Metabolomic Analysis of Key Metabolites and Regulatory Mechanisms in the Transition of Uterine Receptivity in Water Buffalo (Bubalus bubalis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Blood Cell Assays
2.4. LC-MS Analysis
2.5. Non-Targeted Metabolomic Analysis
2.6. Statistical Analysis
3. Results
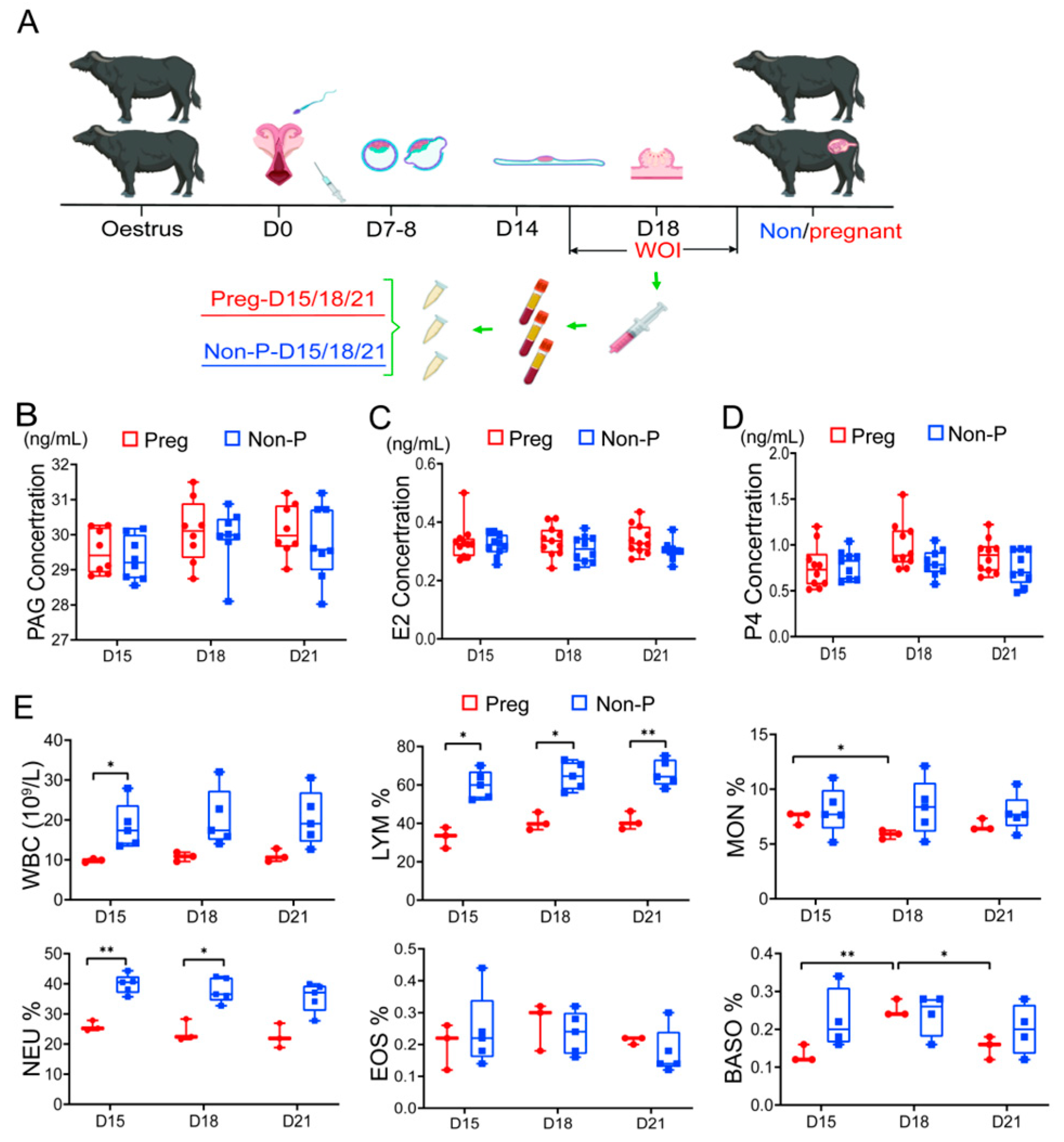
3.1. Changes in Serum Hormones and Blood Leukocytes During the Buffalo Window of Implantation (WOI)
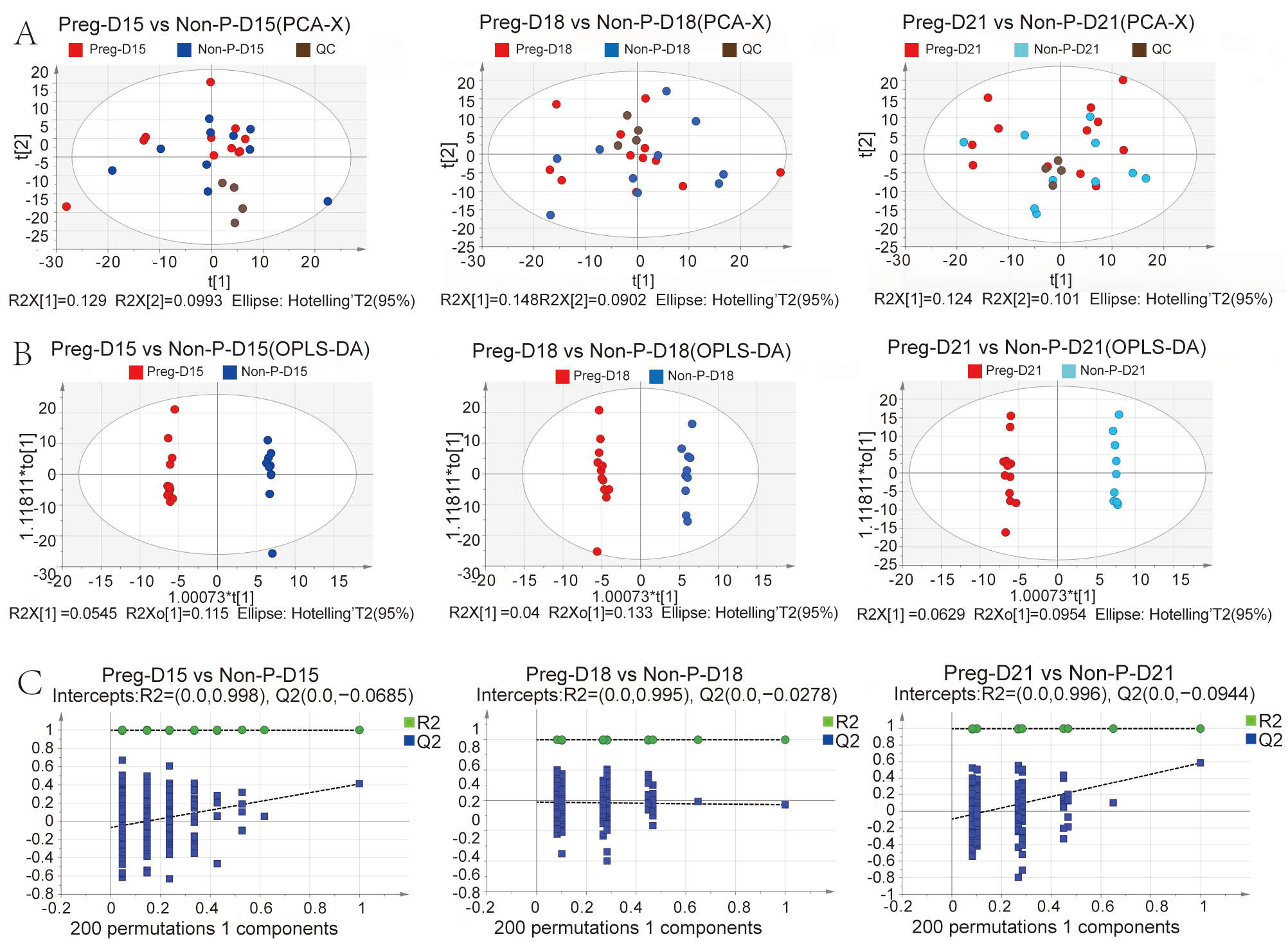
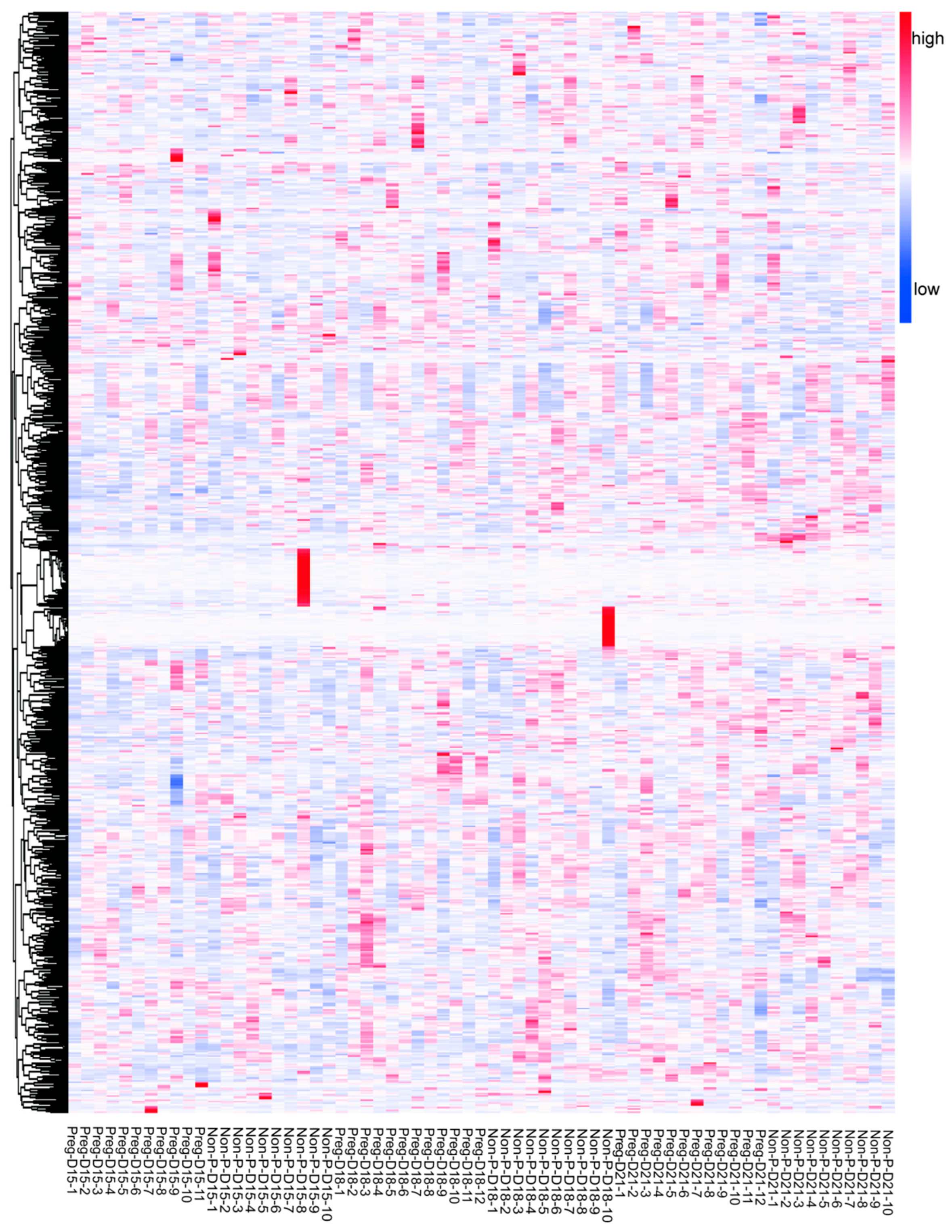
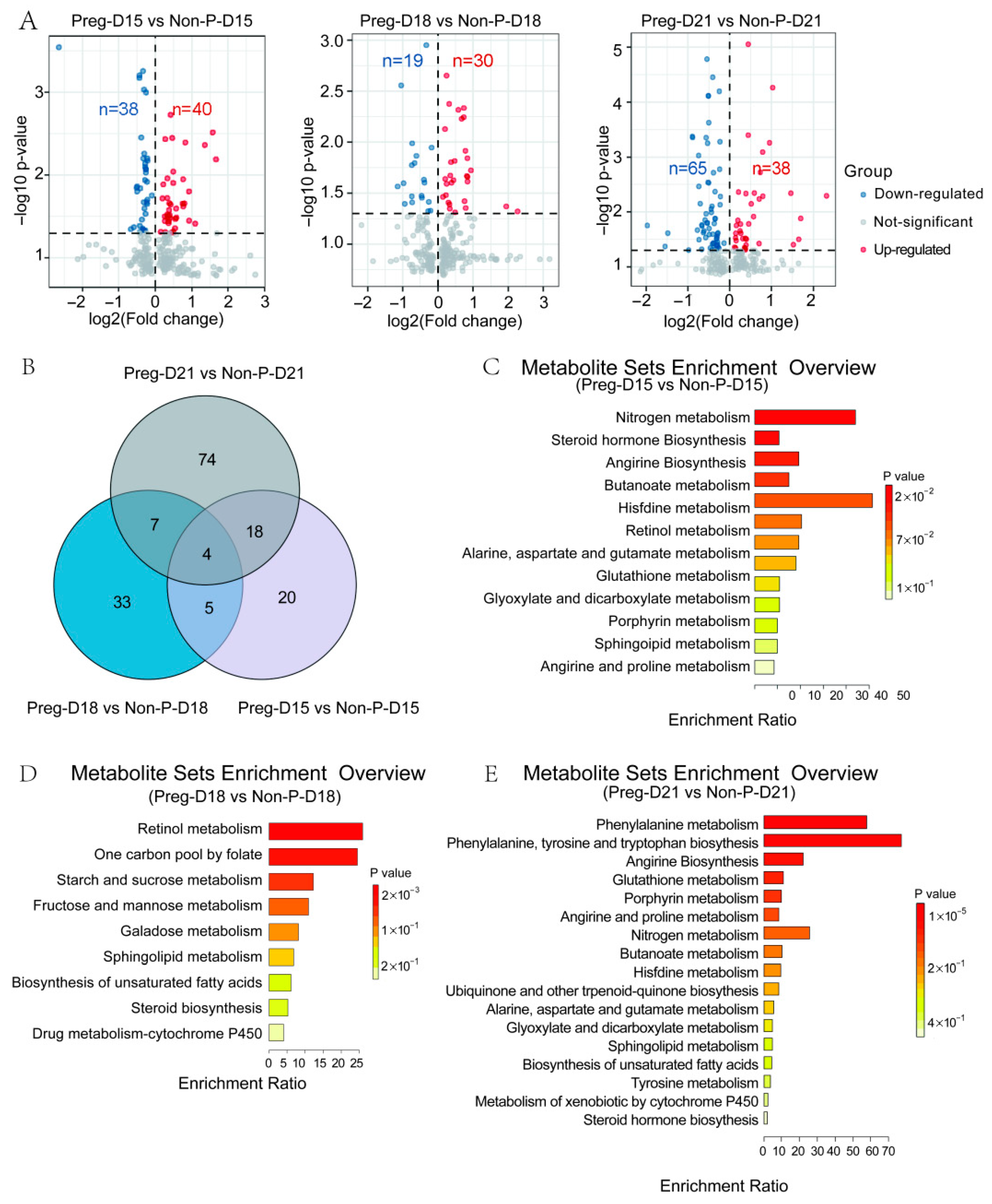
3.2. Changes in Serum Metabolites in Pregnant and Non-Pregnant Buffalo
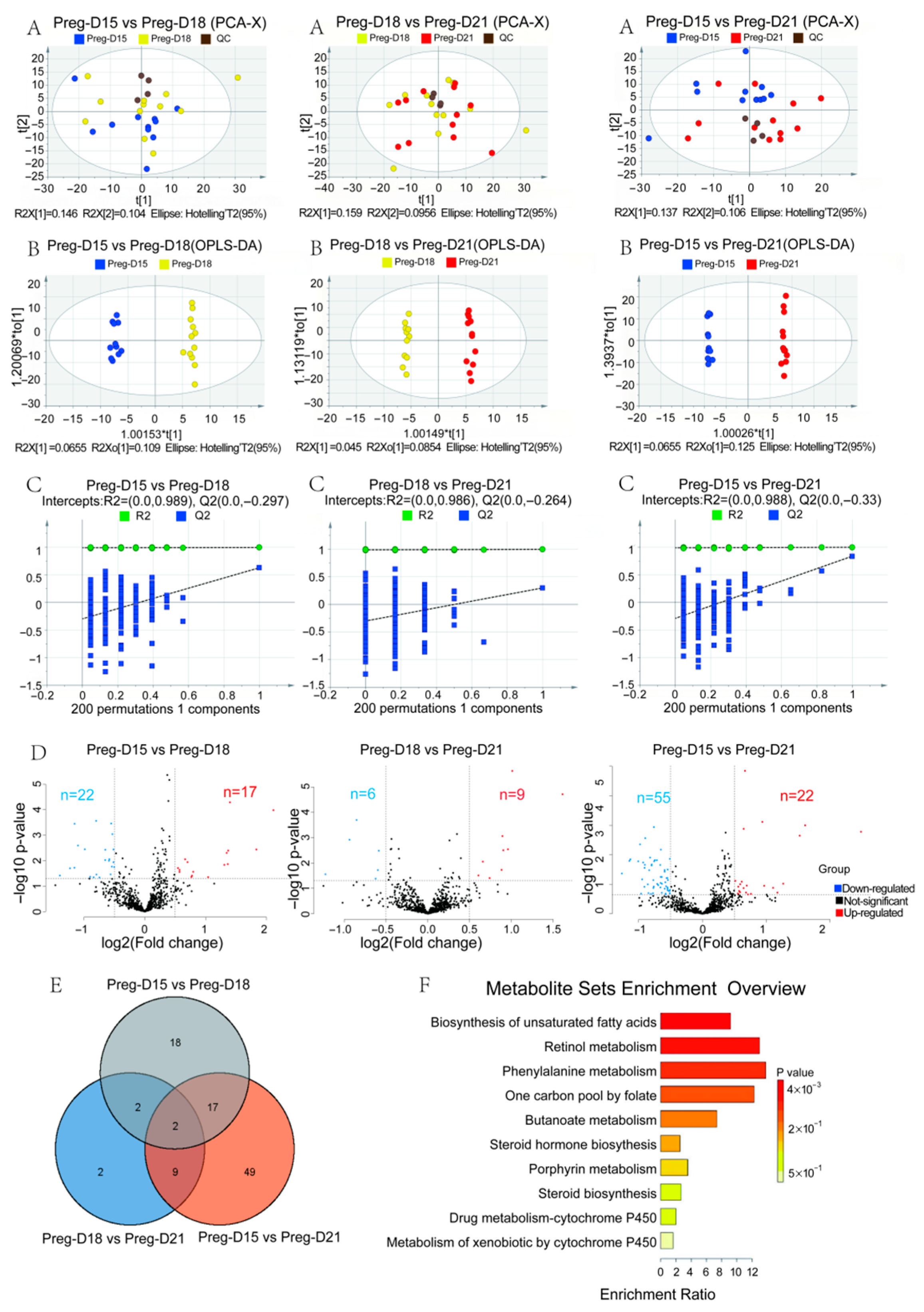
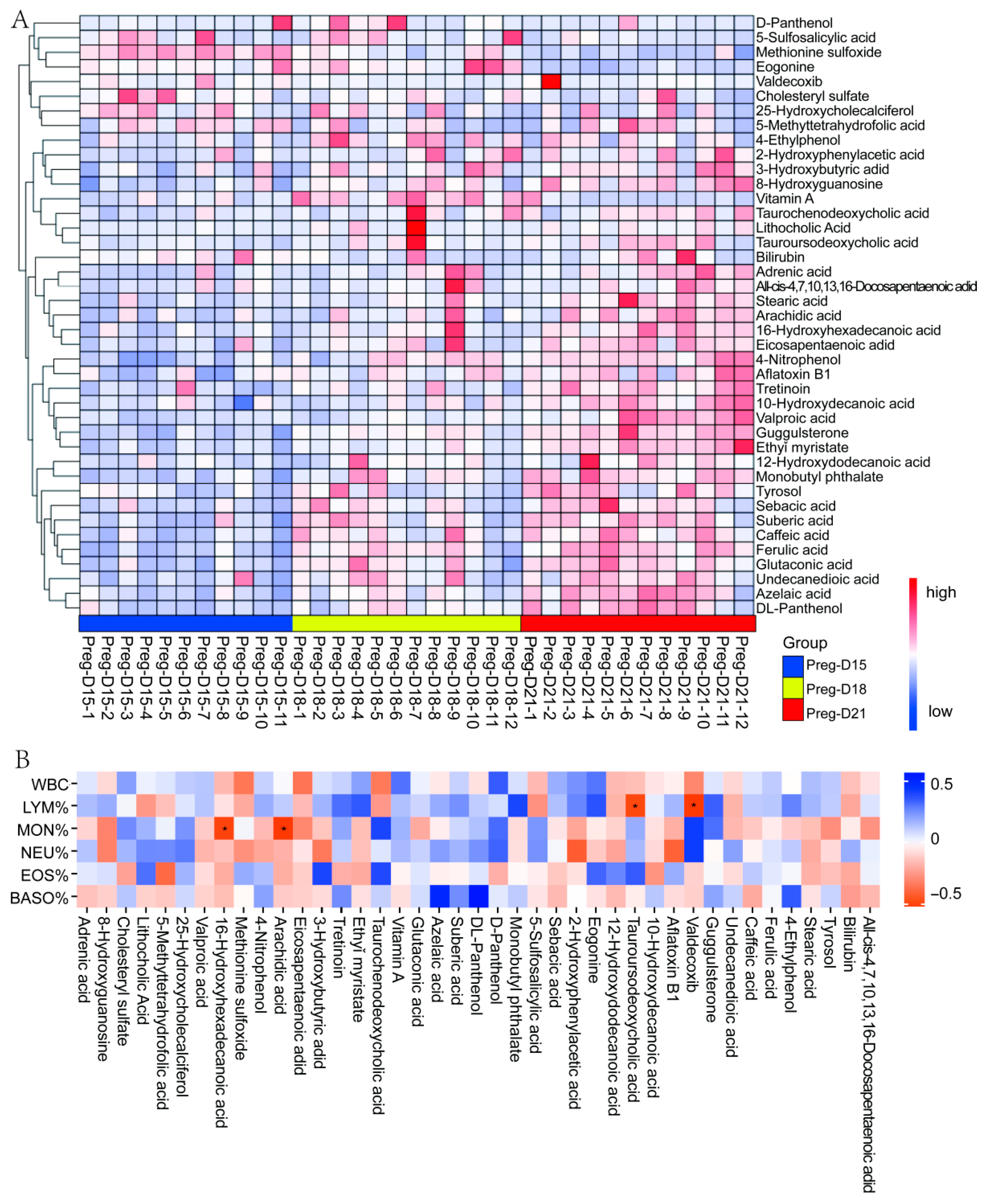
3.3. Changes in Serum Metabolites During the Buffalo WOI
3.4. Correlation Analysis of Metabolomics and Leukocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WOI | Window of Implantation |
| D15 | D15 of artificial insemination |
| D18 | D18 of artificial insemination |
| D21 | D21 of artificial insemination |
| Preg | pregnant |
| Non-P | non-pregnant |
References
- Harper, M.J. The implantation window. Bailliere’s Clin. Obstet. Gynaecol. 1992, 6, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.A.; Zyju, D.P.; Subramaniam, A.G.; Nautiyal, J.; Laloraya, M. Son of sevenless 1 (sos1), the rasgef, interacts with erα and stat3 during embryo implantation. J. Mol. Endocrinol. 2023, 70, e220089. [Google Scholar] [CrossRef]
