Impact of Dietary Interventions on the Human Plasma and Lipoprotein Lipidome
Abstract
1. Introduction
2. Review Methodology
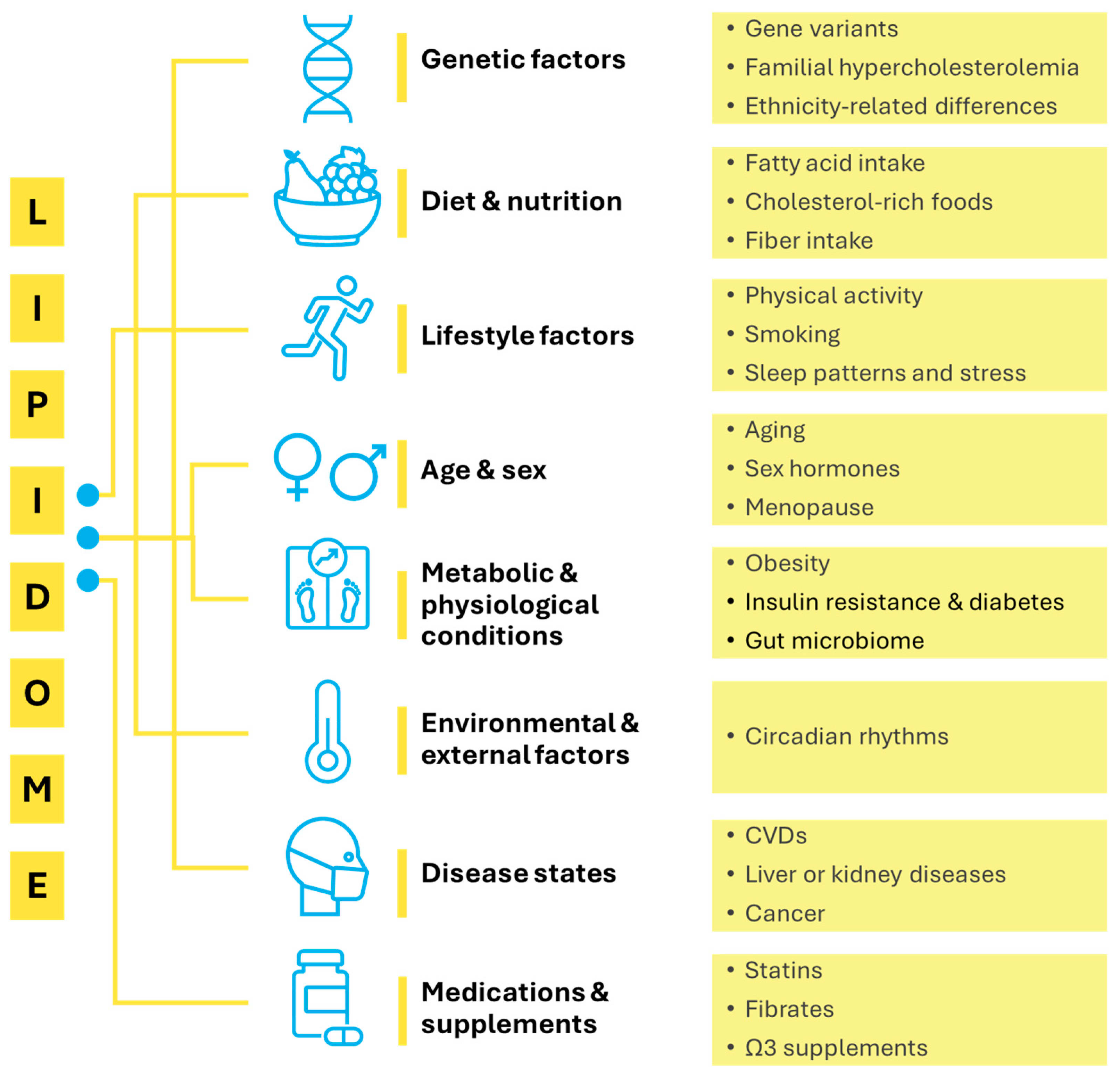
3. Factors Affecting the Human Lipidome
3.1. Genetic and Physiological Determinants
3.2. Metabolic and Inflammatory Conditions
3.3. Gut Microbiota
3.4. Lifestyle Factors
3.5. Diet and Food Matrix Effects
4. Effect of Diet on Lipidome
4.1. Plasma Lipidome
4.1.1. Dietary Patterns
4.1.2. Dietary Fats
4.1.3. Other Food Products
4.2. Serum
4.3. HDL Lipidome
4.4. LDL Lipidome
5. The Relevance of Lipidome in Health and Disease
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Hornburg, D.; Wu, S.; Moqri, M.; Zhou, X.; Contrepois, K.; Bararpour, N.; Traber, G.M.; Su, B.; Metwally, A.A.; Avina, M.; et al. Dynamic lipidome alterations associated with human health, disease and ageing. Nat. Metab. 2023, 5, 1578–1594. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.; Ouyang, Z. Marching Toward Human Lipidome Project–Advancement of Structural Lipidomics. TrAC Trends Anal. 2024, 177, 117765. [Google Scholar] [CrossRef]
- Tabassum, R.; Rämö, J.T.; Ripatti, P.; Koskela, J.T.; Kurki, M.; Karjalainen, J.; Palta, P.; Hassan, S.; Nunez-Fontarnau, J.; Kiiskinen, T.T.J. Genetic Architecture of Human Plasma Lipidome and Its Link to Cardiovascular Disease. Nat. Commun. 2019, 10, 4329. [Google Scholar] [CrossRef]
- Cadby, G.; Giles, C.; Melton, P.E.; Huynh, K.; Mellett, N.A.; Duong, T.; Nguyen, A.; Cinel, M.; Smith, A.; Olshansky, G. Comprehensive Genetic Analysis of the Human Lipidome Identifies Loci Associated with Lipid Homeostasis with Links to Coronary Artery Disease. Nat. Commun. 2022, 13, 3124. [Google Scholar] [CrossRef]
- Huang, Y.; Stinson, S.E.; Thodberg, M.; Holm, L.A.; Thielemann, R.; Sulek, K.; Lund, M.A.V.; Fonvig, C.E.; Kim, M.; Trost, K. Genetic Factors Shaping the Plasma Lipidome and the Relations to Cardiometabolic Risk in Children and Adolescents. EBioMedicine 2025, 112, 105537. [Google Scholar] [CrossRef]
- Padro, T.; López-Yerena, A.; Pérez, A.; Vilahur, G.; Badimon, L. Dietary Ω3 Fatty Acids and Phytosterols in the Modulation of the HDL Lipidome: A Longitudinal Crossover Clinical Study. Nutrients 2023, 15, 3637. [Google Scholar] [CrossRef]
- Wei, F.; Lamichhane, S.; Orešič, M.; Hyötyläinen, T. Lipidomes in Health and Disease: Analytical Strategies and Considerations. TrAC Trends Anal. 2019, 120, 115664. [Google Scholar] [CrossRef]
