The Metabolic Vulnerability Index (MVX) in Subclinical Thyroid Disorders and Euthyroidism: A Cross-Sectional Exploratory Analysis from the ELSA-Brasil Study
Abstract
1. Introduction
2. Methods
2.1. Design and Participants
2.2. MVX, MMX and IVX Measurement
2.3. Thyroid-Related Parameters
2.4. Other Baseline Variables
2.5. Data Analyses
- Model 1: Adjusted for age, sex, race, smoking, diabetes, hypertension, BMI, dyslipidemia, estimated glomerular filtration rate, previous coronary heart disease, and family history of cardiovascular disease.
- Model 2: Further adjusted for physical activity, alcohol intake, and diet.
3. Results
Non-Linear Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Praw, S.S.; Brent, G.A. Approach to the Patient with a Suppressed TSH. J. Clin. Endocrinol. Metab. 2022, 108, 472–482. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Jabbar, A.; Pingitore, A.; Danzi, S.; Biondi, B.; Klein, I.; Peeters, R.; Zaman, A.; Iervasi, G. Thyroid Hormones and Cardiovascular Function and Diseases. J. Am. Coll. Cardiol. 2018, 71, 1781–1796. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cooper, D.S. Subclinical Hyperthyroidism. N. Engl. J. Med. 2018, 378, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Flaim, K.E.; Li, J.B.; Jefferson, L.S. Effects of thyroxine on protein turnover in rat skeletal muscle. Am. J. Physiol. 1978, 235, E231. [Google Scholar] [CrossRef]
- Schutz, Y. Protein Turnover, Ureagenesis and Gluconeogenesis. Int. J. Vitam. Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Brown, J.G.; Bates, P.C.; Holliday, M.A.; Millward, D.J. Thyroid hormones and muscle protein turnover. The effect of thyroid-hormone deficiency and replacement in thryoidectomized and hypophysectomized rats. Biochem. J. 1981, 194, 771–782. [Google Scholar] [CrossRef]
- Tsai, K.; Leung, A.M. Subclinical Hyperthyroidism: A Review of the Clinical Literature. Endocr. Pract. 2021, 27, 254–260. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Biondi, B.; Bartalena, L.; Cooper, D.S.; Hegedüs, L.; Laurberg, P.; Kahaly, G.J. The 2015 European Thyroid Association Guidelines on Diagnosis and Treatment of Endogenous Subclinical Hyperthyroidism. Eur. Thyroid J. 2015, 4, 149–163. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Cardiovascular risks in patients with subclinical thyroid dysfunction. Ned. Tijdschr. Geneeskd. 2012, 156, A5477. [Google Scholar]
- Morrison, W.L.; Gibson, J.N.; Jung, R.T.; Rennie, M.J. Skeletal muscle and whole body protein turnover in thyroid disease. Eur. J. Clin. Investig. 1988, 18, 62–68. [Google Scholar] [CrossRef]
