Paradoxical Regulation of α7nAChR and NLRP3 Inflammasome in Gastrointestinal Cancers and Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Definition of Study Groups
2.3. Study Groups
- Healthy Control-Stomach (S-C) (n = 10): Healthy stomach tissue without inflammatory or tumorous pathology;
- Healthy Control-Colorectal Tissue (C-C) (n = 10): Colorectal tissue samples without inflammatory or tumorous pathology;
- Gastric Cancer (S-Tm) (n = 10): Tumor-containing stomach tissue from patients diagnosed with stomach cancer;
- Colorectal Cancer (C-Tm) (n = 10): Tumor-containing colorectal tissue from patients diagnosed with colorectal cancer;
- Colitis (UC) (n = 10): Colorectal tissue with inflammatory changes from patients diagnosed with colitis.
2.4. Endoscopy and Colonoscopy and Sample Collection
2.5. Tissue Homogenization
2.6. ELISA Method
2.7. Statistical Analysis of Data
3. Results
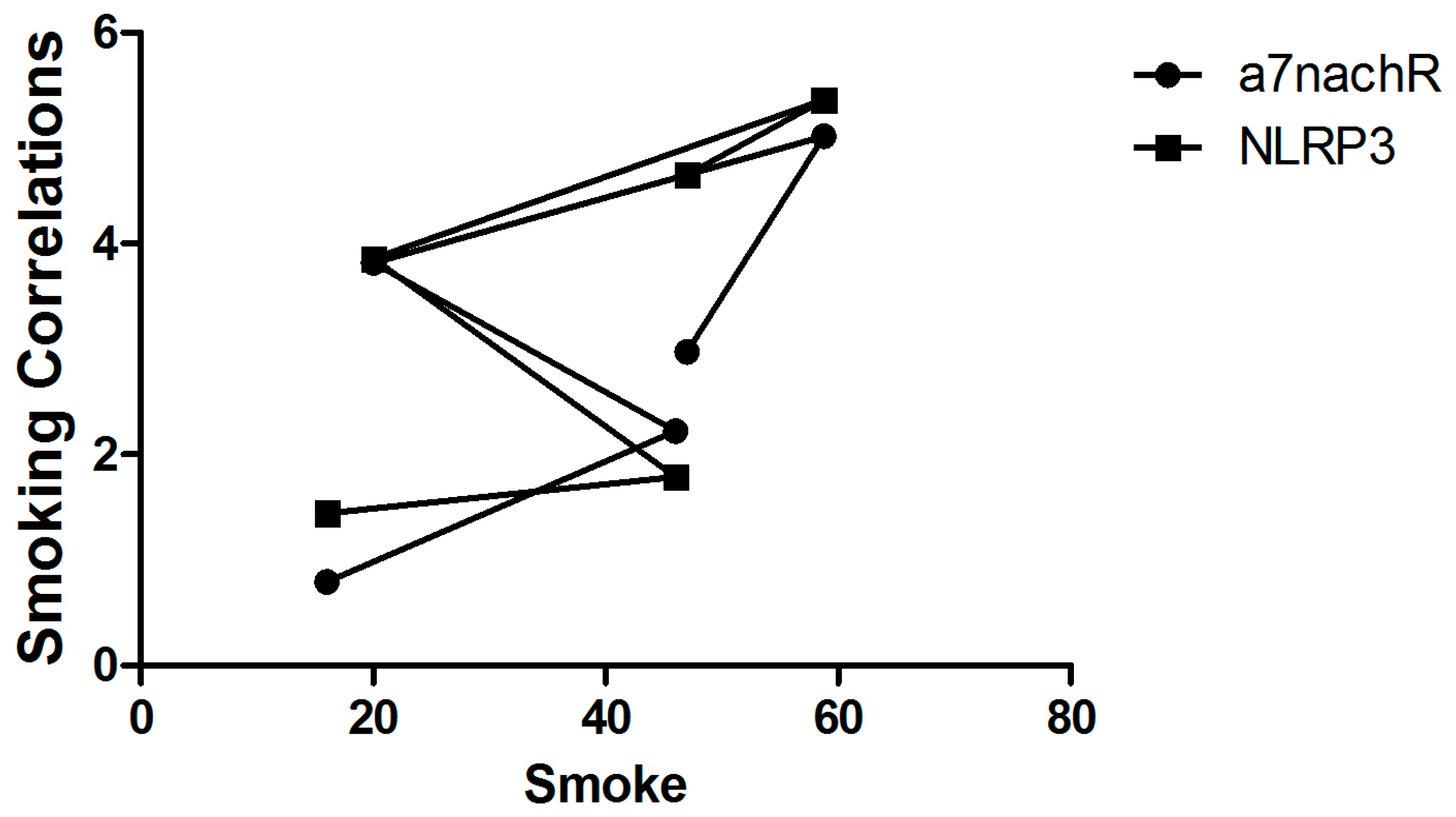
3.1. Determining the Correlation Between Healthy Individuals and Patients with Cancer and Colitis Who Smoke
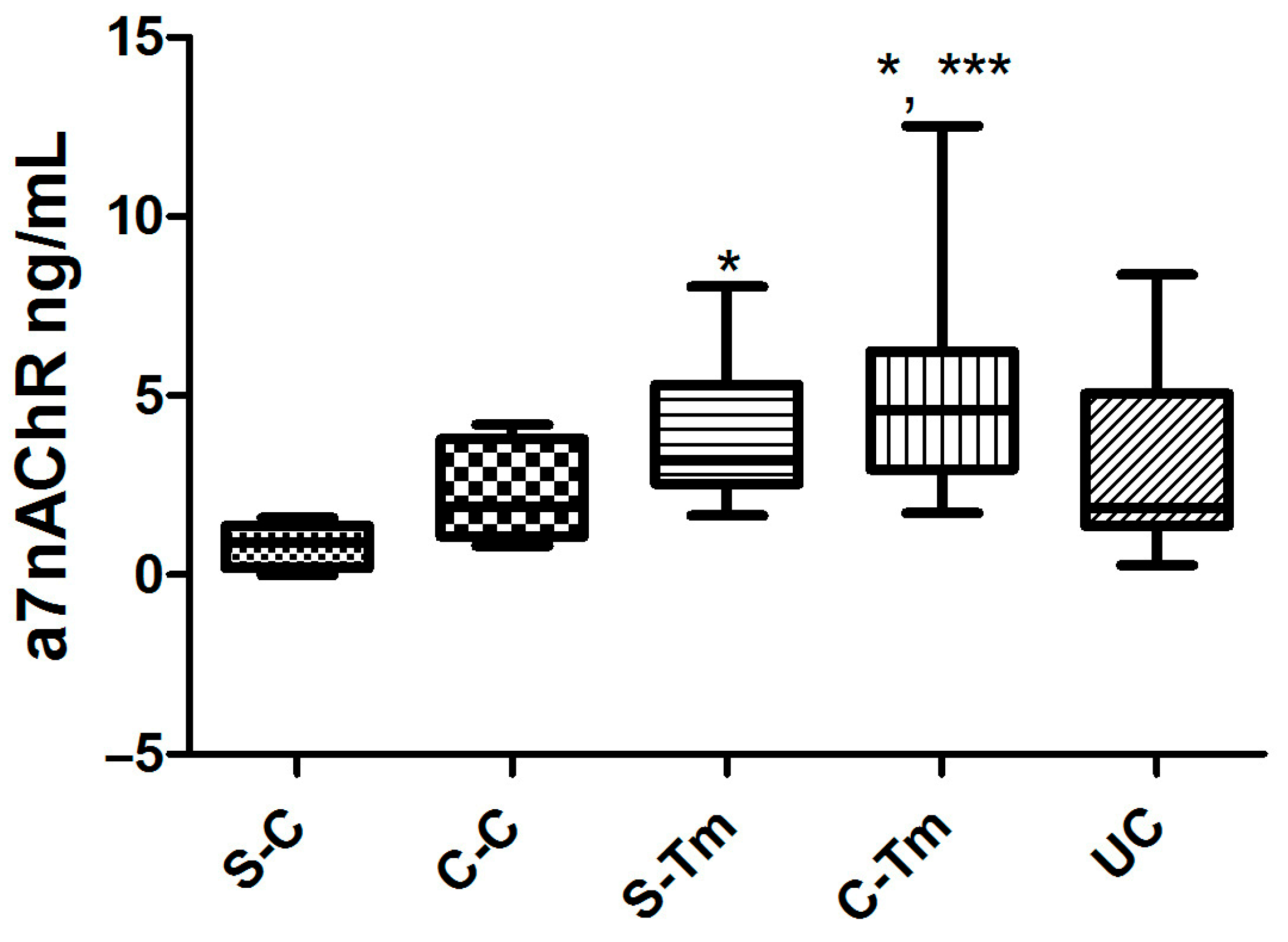
3.2. Findings of α7nAChR Levels
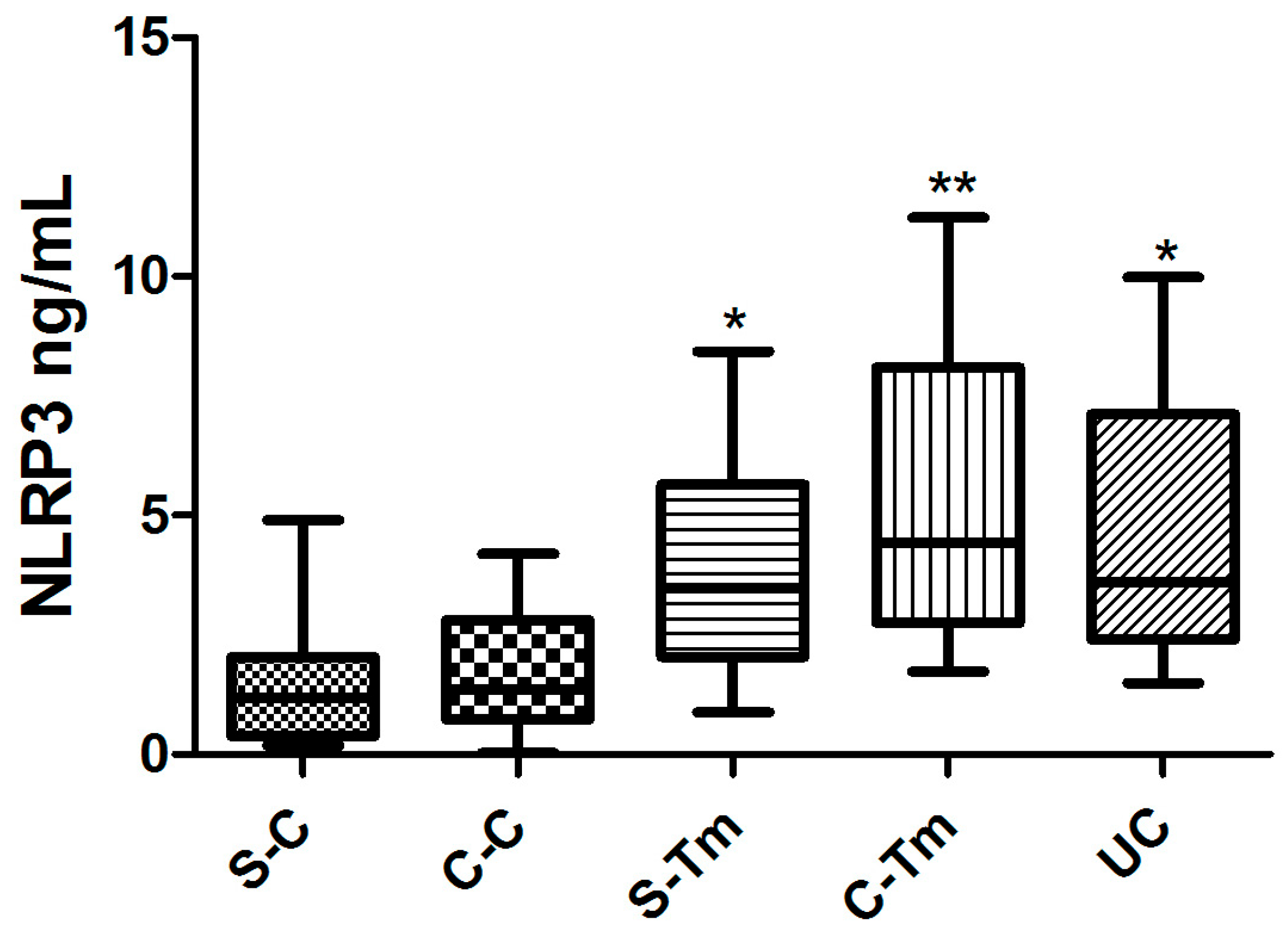