- Quehenberger, O.; Dennis, E.A. The Human Plasma Lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics Profiling and Risk of Cardiovascular Disease in the Prospective Population-Based Bruneck Study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Liang, L.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Zheng, Y.; Wang, D.D.; Corella, D.; Castaner, O.; Ros, E.; et al. Plasma lipidome patterns associated with cardiovascular risk in the PREDIMED trial: A case-cohort study. Int. J. Cardiol. 2018, 253, 126–132. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma Ceramides Predict Cardiovascular Death in Patients with Stable Coronary Artery Disease and Acute Coronary Syndromes beyond LDL-Cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Tabassum, R.; Ruotsalainen, S.; Ottensmann, L.; Gerl, M.J.; Klose, C.; Tukiainen, T.; Pirinen, M.; Simons, K.; Widén, E.; Ripatti, S. Lipidome-and Genome-wide Study to Understand Sex Differences in Circulatory Lipids. J. Am. Heart Assoc. 2022, 11, e027103. [Google Scholar] [CrossRef]
- Beyene, H.B.; Olshansky, G.; T. Smith, A.A.; Giles, C.; Huynh, K.; Cinel, M.; Mellett, N.A.; Cadby, G.; Hung, J.; Hui, J.; et al. High-Coverage Plasma Lipidomics Reveals Novel Sex-Specific Lipidomic Fingerprints of Age and BMI: Evidence from Two Large Population Cohort Studies. PLoS Biol. 2020, 18, e3000870. [Google Scholar] [CrossRef]
- West, A.L.; Michaelson, L.V.; Miles, E.A.; Haslam, R.P.; Lillycrop, K.A.; Georgescu, R.; Han, L.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Lipidomic Analysis of Plasma from Healthy Men and Women Shows Phospholipid Class and Molecular Species Differences between Sexes. Lipids 2020, 56, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. [Google Scholar] [CrossRef]
- Tabassum, R.; Widén, E.; Ripatti, S. Effect of Biological Sex on Human Circulating Lipidome: An Overview of the Literature. Atherosclerosis 2023, 384, 117274. [Google Scholar] [CrossRef]
- Krishnan, K.C.; Mehrabian, M.; Lusis, A.J. Sex Differences in Metabolism and Cardiometabolic Disorders. Curr. Opin. Infect. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Nogueira, I.A.L.; da Cruz, É.J.S.N.; Fontenele, A.M.M.; Figueiredo Neto, J.A. Alterations in Postmenopausal Plasmatic Lipidome. PLoS ONE 2018, 13, e0203027. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.; Gall, J.; Byrne, R.; Yvan-Charvet, L.; McGillicuddy, F.C. Metabolic Inflammation in Obesity—At the Crossroads between Fatty Acid and Cholesterol Metabolism. Mol. Nutr. Food Res. 2020, 65, 1900482. [Google Scholar] [CrossRef]
- Anand, P.K. Lipids, Inflammasomes, Metabolism, and Disease. Immunol. Rev. 2020, 297, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Triglia, L.T.; Gurgoglione, F.L.; Barocelli, F.; Bianconcini, M.; Niccoli, G. Lipids and Inflammation: Novel Molecular Targets and Therapeutic Implications. Curr. Med. Chem. 2025, 32, 2950–2970. [Google Scholar] [CrossRef]