- Hasselgren, P.O.; Chen, I.W.; James, J.H.; Sperling, M.; Warner, B.W.; Fischer, J.E. Studies on the Possible Role of Thyroid Hormone in Altered Muscle Protein Turnover During Sepsis. Ann. Surg. 1987, 206, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsujimoto, T.; Saito, J.; Sugiyama, T. Association Between Serum Thyrotropin Levels and Mortality Among Euthyroid Adults in the United States. Thyroid 2016, 26, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Gentile, A.; Silvestri, E.; Goglia, F.; Lombardi, A. Effect of Iodothyronines on Thermogenesis: Focus on Brown Adipose Tissue. Front. Endocrinol. 2018, 9, 254. [Google Scholar] [CrossRef]
- Mendoza, A.; Hollenberg, A.N. New insights into thyroid hormone action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef]
- Lee, J.Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.I.; Hashizaki, H.; Kim, M.J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am. J. Physiol.-Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef] [PubMed]
- Iervasi, G.; Pingitore, A.; Landi, P.; Raciti, M.; Ripoli, A.; Scarlattini, M.; L’Abbate, A.; Donato, L. Low-T3 Syndrome. Circulation 2003, 107, 708–713. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Brent, G.A.; Ebrahimi, R.; Rhee, C.M.; Leung, A.M. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw. Open 2020, 3, e1920745. [Google Scholar] [CrossRef]
- Galvao, S.; Bensenor, I.M.; Blaha, M.J.; Jones, S.; Toth, P.P.; Santos, R.D.; Bittencourt, M.; Lotufo, P.A.; Teixeira, P.F.D.S. GlycA as a novel biomarker of systemic inflammation in hypothyroidism. Thyroid 2023, 33, 1171–1181. [Google Scholar] [CrossRef]
- Peng, C.C.; Huang, H.K.; Wu, B.B.; Chang, R.H.; Tu, Y.K.; Munir, K.M. Association of Thyroid Hormone Therapy with Mortality in Subclinical Hypothyroidism: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 106, 292–303. [Google Scholar] [CrossRef]
- Laulund, A.S.; Nybo, M.; Brix, T.H.; Abrahamsen, B.; Jørgensen, H.L.; Hegedüs, L. Duration of Thyroid Dysfunction Correlates with All-Cause Mortality. The OPENTHYRO Register Cohort. PLoS ONE 2014, 9, e110437. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W., 3rd; Kraus, W.E. Multimarkers of metabolic malnutrition and inflammation and their association with mortality risk in cardiac catheterisation patients: A prospective, longitudinal, observational, cohort study. Lancet Healthy Longev. 2023, 4, e72–e82. [Google Scholar] [CrossRef]
- Conners, K.M.; Shearer, J.J.; Joo, J.; Park, H.; Manemann, S.M.; Remaley, A.T.; Otvos, J.D.; Connelly, M.A.; Sampson, M.; Bielinski, S.J.; et al. The Metabolic Vulnerability Index A Novel Marker for Mortality Prediction in Heart Failure. JACC Heart Fail. 2024, 12, 290–300. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Generoso, G.; Goulart, A.C.; Santos, R.D.; Blaha, M.J.; Jones, S.; Toth, P.P.; Lotufo, P.A.; Bittencourt, M.S.; Benseñor, I.M. Differences in HDL particle size in the presence of subclinical thyroid dysfunctions: The ELSA-Brasil study. Atherosclerosis 2020, 312, 60–65. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Meneghini, V.; Tebar, W.; Martins, J.R.M.; Sgarbi, J.A.; Teixeira, P.F.D.S.; Jones, S.R.; Blaha, M.J.; Toth, P.P.; Lotufo, P.A.; et al. Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil. Metabolites 2024, 14, 437. [Google Scholar] [CrossRef]