3.3. Findings of NLRP3 Inflammasome Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grierson, P.; Lim, K.H.; Amin, M. Immunotherapy in gastrointestinal cancers. J. Gastrointest. Oncol. 2017, 8, 474–484. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Savadi, P.; Khaze, V.; Minouei, M.; McMillan, N.A.J.; Hallaj-Nezhadi, S.; Baradaran, B. Targeting of high mobility group A2 by small interfering RNA-loaded nanoliposome-induced apoptosis and migration inhibition in gastrointestinal cancer cells. J. Cell. Biochem. 2019, 120, 9203–9212. [Google Scholar] [CrossRef]
- Zhao, Y. The Oncogenic Functions of Nicotinic Acetylcholine Receptors. J. Oncol. 2016, 2016, 9650481. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Regulatory role of the α7nAChR in cancer. Curr. Drug Targets 2012, 13, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Sampaio Moura, N.; Schledwitz, A.; Alizadeh, M.; Kodan, A.; Njei, L.P.; Raufman, J.P. Cholinergic Mechanisms in Gastrointestinal Neoplasia. Int. J. Mol. Sci. 2024, 25, 5316. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Nilsson, L.; Nylund, G.; Khorram-Manesh, A.; Nordgren, S.; Delbro, D.S. Is acetylcholine an autocrine/paracrine growth factor via the nicotinic alpha7-receptor subtype in the human colon cancer cell line HT-29? Eur. J. Pharmacol. 2009, 609, 27–33. [Google Scholar] [CrossRef]