- Ito, A. Lipid Metabolic Reprogramming in Immune Regulation and Chronic Inflammatory Diseases. Endocr. J. 2025, 72, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid Signaling and Lipotoxicity in Metaflammation: Indications for Metabolic Disease Pathogenesis and Treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Dyslipidemia and Inflammation: An Evolutionary Conserved Mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef]
- Allayee, H.; Hazen, S.L. Contribution of Gut Bacteria to Lipid Levels: Another Metabolic Role for Microbes? Circ. Res. 2015, 117, 750–754. [Google Scholar] [CrossRef]
- Xu, H.; Fang, F.; Wu, K.; Song, J.; Li, Y.; Lu, X.; Liu, J.; Zhou, L.; Yu, W.; Yu, F.; et al. Gut Microbiota-Bile Acid Crosstalk Regulates Murine Lipid Metabolism via the Intestinal FXR-FGF19 Axis in Diet-Induced Humanized Dyslipidemia. Microbiome 2023, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Raka, F.; Adeli, K. The Role of the Gut Microbiota in Lipid and Lipoprotein Metabolism. J. Clin. Med. 2019, 8, 2227. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Tsiantas, K.; Konteles, S.J.; Kritsi, E.; Sinanoglou, V.J.; Tsiaka, T.; Zoumpoulakis, P. Effects of Non-Polar Dietary and Endogenous Lipids on Gut Microbiota Alterations: The Role of Lipidomics. Int. J. Mol. Sci. 2022, 23, 4070. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolontà, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef]
- Nivukoski, U.; Niemelä, M.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O.; Tauler, P. Impacts of Unfavourable Lifestyle Factors on Biomarkers of Liver Function, Inflammation and Lipid Status. PLoS ONE 2019, 14, e0218463. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.C.-P.; Shui, G.; Cazenave-Gassiot, A.; Wenk, M.R.; Gooley, J.J. Changes in Plasma Lipids during Exposure to Total Sleep Deprivation. Sleep 2015, 38, 1683–1691. [Google Scholar] [CrossRef]
- Miranda, A.M.; Oliveira, T.G. Lipids under Stress–a Lipidomic Approach for the Study of Mood Disorders. BioEssays 2015, 37, 1226–1235. [Google Scholar] [CrossRef]
- O’Gorman, A.; Morris, C.; Ryan, M.; O’Grada, C.M.; Roche, H.M.; Gibney, E.R.; Gibney, M.J.; Brennan, L. Habitual Dietary Intake Impacts on the Lipidomic Profile. J. Chromatogr. B 2014, 966, 140–146. [Google Scholar] [CrossRef]
- Si, J.; Li, J.; Yu, C.; Guo, Y.; Bian, Z.; Millwood, I.; Yang, L.; Walters, R.; Chen, Y.; Du, H.; et al. Improved Lipidomic Profile Mediates the Effects of Adherence to Healthy Lifestyles on Coronary Heart Disease. eLife 2021, 10, e60999. [Google Scholar] [CrossRef]