- van den Heuvel, M.J.; Xie, X.; Tayade, C.; Peralta, C.; Fang, Y.; Leonard, S.; Paffaro, V.A., Jr.; Sheikhi, A.K.; Murrant, C.; Croy, B.A. A review of trafficking and activation of uterine natural killer cells. Am. J. Reprod. Immunol. 2005, 54, 322–331. [Google Scholar] [CrossRef]
- Rubel, C.A.; Franco, H.L.; Jeong, J.W.; Lydon, J.P.; DeMayo, F.J. Gata2 is expressed at critical times in the mouse uterus during pregnancy. Gene. Expr. Patterns 2012, 12, 196–203. [Google Scholar] [CrossRef]
- Ye, X.; Hama, K.; Contos, J.J.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. Lpa3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Krug, S.; Kastenmüller, G.; Stückler, F.; Rist, M.J.; Skurk, T.; Sailer, M.; Raffler, J.; Römisch-Margl, W.; Adamski, J.; Prehn, C.; et al. The dynamic range of the human metabolome revealed by challenges. FASEB J. 2012, 26, 2607–2619. [Google Scholar] [CrossRef]
- Ye, Q.; Hu, Y.; Jiang, H.; Luo, T.; Han, L.; Chen, Y.; Chen, J.; Ma, L.; He, Z.; Yan, X. Maternal intestinal l. Vaginalis facilitates embryo implantation and survival through enhancing uterine receptivity in sows. Microbiome 2025, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Labarta, E.; Sebastian-Leon, P.; Devesa-Peiro, A.; Celada, P.; Vidal, C.; Giles, J.; Rodriguez-Varela, C.; Bosch, E.; Diaz-Gimeno, P. Analysis of serum and endometrial progesterone in determining endometrial receptivity. Hum. Reprod. 2021, 36, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhou, H.; Liu, H.; Li, X.; Cao, L.; Zheng, W. Sanleng wan inhibits endometriosis progression by regulating sphingolipid metabolism via the s1p/s1pr1-akt axis based on serum metabolomics and network pharmacology. J. Ethnopharmacol. 