- Wolak-Dinsmore, J.; Gruppen, E.G.; Shalaurova, I.; Matyus, S.P.; Grant, R.P.; Gegen, R.; Bakker, S.J.L.; Otvos, J.D.; Connelly, M.A.; Dullaart, R.P.F. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Emeasoba, E.U.; Ibeson, E.; Nwosu, I.; Montemarano, N.; Shani, J.; Shetty, V.S. Clinical Relevance of Nuclear Magnetic Resonance LipoProfile. Front. Nucl. Med. 2022, 2, 960522. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, S.E.; Jung, S.W.; Jin, H.S.; Park, I.B.; Ahn, S.V.; Lee, S. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin. Endocrinol. 2017, 87, 87–96. [Google Scholar] [CrossRef]
- Abbey, E.; McGready, J.; Simonsick, E.; Mammen, J. T3:T4 Ratio Can Distinguish Between Adaptive Changes and True Subclinical Hypothyroidism in Older Adults. Innov. Aging 2020, 4 (Suppl. S1), 229. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef]
- Aquino, E.M.; Barreto, S.M.; Bensenor, I.M.; Carvalho, M.S.; Chor, D.; Duncan, B.B.; Lotufo, P.A.; Mill, J.G.; Molina Mdel, C.; Mota, E.L.; et al. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): Objectives and Design. Am. J. Epidemiol. 2012, 175, 315–324. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Chor, D.; Barreto, S.M.; Aquino, E.M.; Passos, V.M.; Matos, S.M.; Molina Mdel, C.; et al. Cohort Profile: Longitudinal Study of Adult Health (ELSA-Brasil). Int. J. Epidemiol. 2015, 44, 68–75. [Google Scholar] [CrossRef]
- Pereira, A.C.; Bensenor, I.M.; Fedeli, L.M.; Castilhos, C.; Vidigal, P.G.; Maniero, V.; Leite, C.M.; Pimentel, R.A.; Duncan, B.B.; Mill, J.G.; et al. Delineamento e implementação do biobanco do ELSA-Brasil: Estudo prospectivo na população brasileira. Rev. Saúde Pública 2012, 47 (Suppl. S2), 72–78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Griep, R.H.; Passos, V.M.; Luft, V.C.; Goulart, A.C.; Menezes, G.M.; Molina Mdel, C.; Vigo, A.; Nunes, M.A. Estratégias e desenvolvimento de garantia e controle de qualidade no ELSA-Brasil. Rev. Saúde Pública 2012, 47 (Suppl. S2), 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bensenor, I.M.; Griep, R.H.; Pinto, K.A.; de Faria, C.P.; Felisbino-Mendes, M.; Caetano, E.I.; Albuquerque, L.S.; Schmidt, M.I. Rotinas de organização de exames e entrevistas no centro de investigação ELSA-Brasil. Rev. Saúde Pública 2012, 47 (Suppl. S2), 37–47. [Google Scholar] [CrossRef]