- Nieman, D.C.; Sakaguchi, C.A.; Pelleigrini, M.; Thompson, M.J.; Sumner, S.; Zhang, Q. Healthy Lifestyle Linked to Innate Immunity and Lipoprotein Metabolism: A Cross-Sectional Comparison Using Untargeted Proteomics. Sci. Rep. 2023, 13, 16728. [Google Scholar] [CrossRef]
- Pathmasiri, W.; Rushing, B.R.; McRitchie, S.; Choudhari, M.; Du, X.; Smirnov, A.; Pelleigrini, M.; Thompson, M.J.; Sakaguchi, C.A.; Nieman, D.C.; et al. Untargeted Metabolomics Reveal Signatures of a Healthy Lifestyle. Sci. Rep. 2024, 14, 13630. [Google Scholar] [CrossRef]
- Mietus-Snyder, M.; Perak, A.M.; Cheng, S.; Hayman, L.L.; Haynes, N.; Meikle, P.J.; Shah, S.H.; Suglia, S.F. Next Generation, Modifiable Cardiometabolic Biomarkers: Mitochondrial Adaptation and Metabolic Resilience: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Peña-de-la-Sancha, P.; Muñoz-García, A.; Espínola-Zavaleta, N.; Bautista-Pérez, R.; Mejía, A.M.; Luna-Luna, M.; López-Olmos, V.; Rodríguez-Pérez, J.-M.; Fragoso, J.-M.; Carreón-Torres, E.; et al. Eicosapentaenoic and Docosahexaenoic Acid Supplementation Increases HDL Content in N-3 Fatty Acids and Improves Endothelial Function in Hypertriglyceridemic Patients. Int. J. Mol. Sci. 2023, 24, 5390. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castillejo, S.; Pedret, A.; Catalán, Ú.; Valls, R.; Farràs, M.; Rubió, L.; Castañer, O.; Macià, A.; Fitó, M.; Motilva, M.J.; et al. Virgin Olive Oil Phenolic Compounds Modulate the HDL Lipidome in Hypercholesterolaemic Subjects: A Lipidomic Analysis of the VOHF Study. Mol. Nutr. Food Res. 2021, 65, 2001192. [Google Scholar] [CrossRef]
- Khan, A.A.; Mundra, P.A.; Straznicky, N.E.; Nestel, P.J.; Wong, G.; Tan, R.; Huynh, K.; Ng, T.W.; Mellett, N.A.; Weir, J.M.; et al. Weight Loss and Exercise Alter the High-Density Lipoprotein Lipidome and Improve High-Density Lipoprotein Functionality in Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2018, 38, 438–447. [Google Scholar] [CrossRef]
- Michalski, M.-C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.-L.; Bouvier, J.; Chardigny, J.-M.; Raynal-Ljutovac, K. Multiscale Structures of Lipids in Foods as Parameters Affecting Fatty Acid Bioavailability and Lipid Metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef]
- Toledo, E.; Wang, D.D.; Ruiz-Canela, M.; Clish, C.B.; Razquin, C.; Zheng, Y.; Guasch-Ferré, M.; Hruby, A.; Corella, D.; Gómez-Gracia, E.; et al. Plasma Lipidomic Profiles and Cardiovascular Events in a Randomized Intervention Trial with the Mediterranean Diet. Am. J. Clin. Nutr. 2017, 106, 973–983. [Google Scholar] [CrossRef]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Nygren, H.; Seppänen-Laakso, T.; Poutanen, K.; Hyötyläinen, T.; Risérus, U.; Savolainen, M.J.; et al. A Healthy Nordic Diet Alters the Plasma Lipidomic Profile in Adults with Features of Metabolic Syndrome in a Multicenter Randomized Dietary Intervention. J. Nutr. 2015, 146, 662–672. [Google Scholar] [CrossRef]