2025, 352, 120153. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Cai, J.; Wang, Y.; Chai, D.; Sun, Z.; Chen, J.; Li, M.; Xiao, T.; Shan, C.; et al. St6galnac1-mediated sialylation in uterine endometrial epithelium facilitates the epithelium-embryo attachment. J. Adv. Res. 2025, 72, 197–212. [Google Scholar] [CrossRef]
- Paule, S.G.; Heng, S.; Samarajeewa, N.; Li, Y.; Mansilla, M.; Webb, A.I.; Nebl, T.; Young, S.L.; Lessey, B.A.; Hull, M.L.; et al. Podocalyxin is a key negative regulator of human endometrial epithelial receptivity for embryo implantation. Hum. Reprod. 2021, 36, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Molina, N.M.; Jurado-Fasoli, L.; Sola-Leyva, A.; Sevilla-Lorente, R.; Canha-Gouveia, A.; Ruiz-Durán, S.; Fontes, J.; Aguilera, C.M.; Altmäe, S. Endometrial whole metabolome profile at the receptive phase: Influence of mediterranean diet and infertility. Front. Endocrinol. 2023, 14, 1120988. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; He, Y.; Liu, Q.; Yang, S. Exploring the mechanism of action of aspirin in improving endometrial receptivity in pcos rats based on uterine lavage fluid metabolomics. PLoS ONE 2025, 20, e0324432. [Google Scholar] [CrossRef] [PubMed]
- Craciunas, L.; Chu, J.; Pickering, O.; Mohiyiddeen, L.; Coomarasamy, A. The metabolomic profile of endometrial receptivity in recurrent miscarriage. Minerva Obstet. Gynecol. 2023, 75, 526–534. [Google Scholar] [CrossRef]
