56 [22.48, 23.44] | 24.13 ± 1.57 [23.64, 24.62] |
| BF | 23.03 ± 0.79 [22.78, 23.28] | 23.14 ± 0.90 [22.86, 23.42] | 23.04 ± 0.79 [22.79, 23.29] | 20.96 ± 0.59 [20.78, 21.14] | 20.73 ± 0.87 [20.46, 21.00] | 23.10 ± 0.77 [22.86, 23.34] |
| GL | 4.69 ± 0.29 [4.60, 4.78] | 4.88 ± 0.41 [4.75, 5.01] | 4.71 ± 0.31 [4.61, 4.81] | 4.49 ± 0.25 [4.41, 4.57] | 4.33 ± 0.38 [4.21, 4.45] | 4.75 ± 0.32 [4.65, 4.85] |
| TG | 125.13 ± 1.68 [124.61, 125.65] | 125.25 ± 1.50 [124.78, 125.72] | 125.14 ± 1.69 [124.62, 125.66] | 88.09 ± 4.63 [86.66, 89.52] | 85.25 ± 0.94 [84.96, 85.54] | 125.15 ± 1.71 [124.62, 125.68] |
| SBP | 129.10 ± 1.84 [128.53, 129.67] | 129.34 ± 1.70 [128.81, 129.87] | 129.15 ± 1.86 [128.57, 129.73] | 121.59 ± 2.85 [120.71, 122.47] | 119.84 ± 1.87 [119.26, 120.42] | 129.59 ± 2.09 [128.94, 130.24] |
| DBP | 78.26 ± 1.60 [77.76, 78.76] | 78.18 ± 1.57 [77.69, 78.67] | 77.43 ± 3.73 [76.27, 78.59] | 66.00 ± 3.11 [65.04, 66.96] | 64.82 ± 1.20 [64.45, 65.19] | 78.25 ± 1.69 [77.73, 78.77] |
| TC | 170.00 ± 1.53 [169.53, 170.47] | 169.88 ± 1.54 [169.40, 170.36] | 170.02 ± 1.54 [169.54, 170.50] | 148.31 ± 2.80 [147.44, 149.18] | 145.70 ± 3.48 [144.62, 146.78] | 169.51 ± 3.86 [168.31, 170.71] |
| HDL | 34.66 ± 0.77 [34.42, 34.90] | 34.59 ± 0.63 [34.39, 34.79] | 34.66 ± 0.77 [34.42, 34.90] | 34.82 ± 0.60 [34.63, 35.01] | 35.05 ± 0.80 [34.80, 35.30] | 34.83 ± 0.60 [34.64, 35.02] |
| LDL | 112.26 ± 1.33 [111.85, 112.67] | 112.18 ± 1.35 [111.76, 112.60] | 113.00 ± 3.06 [112.05, 113.95] | 96.04 ± 1.17 [95.68, 96.40] | 94.92 ± 2.02 [94.29, 95.55] | 112.52 ± 3.51 [111.43, 113.61] |
| VO2max | 39.45 ± 3.44 [38.38, 40.52] | 39.55 ± 3.32 [38.52, 40.58] | 39.73 ± 3.40 [38.68, 40.78] | 45.88 ± 2.53 [45.10, 46.66] | 46.74 ± 3.26 [45.73, 47.75] | 39.78 ± 3.26 [38.77, 40.79] |
| Measurement Effect | Group Effect | ||||||
|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | Bonferroni Post Hoc Cohen’s d | |
| BM | 374.814 | <0.001 | 0.599 | 12.418 | <0.001 | 0.153 | 0.372; 0.426; 0.229 |
| BMI | 262.113 | <0.001 | 0.691 | 17.235 | <0.001 | 0.233 | 0.395; 0.471 |
| BF ¥ | 497.225 | <0.001 | 0.816 | 30.622 | <0.001 | 0.351 | 0.197; 0.263 |
| GL | 96.179 | <0.001 | 0.455 | 2.612 | 0.078 | 0.043 | None |
| TG | 6916.821 | <0.001 | 0.894 | 1755.365 | <0.001 | 0.968 | 0.671; 0.791; 0.421 |
| SBP ¥ | 742.319 | <0.001 | 0.868 | 83.709 | <0.001 | 0.593 | 0.242; 0.199 |
| DBP ¥ | 865.912 | <0.001 | 0.885 | 141.735 | <0.001 | 0.700 | 0.138; 0.244 |
| TC | 2001.049 | <0.001 | 0.946 | 491.477 | <0.001 | 0.895 | 0.411; 0.492; 0.221 |
| HDL | 32.091 | <0.001 | 0.218 | 0.193 | 0.825 | 0.003 | None |
| LDL | 1668.500 | <0.001 | 0.936 | 363.755 | <0.001 | 0.864 | 0.265; 0.334 |
| VO2max | 138.564 | <0.001 | 0.546 | 22.577 | <0.001 | 0.282 | 0.183; 0.544 |
| HIIT 1 (6 Weeks) Intensity/Recovery | HIIT 2 (8 Weeks) Intensity/Recovery | |
|---|---|---|
| Warm-up | 5 min of jogging 5 min of acceleration running 5 min of dynamic stretching | 5 min of jogging 5 min of acceleration running 5 min of dynamic stretching |
| Main stimuli | 90–95%/50–55% HRmax Week 1–2: 2 sets × 6 (30/30 s) Week 3–4: 2 sets × 7 (30/30 s) Week 5–6: 2 sets × 8 (30/30 s) Passive recovery between sets: 4 min | 90–95%/50–55% HRmax Week 1–2: 2 sets × 6 (30/30 s) Week 3–4: 2 sets × 7 (30/30 s) Week 5–6: 2 sets × 8 (30/30 s) Week 7–8: 2 sets × 9 (30/30 s) Passive recovery between sets: 4 min |
| Cooldown | 10 min of running 5 min of dynamic stretching exercises | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankovic, M.; Čaprić, I.; Pezelj, L.; Biševac, E.; Mekić, R.; Zećirović, A.; Salihagić, Z.; Ajdinović, A.; Jelaska, I. High-Intensity Interval Training (HIIT): Impact of Duration on Body Composition, Cardiometabolic Health, and Aerobic Capacity in Adolescent Women. Metabolites 2025, 15, 623. https://doi.org/10.3390/metabo15090623
Stankovic M, Čaprić I, Pezelj L, Biševac E, Mekić R, Zećirović A, Salihagić Z, Ajdinović A, Jelaska I. High-Intensity Interval Training (HIIT): Impact of Duration on Body Composition, Cardiometabolic Health, and Aerobic Capacity in Adolescent Women. Metabolites. 2025; 15(9):623. https://doi.org/10.3390/metabo15090623
Chicago/Turabian StyleStankovic, Mima, Ilma Čaprić, Luka Pezelj, Emir Biševac, Raid Mekić, Armin Zećirović, Zerina Salihagić, Aldina Ajdinović, and Igor Jelaska. 2025. "High-Intensity Interval Training (HIIT): Impact of Duration on Body Composition, Cardiometabolic Health, and Aerobic Capacity in Adolescent Women" Metabolites 15, no. 9: 623. https://doi.org/10.3390/metabo15090623
APA StyleStankovic, M., Čaprić, I., Pezelj, L., Biševac, E., Mekić, R., Zećirović, A., Salihagić, Z., Ajdinović, A., & Jelaska, I. (2025). High-Intensity Interval Training (HIIT): Impact of Duration on Body Composition, Cardiometabolic Health, and Aerobic Capacity in Adolescent Women. Metabolites, 15(9), 623. https://doi.org/10.3390/metabo15090623