- Lu, B.; Kwan, K.; Levine, Y.A.; Olofsson, P.S.; Yang, H.; Li, J.; Joshi, S.; Wang, H.; Andersson, U.; Chavan, S.S.; et al. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol. Med. 2014, 20, 350–358. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B.; Gupta, S.C. Inflammation and Cancer; Springer: Basel, Switzerland, 2014. [Google Scholar]
- Echizen, K.; Oshima, H.; Nakayama, M.; Oshima, M. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv. Biol. Regul. 2018, 68, 39–45. [Google Scholar] [CrossRef]
- Zhu, X.; Dai, S.; Xia, B.; Gong, J.; Ma, B. Activation of the alpha 7 nicotinic acetylcholine receptor mitigates osteoarthritis progression by inhibiting NF-κB/NLRP3 inflammasome activation and enhancing autophagy. PLoS ONE 2021, 16, e0256507. [Google Scholar] [CrossRef]
- Ke, P.; Shao, B.Z.; Xu, Z.Q.; Chen, X.W.; Wei, W.; Liu, C. Activating α7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of β-arrestin-1. CNS Neurosci. Ther. 2017, 23, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Pennel, K.A.F.; Park, J.H.; McMillan, D.C.; Roseweir, A.K.; Edwards, J. Signal interaction between the tumour and inflammatory cells in patients with gastrointestinal cancer: Implications for treatment. Cell Signal 2019, 54, 81–90. [Google Scholar] [CrossRef]