- Drost, M. Bubaline versus bovine reproduction. Theriogenology 2007, 68, 447–449. [Google Scholar] [CrossRef]
- Srirattana, K.; Hufana-Duran, D.; Atabay, E.P.; Duran, P.G.; Atabay, E.C.; Lu, K.; Liang, Y. Current status of assisted reproductive technologies in buffaloes. Anim. Sci. J. 2022, 93, e13767. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Carvalho, J.G.S.; Elliff, F.M.; Silva, J.; Chello, D.; Carvalho, N.A.T. Embryo transfer in buffalo (bubalus bubalis). Theriogenology 2020, 150, 221–228. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, N.; Chen, B.; Dai, J.; Bai, J.; Huang, B.; Jin, L.; Dong, X.; Li, Z. Novel endometrial receptivity test increases clinical pregnancy and live birth rates in patients with recurrent implantation failure: Secondary analysis of a prospective clinical trial. Int. J. Gynaecol. Obstet. 2025, 00, 1-7. [Google Scholar] [CrossRef]
- Maziotis, E.; Kalampokas, T.; Giannelou, P.; Grigoriadis, S.; Rapani, A.; Anifantakis, M.; Kotsifaki, A.; Pantou, A.; Triantafyllidou, O.; Tzanakaki, D.; et al. Commercially available molecular approaches to evaluate endometrial receptivity: A systematic review and critical analysis of the literature. Diagnostics 2022, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.B.; Alves, M.B.R.; Bridi, A.; Bohrer, R.C.; Escobar, G.S.L.; de Carvalho, J.; Binotti, W.A.B.; Pugliesi, G.; Lemes, K.M.; Chello, D.; et al. Reproductive seasonality influences oocyte retrieval and embryonic competence but not uterine receptivity in buffaloes. Theriogenology 2021, 170, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Karanwal, S.; Chera, J.S.; Batra, V.; Kumaresan, A.; Sarwalia, P.; Datta, T.K.; Kumar, R. Circulatory extracellular vesicle derived mir-195-5p promotes cellular apoptosis and suppresses cell proliferation in the buffalo endometrial primary cell culture. Sci. Rep. 2023, 13, 16703. [Google Scholar] [CrossRef]
- Casano, A.B.; Menchetti, L.; Trabalza-Marinucci, M.; Riva, F.; De Matteis, G.; Brecchia, G.; Inglesi, A.; Rossi, E.; Signorelli, F.; Barile, V.L.; et al. Gene expression of pregnancy-associated glycoproteins-1 (pag-1), interferon-tau (ifnt) and interferon stimulated genes (isgs) as diagnostic and prognostic markers of maternal-fetal cellular interaction in buffalo cows. Theriogenology 2023, 209, 89–97. [Google Scholar] [CrossRef]
- Klisch, K.; De Sousa, N.M.; Beckers, J.F.; Leiser, R.; Pich, A. Pregnancy associated glycoprotein-1, -6, -7, and -17 are major products of bovine binucleate trophoblast giant cells at midpregnancy. Mol. Reprod. Dev. 2005, 71, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via uplc-ms. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Bian, X.; Zhao, Y.; Xiao, S.; Yang, H.; Han, Y.; Zhang, L. Metabolome and transcriptome analysis reveals the molecular profiles underlying the ginseng response to rusty root symptoms. BMC. Plant. Biol. 2021, 21, 215. [Google Scholar] [CrossRef]
- Kumar, P.; Saxena, A.; Singh, S.K.; Sharma, R.K.; Singh, I.; Agarwal, S.K. Identification of pregnancy-associated glycoproteins by peptide mass fingerprinting in water buffalo (bubalus bubalis). Indian. J. Biochem. Biophys. 2014, 51, 326–330. [Google Scholar] [PubMed]