- Dibay Moghadam, S.; Navarro, S.L.; Shojaie, A.; Randolph, T.W.; Bettcher, L.F.; Le, C.B.; Hullar, M.A.; Kratz, M.; Neuhouser, M.L.; Lampe, P.D.; et al. Plasma Lipidomic Profiles after a Low and High Glycemic Load Dietary Pattern in a Randomized Controlled Crossover Feeding Study. Metabolomics 2020, 16, 121. [Google Scholar] [CrossRef]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Poutanen, K.; Mykkänen, H.; Seppänen-Laakso, T.; Gylling, H.; Uusitupa, M.; Orešič, M.; et al. Whole Grain Products, Fish and Bilberries Alter Glucose and Lipid Metabolism in a Randomized, Controlled Trial: The Sysdimet Study. PLoS ONE 2011, 6, e22646. [Google Scholar] [CrossRef]
- Madkour, M.I.; Islam, M.T.; Tippetts, T.S.; Chowdhury, K.H.; Lesniewski, L.A.; Summers, S.A.; Zeb, F.; Abdelrahim, D.N.; AlKurd, R.; Khraiwesh, H.M.; et al. Ramadan Intermittent Fasting Is Associated with Ameliorated Inflammatory Markers and Improved Plasma Sphingolipids/Ceramides in Subjects with Obesity: Lipidomics Analysis. Sci. Rep. 2023, 13, 17322. [Google Scholar] [CrossRef]
- Djekic, D.; Shi, L.; Calais, F.; Carlsson, F.; Landberg, R.; Hyötyläinen, T.; Frøbert, O. Effects of a Lacto-Ovo-Vegetarian Diet on the Plasma Lipidome and Its Association with Atherosclerotic Burden in Patients with Coronary Artery Disease—A Randomized, Open-Label, Cross-over Study. Nutrients 2020, 12, 3586. [Google Scholar] [CrossRef]
- Shabrina, A.; Tung, T.-H.; Nguyen, N.T.K.; Lee, H.-C.; Wu, H.-T.; Wang, W.; Huang, S.-Y. N-3 PUFA and Caloric Restriction Diet Alters Lipidomic Profiles in Obese Men with Metabolic Syndrome: A Preliminary Open Study. Eur. J. Nutr. 2019, 59, 3103–3112. [Google Scholar] [CrossRef]
- Ottestad, I.; Hassani, S.; Borge, G.I.; Kohler, A.; Vogt, G.; Hyötyläinen, T.; Orešič, M.; Brønner, K.W.; Holven, K.B.; Ulven, S.M.; et al. Fish Oil Supplementation Alters the Plasma Lipidomic Profile and Increases Long-Chain PUFAs of Phospholipids and Triglycerides in Healthy Subjects. PLoS ONE 2012, 7, e42550. [Google Scholar] [CrossRef]
- Eichelmann, F.; Prada, M.; Sellem, L.; Jackson, K.G.; Salas Salvadó, J.; Razquin Burillo, C.; Estruch, R.; Friedén, M.; Rosqvist, F.; Risérus, U.; et al. Lipidome Changes Due to Improved Dietary Fat Quality Inform Cardiometabolic Risk Reduction and Precision Nutrition. Nat. Med. 2024, 30, 2867–2877. [Google Scholar] [CrossRef]