- Wang, H.; Cho, C.H. Effect of NF-κB signaling on apoptosis in chronic inflammation-associated carcinogenesis. Curr. Cancer Drug Targets 2010, 10, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour necrosis factor and cancer. J. Pathol. 2013, 230, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef]
- de Castro Barbosa, M.L.; da Conceicao, R.A.; Fraga, A.G.M.; Camarinha, B.D.; de Carvalho Silva, G.C.; Lima, A.G.F.; Cardoso, E.A.; de Oliveira Freitas Lione, V. NF-κB signaling pathway inhibitors as anticancer drug candidates. Anti-Cancer Agents Med. Chem. 2017, 17, 483–490. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Kelly, M.J.; Breathnach, C.; Tracey, K.J.; Donnelly, S.C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 2022, 3, 100696. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef]
- Tang, H.; Li, J.; Zhou, Q.; Li, S.; Xie, C.; Niu, L.; Ma, J.; Li, C. Vagus nerve stimulation alleviated cerebral ischemia and reperfusion injury in rats by inhibiting pyroptosis via α7 nicotinic acetylcholine receptor. Cell Death Discov. 2022, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Andrés, A.; Panisello-Roselló, A.; Roselló-Catafau, J.; Folch-Puy, E. NLRP3 Inflammasome-Mediated Inflammation in Acute Pancreatitis. Int. J. Mol. Sci. 2020, 29, 5386. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | S-C | C-C | S-Tm | C-Tm | UC |
|---|---|---|---|---|---|