- Harmon, Q.E.; Kissell, K.; Jukic, A.M.Z.; Kim, K.; Sjaarda, L.; Perkins, N.J.; Umbach, D.M.; Schisterman, E.F.; Baird, D.D.; Mumford, S.L. Vitamin d and reproductive hormones across the menstrual cycle. Hum. Reprod. 2020, 35, 413–423. [Google Scholar] [CrossRef]
- Derricott, H.; Heazell, A.E.P.; Greenwood, S.L.; Jones, R.L. A novel in vitro model of villitis of unknown etiology demonstrates altered placental hormone and cytokine profile. Am. J. Reprod. Immunol. 2017, 78. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, W.; Yu, Q.; Xing, T.; Chen, S.; Liu, L.; Yu, L.; Du, J.; Luo, Q.; Shen, J.; et al. Toxoplasma chinese 1 strain of wh3δrop16(i/iii)/gra15(ii) genetic background contributes to abnormal pregnant outcomes in murine model. Front. Immunol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Kwit, K.; Pomorska-Mól, M.; Markowska-Daniel, I. Pregnancy outcome and clinical status of gilts following experimental infection by h1n2, h3n2 and h1n1pdm09 influenza a viruses during the last month of gestation. Arch. Virol. 2015, 160, 2415–2425. [Google Scholar] [CrossRef]
- Bhat, P.V.; Manolescu, D.C. Role of vitamin a in determining nephron mass and possible relationship to hypertension. J. Nutr. 2008, 138, 1407–1410. [Google Scholar] [CrossRef]