- Connelly, M.A.; Gruppen, E.G.; Wolak-Dinsmore, J.; Matyus, S.P.; Riphagen, I.J.; Shalaurova, I.; Bakker, S.J.L.; Otvos, J.D.; Dullaart, R.P.F. GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin. Chim. Acta 2016, 452, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Shalaurova, I.; Wolak-Dinsmore, J.; Connelly, M.A.; Mackey, R.H.; Stein, J.H.; Tracy, R.P. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015, 61, 714–723. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef]
- Ferreira, N.V.; Gonçalves, N.G.; Szlejf, C.; Goulart, A.C.; de Souza Santos, I.; Duncan, B.B.; Schmidt, M.I.; Barreto, S.M.; Caramelli, P.; Feter, N.; et al. Optimal cardiovascular health is associated with slower cognitive decline. Eur. J. Neurol. 2024, 31, e16139. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, X.; Qian, Z.; Xu, W.; Zhou, X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics 2019, 9, 4019–4029. [Google Scholar] [CrossRef] [PubMed]
- Conraads, V.M.; Bosmans, J.M.; Vrints, C.J. Chronic heart failure: An example of a systemic chronic inflammatory disease resulting in cachexia. Int. J. Cardiol. 2002, 85, 33–49. [Google Scholar] [CrossRef]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Kelly, J.P.; Craig, D.M.; Haynes, C.; Jessee, R.C.; Huffman, K.M.; Kraus, W.E.; Shah, S.H. A Novel Protein Glycan–Derived Inflammation Biomarker Independently Predicts Cardiovascular Disease and Modifies the Association of HDL Subclasses with Mortality. Clin. Chem. 2017, 63, 288–296. [Google Scholar] [CrossRef]
- Malo, A.-I.; Rull, A.; Girona, J.; Domingo, P.; Fuertes-Martin, R.; Amigo, N.; Rodriguez-Borjabad, C.; Martinez-Micaelo, N.; Leal, M.; Peraire, J.; et al. Glycoprotein Profile Assessed by 1H-NMR as a Global Inflammation Marker in Patients with HIV Infection. A Prospective Study. J. Clin. Med. 2020, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Puig-Jové, C.; Julve, J.; Castelblanco, E.; Julián, M.T.; Amigó, N.; Andersen, H.U.; Ahluwalia, T.S.; Rossing, P.; Mauricio, D.; Jensen, M.T.; et al. The novel inflammatory biomarker GlycA and triglyceride-rich lipoproteins are associated with the presence of subclinical myocardial dysfunction in subjects with type 1 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 257. [Google Scholar] [CrossRef]
- Moreno-Vedia, J.; Rosales, R.; Ozcariz, E.; Llop, D.; Lahuerta, M.; Benavent, M.; Rodriguez-Calvo, R.; Plana, N.; Pedragosa, A.; Masana, L.; et al. Triglyceride-Rich Lipoproteins and Glycoprotein A and B Assessed by 1H-NMR in Metabolic-Associated Fatty Liver Disease. Front. Endocrinol. 2022, 12, 775677. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Craig, D.M.; Haynes, C.; Dowdy, Z.E.; Shah, S.H.; Kraus, W.E. High-density lipoprotein subclass measurements improve mortality risk prediction, discrimination and reclassification in a cardiac catheterization cohort. Atherosclerosis 2016, 246, 229–235. [Google Scholar] [CrossRef]