| Age (Years) | 47.4 ± 3.85 | 59.1 ± 4.2 | 54.2 ± 2.30 | 62.3 ± 3.4 | 49.5 ± 6.8 |
| Sex (F/M) | 5/5 | 6/4 | 5/5 | 4/6 | 2/8 |
| Smoking (%) | 16 | 20 | 46 * | 58.8 * | 47 * |
| ⍺7nAChR (ng/mL) (Mean ± SD) | 0.79 ± 0.60 | 2.22 ± 1.3 * | 3.82 ± 1.9 *,** | 5.02 ± 3.1 | 2.97 ± 2.5 |
| NLPR3 (ng/mL) (Mean ± SD) | 1.44 ± 1.4 | 1.79 ± 1.38 | 3.86 ± 2.36 * | 5.36 ± 3.17 ** | 4.65 ± 2.83 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ates, G.; Ozgur, I.; Sormaz, I.C. Paradoxical Regulation of α7nAChR and NLRP3 Inflammasome in Gastrointestinal Cancers and Ulcerative Colitis. Metabolites 2025, 15, 622. https://doi.org/10.3390/metabo15090622
Ates G, Ozgur I, Sormaz IC. Paradoxical Regulation of α7nAChR and NLRP3 Inflammasome in Gastrointestinal Cancers and Ulcerative Colitis. Metabolites. 2025; 15(9):622. https://doi.org/10.3390/metabo15090622
Chicago/Turabian StyleAtes, Gulten, Ilker Ozgur, and Ismail Cem Sormaz. 2025. "Paradoxical Regulation of α7nAChR and NLRP3 Inflammasome in Gastrointestinal Cancers and Ulcerative Colitis" Metabolites 15, no. 9: 622. https://doi.org/10.3390/metabo15090622
APA StyleAtes, G., Ozgur, I., & Sormaz, I. C. (2025). Paradoxical Regulation of α7nAChR and NLRP3 Inflammasome in Gastrointestinal Cancers and Ulcerative Colitis. Metabolites, 15(9), 622. https://doi.org/10.3390/metabo15090622