- Isales, G.M.; Hipszer, R.A.; Raftery, T.D.; Chen, A.; Stapleton, H.M.; Volz, D.C. Triphenyl phosphate-induced developmental toxicity in zebrafish: Potential role of the retinoic acid receptor. Aquat. Toxicol. 2015, 161, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sobral, A.F.; Cunha, A. Unveiling the therapeutic potential of folate-dependent one-carbon metabolism in cancer and neurodegeneration. Int. J. Mol. Sci. 2024, 25, 9339. [Google Scholar] [CrossRef]
- Pogson, C.I.; Knowles, R.G.; Salter, M. The control of aromatic amino acid catabolism and its relationship to neurotransmitter amine synthesis. Crit. Rev. Neurobiol. 1989, 5, 29–64. [Google Scholar] [PubMed]
- Felaco, F.M.; Pagliano, P. hepatic encephalopathy. Pathogenesis and therapy. Infez. Med. 1997, 5, 14–19. [Google Scholar] [PubMed]
- Kudo, Y.; Boyd, C.A. Human placental indoleamine 2,3-dioxygenase: Cellular localization and characterization of an enzyme preventing fetal rejection. Biochim. Biophys. Acta. 2000, 1500, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and t-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/th2/th17 and regulatory t-cell paradigm in pregnancy. Am. J. Reprod. Immunol 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, D.; Monfardini, E.; Opsomer, G.; Burvenich, C.; Dosogne, H.; De Kruif, A.; Beckers, J.F. Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J. Dairy Res. 2000, 67, 249–259. [Google Scholar] [CrossRef]
- Dosogne, H.; Burvenich, C.; Freeman, A.E.; Kehrli, M.E., Jr.; Detilleux, J.C.; Sulon, J.; Beckers, J.F.; Hoeben, D. Pregnancy-associated glycoprotein and decreased polymorphonuclear leukocyte function in early post-partum dairy cows. Vet. Immunol. Immunopathol. 1999, 67, 47–54. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Long, E.O. Kir2dl4 (cd158d): An activation receptor for hla-g. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef] [PubMed]