- Aquilani, R.; Rovere, M.T.L.; Corbellini, D.; Pasini, E.; Verri, M.; Barbieri, A.; Condino, A.M.; Boschi, F. Plasma Amino Acid Abnormalities in Chronic Heart Failure. Mechanisms, Potential Risks and Targets in Human Myocardium Metabolism. Nutrients 2017, 9, 1251. [Google Scholar] [CrossRef]
- Stryeck, S.; Gastrager, M.; Degoricija, V.; Trbušić, M.; Potočnjak, I.; Radulović, B.; Pregartner, G.; Berghold, A.; Madl, T.; Frank, S. Serum Concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate are Associated with Increased 3-Month Mortality in Acute Heart Failure Patients. Sci. Rep. 2019, 9, 6743. [Google Scholar] [CrossRef]
- van der Boom, T.; Gruppen, E.G.; Lefrandt, J.D.; Connelly, M.A.; Links, T.P.; Dullaart, R.P. Plasma branched chain amino acids are lower in short-term profound hypothyroidism and increase in response to thyroid hormone supplementation. Scand. J. Clin. Lab. Investig. 2020, 80, 562–566. [Google Scholar] [CrossRef]
- Sun, L.; Goh, H.J.; Verma, S.; Govindharajulu, P.; Sadananthan, S.A.; Michael, N.; Henry, C.J.; Goh, J.P.N.; Velan, S.S.; Leow, M.K.S. Brown adipose tissues mediate the metabolism of branched chain amino acids during the transitioning from hyperthyroidism to euthyroidism (TRIBUTE). Sci. Rep. 2022, 12, 3693. [Google Scholar] [CrossRef]
- Calcaterra, V.; Nappi, R.E.; Regalbuto, C.; De Silvestri, A.; Incardona, A.; Amariti, R.; Bassanese, F.; Clemente, A.M.; Vinci, F.; Albertini, R.; et al. Gender Differences at the Onset of Autoimmune Thyroid Diseases in Children and Adolescents. Front. Endocrinol. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nishio, S.; Takeda, T.; Komatsu, M. Gender-specific regulation of response to thyroid hormone in aging. Thyroid Res. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Luca, R.D.; Davis, P.J.; Lin, H.-Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction with Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.E.; Shaw, M.H.; Becker, J.T. Amino Acids in Nutrition and Health, Amino acids in systems function and health. Adv. Exp. Med. Biol. 2020, 1265, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Pałkowska-Goździk, E.; Lachowicz, K.; Rosołowska-Huszcz, D. Effects of Dietary Protein on Thyroid Axis Activity. Nutrients 2017, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Castel, H.; Shahar, D.; Harman-Boehm, I. Gender Differences in Factors Associated with Nutritional Status of Older Medical Patients. J. Am. Coll. Nutr. 2006, 25, 128–134. [Google Scholar] [CrossRef]
- Short, K.R.; Nair, K.S. Muscle Protein Metabolism and the Sarcopenia of Aging. Int. J. Sport. Nutr. Exerc. Metab. 2001, 11, S119–S127. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; Lacey, S.; Arsenic, T.; McCarthy, A.L. The Gender-Specific Relationship between Nutritional Status, Physical Activity and Functional Mobility in Irish Community-Dwelling Older Adults. Int. J. Environ. Res. Public. Health 2021, 18, 8427. [Google Scholar] [CrossRef]