- Rouas-Freiss, N.; Moreau, P.; LeMaoult, J.; Papp, B.; Tronik-Le Roux, D.; Carosella, E.D. Role of the hla-g immune checkpoint molecule in pregnancy. Hum. Immunol. 2021, 82, 353–361. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Song, J.; Liang, G.; Zhong, H.; Xu, Y.; Xie, Y.; Shi, D.; Luo, C. Metabolomic Analysis of Key Metabolites and Regulatory Mechanisms in the Transition of Uterine Receptivity in Water Buffalo (Bubalus bubalis). Metabolites 2025, 15, 615. https://doi.org/10.3390/metabo15090615
Lu X, Song J, Liang G, Zhong H, Xu Y, Xie Y, Shi D, Luo C. Metabolomic Analysis of Key Metabolites and Regulatory Mechanisms in the Transition of Uterine Receptivity in Water Buffalo (Bubalus bubalis). Metabolites. 2025; 15(9):615. https://doi.org/10.3390/metabo15090615
Chicago/Turabian StyleLu, Xingrong, Jingyuan Song, Gan Liang, Huapei Zhong, Yuanyuan Xu, Yingxue Xie, Deshun Shi, and Chan Luo. 2025. "Metabolomic Analysis of Key Metabolites and Regulatory Mechanisms in the Transition of Uterine Receptivity in Water Buffalo (Bubalus bubalis)" Metabolites 15, no. 9: 615. https://doi.org/10.3390/metabo15090615
APA StyleLu, X., Song, J., Liang, G., Zhong, H., Xu, Y., Xie, Y., Shi, D., & Luo, C. (2025). Metabolomic Analysis of Key Metabolites and Regulatory Mechanisms in the Transition of Uterine Receptivity in Water Buffalo (Bubalus bubalis). Metabolites, 15(9), 615. https://doi.org/10.3390/metabo15090615