- Khan, S.R.; Peeters, R.P.; van Hagen, P.M.; Dalm, V.; Chaker, L. Determinants and Clinical Implications of Thyroid Peroxidase Antibodies in Middle-Aged and Elderly Individuals: The Rotterdam Study. Thyroid 2022, 32, 78–89. [Google Scholar] [CrossRef]
- Meneghini, V.; Tebar, W.R.; Santos, I.S.; Janovsky, C.C.P.S.; de Almeida-Pititto, B.; Birck, M.G.; Lotufo, P.A.; Goulart, A.C.; Sgarbi, J.A.; Teixeira, P.D.F.D.S.; et al. Potential determinants of thyroid peroxidase antibodies and mortality risk: Results from the ELSA-Brasil study. J. Clin. Endocrinol. Metab. 2024, 109, e698–e710. [Google Scholar] [CrossRef]
- Benseñor, I.M.; Janovsky, C.C.P.S.; Goulart, A.C.; Santos, I.D.S.; Diniz, M.D.F.H.S.; Almeida-Pititto, B.D.; Sgarbi, J.A.; Lotufo, P.A. Incidence of TPOAb over a 4-year follow-up period: Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Arch. Endocrinol. Metab. 2021, 65, 832–840. [Google Scholar] [CrossRef]
- Benseñor, I.M.; Goulart, A.C.; Lotufo, P.A.; Menezes, P.R.; Scazufca, M. Prevalence of thyroid disorders among older people: Results from the São Paulo Ageing & Health Study. Cad. Saúde Pública 2011, 27, 155–161. [Google Scholar] [CrossRef]
- Benseñor, I.M.; Sgarbi, J.A.; Janovsky, C.C.P.S.; Pittito, B.A.; Diniz, M.D.F.H.S.; Almeida, M.D.C.C.D.; Alvim, S.M.; Barreto, S.M.; Giatti, L.; Duncan, B.B.; et al. Incidence of thyroid diseases: Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Arch. Endocrinol. Metab. 2021, 65, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Janovsky, C.C.P.S.; Bittencourt, M.S.; Goulart, A.C.; Santos, I.S.; Almeida-Pititto, B.; Lotufo, P.A.; Benseñor, I.M. Prevalence of antithyroperoxidase antibodies in a multiethnic Brazilian population: The ELSA-Brasil Study. Arch. Endocrinol. Metab. 2019, 63, 351–357. [Google Scholar] [CrossRef] [PubMed]
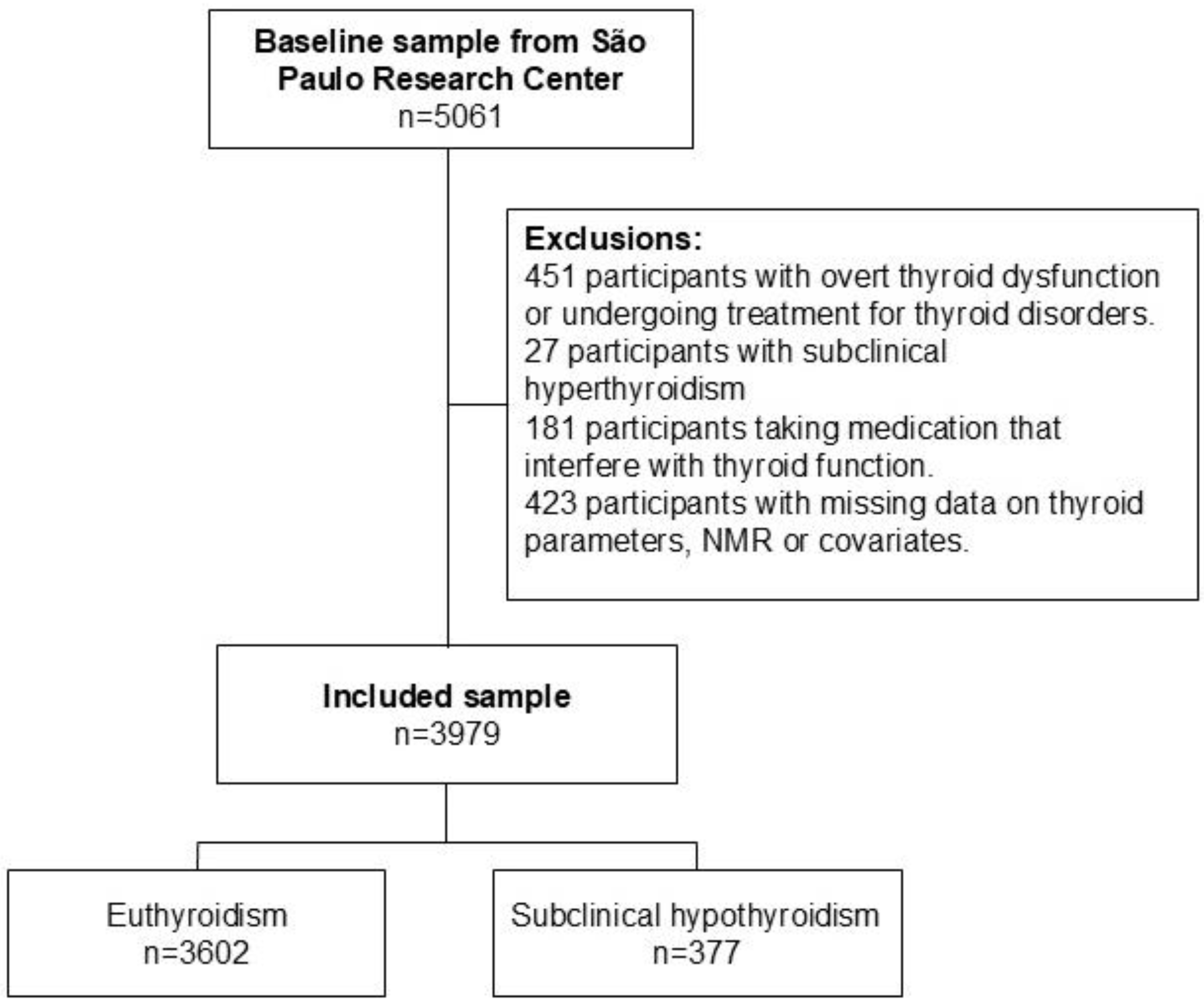
| All Sample (n = 3979) | Thyroid Function | p-Value | ||
|---|---|---|---|---|
| Euthyroid (n = 3602) | Subclinical Hypothyroidism (n = 377) | |||
| Sociodemographic variables | ||||
| Age, years | 51.26 (9.02) | 51.11 (8.99) | 52.72 (9.16) | 0.001 |
| Sex | 0.816 | |||
| Male | 1933 (48.6%) | 1752 (48.6%) | 181 (48.0%) | |
| Female | 2046 (51.4%) | 1850 (51.4%) | 196 (52.0%) | |
| Self-reported race | 0.021 | |||
| Non-white | 1646 (41.4%) | 1511 (41.9%) | 135 (35.8%) | |
| White | 2333 (58.6%) | 2091 (58.1%) | 242 (64.2%) | |
| Risk factors and health conditions | ||||
| BMI, kg/m2 | 27.27 (4.87) | 27.21 (4.81) | 27.82 (5.41) | 0.038 |
| eGFR, mL/min per 1.73 m2 | 85.28 (15.05) | 85.64 (15.06) | 81.83 (14.51) | <0.001 |
| Smoking | 0.071 | |||
| Never | 2125 (53.4%) | 1907 (52.9%) | 218 (57.8%) | |
| Past/current | 1854 (46.6%) | 1695 (47.1%) | 159 (42.2%) | |
| Alcohol intake | 0.134 | |||
| No | 2166 (54.4%) | 1947 (54.1%) | 219 (58.1%) | |
| Yes | 1813 (45.6%) | 1655 (45.9%) | 158 (41.9%) | |
| Physical Activity | 0.412 | |||
| Inactive | 2603 (65.4%) | 2347 (65.2%) | 256 (67.9%) | |
| Not sufficiently active | 454 (11.4%) | 410 (11.4%) | 44 (11.7%) | |
| Active | 922 (23.2%) | 845 (23.5%) | 77 (20.4%) | |
| Diet | 0.162 | |||
| Lower (score < 50) | 950 (23.9%) | 871 (24.2%) | 79 (21.0%) | |
| Higher (score ≥ 50) | 3029 (76.1%) | 2731 (75.8%) | 298 (79.0%) | |
| Diabetes | 688 (17.3%) | 630 (17.5%) | 58 (15.4%) | 0.304 |
| Hypertension | 1232 (31.0%) | 1105 (30.7%) | 127 (33.7%) | 0.229 |
| Dyslipidemia | 1718 (43.2%) | 1556 (43.2%) | 162 (43.0%) | 0.932 |
| Coronary heart disease | 96 (2.4%) | 86 (2.4%) | 10 (2.7%) | 0.724 a |
| Family history of CVD | 1016 (25.5%) | 913 (25.3%) | 103 (27.3%) | 0.403 |
| Cardiometabolic disease | 2695 (67.7%) | 2433 (67.5%) | 262 (69.5%) | 0.441 |
| Positive TPOAb | 371 (9.3%) | 288 (8.0%) | 83 (22.0%) | <0.001 |
| MVX | 43.60 (9.09) | 43.51 (9.11) | 44.42 (8.87) | 0.065 |
| IVX | 40.70 (10.93) | 40.64 (10.99) | 41.25 (10.42) | 0.303 |
| MMX | 50.51 (6.02) | 50.43 (6.02) | 51.21 (6.03) | 0.017 |
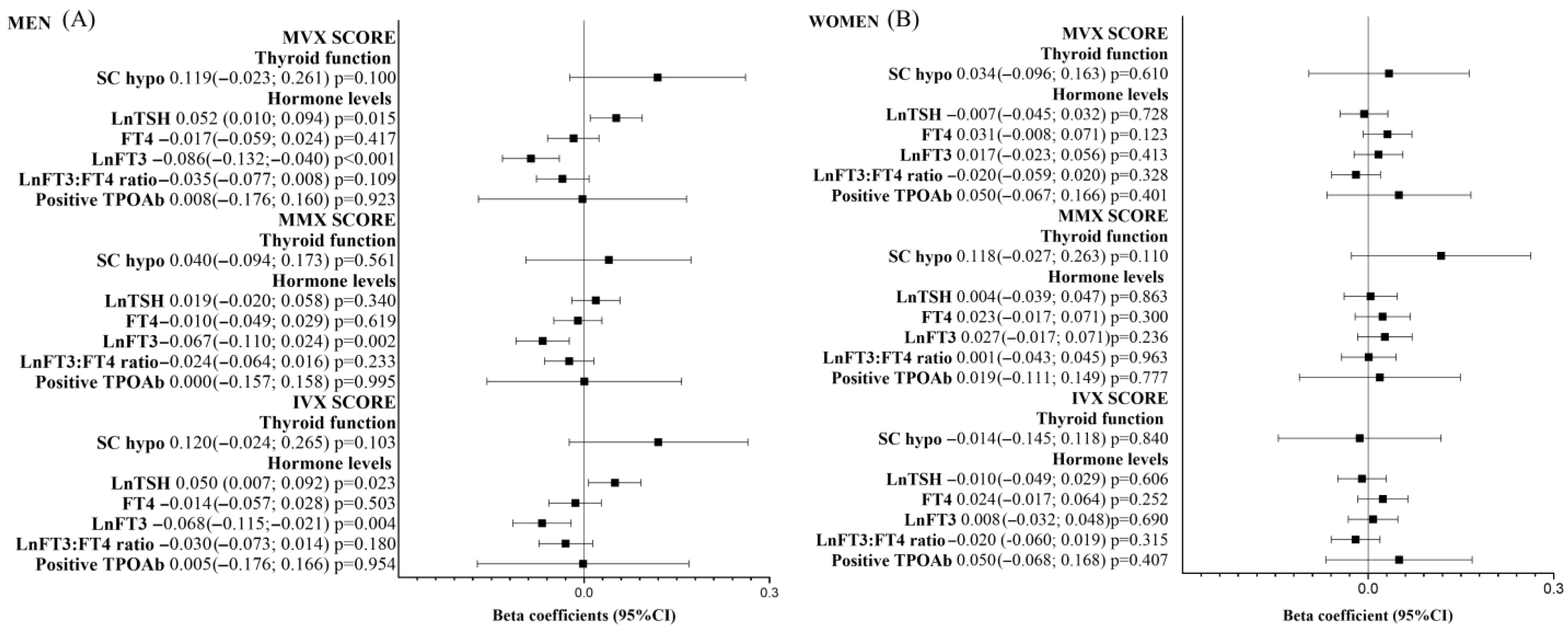
| Crude | Model 1 | Model 2 | Model 2 Including Only Euthyroid Individuals | |||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | |
| MVX SCORE | ||||||||
| Subclinical hypo | 0.10 (−0.01;0.20) | 0.065 | 0.09 (−0.01;0.18) | 0.082 | 0.07 (−0.02;0.17) | 0.146 | - | - |
| LnTSH | 0.04 (−0.04;0.13) | 0.350 | 0.07 (−0.01;0.15) | 0.094 | 0.06 (−0.02;0.14) | 0.137 | 0.01 (−0.10;0.12) | 0.894 |
| FT4 | −0.29 (−0.47;−0.10) | 0.002 | 0.02 (−0.16;0.19) | 0.853 | 0.02 (−0.16;0.19) | 0.856 | 0.03 (−0.16;0.21) | 0.779 |
| LnFT3 | −4.24 (−5.31;−3.17) | <0.001 | −1.17 (−2.24;−0.10) | 0.033 | −1.37 (−2.43;−0.31) | 0.011 | −1.62 (−2.75;−0.50) | 0.004 |
| LnFT3–FT4 ratio | −1.43 (−2.37;−0.49) | 0.003 | −0.78 (−1.68;0.12) | 0.088 | −0.90 (−1.79;−0.01) | 0.047 | −1.10 (−2.04;−0.16) | 0.022 |
| Positive TPOAb | 0.11 (0.01;0.22) | 0.034 | 0.03 (−0.07;0.13) | 0.529 | 0.03 (−0.07;0.12) | 0.596 | 0.01 (−0.10;0.12) | 0.844 |
| MMX SCORE | ||||||||
| Subclinical hypo | 0.13 (0.02;0.23) | 0.017 | 0.08 (−0.02;0.18) | 0.125 | 0.07 (−0.03;0.17) | 0.155 | - | - |
| LnTSH | 0.07 (−0.02;0.15) | 0.130 | 0.03 (−0.05;0.11) | 0.458 | 0.03 (−0.05;0.11) | 0.517 | −0.05 (−0.16;0.06) | 0.370 |
| FT4 | −0.05 (−0.24;0.13) | 0.583 | 0.05 (−0.13;0.23) | 0.574 | 0.04 (−0.14;0.22) | 0.684 | 0.06 (−0.13;0.25) | 0.534 |
| LnFT3 | −4.05 (−5.13;−2.97) | <0.001 | −0.47 (−1.56;0.62) | 0.401 | −0.51 (−1.60;0.57) | 0.354 | −0.51 (−1.66;0.63) | 0.380 |
| LnFT3–FT4 ratio | −2.27 (−3.21;−1.32) | <0.001 | −0.34 (−1.26;0.57) | 0.459 | −0.31 (−1.22;0.60) | 0.507 | −0.40 (−1.36;0.56) | 0.412 |
| Positive TPOAb | 0.12 (0.01;0.23) | 0.029 | 0.01 (−0.09;0.11) | 0.786 | 0.02 (−0.08;0.12) | 0.765 | −0.01 (−0.12;0.10) | 0.851 |
| IVX SCORE | ||||||||
| Subclinical hypo | 0.06 (−0.05;0.16) | 0.302 | 0.07 (−0.03;0.17) | 0.192 | 0.05 (−0.05;0.15) | 0.304 | - | - |
| LnTSH | 0.01 (−0.07;0.10) | 0.775 | 0.06 (−0.02;0.14) | 0.136 | 0.05 (−0.03;0.14) | 0.187 | 0.02 (−0.09;0.13) | 0.662 |
| FT4 | −0.31 (−0.49;−0.12) | 0.001 | −0.01 (−0.19;0.17) | 0.942 | 0.00 (−0.18;0.18) | 0.999 | 0.00 (−0.19;0.19) | 0.991 |
| LnFT3 | −2.91 (−3.99;−1.83) | <0.001 | −1.10 (−2.19;−0.01) | 0.048 | −1.32 (−2.39;−0.24) | 0.017 | −1.61 (−2.75;−0.47) | 0.006 |
| LnFT3–FT4 ratio | −0.53 (−1.47;0.41) | 0.268 | −0.71 (−1.63;0.20) | 0.127 | −0.87 (−1.77;0.04) | 0.060 | −1.05 (−2.01;−0.09) | 0.032 |
| Positive TPOAb | 0.08 (−0.03;0.18) | 0.157 | 0.03 (−0.07;0.13) | 0.527 | 0.03 (−0.07;0.12) | 0.613 | 0.02 (−0.09;0.13) | 0.749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovsky, C.C.P.S.; Meneghini, V.; Tebar, W.; Martins, J.R.M.; Sgarbi, J.A.; Teixeira, P.d.F.d.S.; Santos, I.d.S.; Jones, S.R.; Blaha, M.J.; Toth, P.P.; et al. The Metabolic Vulnerability Index (MVX) in Subclinical Thyroid Disorders and Euthyroidism: A Cross-Sectional Exploratory Analysis from the ELSA-Brasil Study. Metabolites 2025, 15, 606. https://doi.org/10.3390/metabo15090606
Janovsky CCPS, Meneghini V, Tebar W, Martins JRM, Sgarbi JA, Teixeira PdFdS, Santos IdS, Jones SR, Blaha MJ, Toth PP, et al. The Metabolic Vulnerability Index (MVX) in Subclinical Thyroid Disorders and Euthyroidism: A Cross-Sectional Exploratory Analysis from the ELSA-Brasil Study. Metabolites. 2025; 15(9):606. https://doi.org/10.3390/metabo15090606
Chicago/Turabian StyleJanovsky, Carolina Castro Porto Silva, Vandrize Meneghini, William Tebar, Joao Roberto Maciel Martins, José Augusto Sgarbi, Patrícia de Fatima dos Santos Teixeira, Itamar de Souza Santos, Steven R. Jones, Michael J. Blaha, Peter P. Toth, and et al. 2025. "The Metabolic Vulnerability Index (MVX) in Subclinical Thyroid Disorders and Euthyroidism: A Cross-Sectional Exploratory Analysis from the ELSA-Brasil Study" Metabolites 15, no. 9: 606. https://doi.org/10.3390/metabo15090606
APA StyleJanovsky, C. C. P. S., Meneghini, V., Tebar, W., Martins, J. R. M., Sgarbi, J. A., Teixeira, P. d. F. d. S., Santos, I. d. S., Jones, S. R., Blaha, M. J., Toth, P. P., Bittencourt, M. S., Santos, R. D., Lotufo, P. A., Chaker, L., & Bensenor, I. M. (2025). The Metabolic Vulnerability Index (MVX) in Subclinical Thyroid Disorders and Euthyroidism: A Cross-Sectional Exploratory Analysis from the ELSA-Brasil Study. Metabolites, 15(9), 606. https://doi.org/10.3390/metabo15090606