- Sellem, L.; Eichelmann, F.; Jackson, K.G.; Wittenbecher, C.; Schulze, M.B.; Lovegrove, J.A. Replacement of Dietary Saturated with Unsaturated Fatty Acids Is Associated with Beneficial Effects on Lipidome Metabolites: A Secondary Analysis of a Randomized Trial. Am. J. Clin. Nutr. 2023, 117, 1248–1261. [Google Scholar] [CrossRef]
- Dawczynski, C.; Plagge, J.; Jahreis, G.; Liebisch, G.; Höring, M.; Seeliger, C.; Ecker, J. Dietary PUFA Preferably Modify Ethanolamine-Containing Glycerophospholipids of the Human Plasma Lipidome. Nutrients 2022, 14, 3055. [Google Scholar] [CrossRef]
- Lankinen, M.; Schwab, U.; Erkkilä, A.; Seppänen-Laakso, T.; Hannila, M.-L.; Mussalo, H.; Lehto, S.; Uusitupa, M.; Gylling, H.; Orešič, M.; et al. Fatty Fish Intake Decreases Lipids Related to Inflammation and Insulin Signaling—A Lipidomics Approach. PLoS ONE 2009, 4, e5258. [Google Scholar] [CrossRef]
- Sung, H.H.; Sinclair, A.J.; Huynh, K.; Smith, A.A.T.; Mellett, N.A.; Meikle, P.J.; Su, X.Q. Krill Oil Has Different Effects on the Plasma Lipidome Compared with Fish Oil Following 30 Days of Supplementation in Healthy Women: A Randomized Controlled and Crossover Study. Nutrients 2020, 12, 2804. [Google Scholar] [CrossRef]
- Meikle, P.J.; Barlow, C.K.; Mellett, N.A.; Mundra, P.A.; Bonham, M.P.; Larsen, A.; Cameron-Smith, D.; Sinclair, A.; Nestel, P.J.; Wong, G. Postprandial Plasma Phospholipids in Men Are Influenced by the Source of Dietary Fat. J. Nutr. 2015, 145, 2012–2018. [Google Scholar] [CrossRef]
- Sung, H.H.; Sinclair, A.J.; Huynh, K.; Smith, A.T.; Mellett, N.A.; Meikle, P.J.; Su, X.Q. Differential Plasma Postprandial Lipidomic Responses to Krill Oil and Fish Oil Supplementations in Women: A Randomized Crossover Study. Nutrition 2019, 65, 191–201. [Google Scholar] [CrossRef]
- Eichelmann, F.; Sellem, L.; Wittenbecher, C.; Jäger, S.; Kuxhaus, O.; Prada, M.; Cuadrat, R.; Jackson, K.G.; Lovegrove, J.A.; Schulze, M.B. Deep Lipidomics in Human Plasma: Cardiometabolic Disease Risk and Effect of Dietary Fat Modulation. Circulation 2022, 146, 21–35. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, L.; Wu, Q.; Song, B.; Wu, Y.; Yang, X.; Zhou, P.; Niu, Z.; Zheng, H.; Li, H.; et al. Diet-Related Lipidomic Signatures and Changed Type 2 Diabetes Risk in a Randomized Controlled Feeding Study with Mediterranean Diet and Traditional Chinese or Transitional Diets. Diabetes Care 2023, 46, 1691–1699. [Google Scholar] [CrossRef]
- Al-Sari, N.; Schmidt, S.; Suvitaival, T.; Kim, M.; Trošt, K.; Ranjan, A.G.; Christensen, M.B.; Overgaard, A.J.; Pociot, F.; Nørgaard, K.; et al. Changes in the Lipidome in Type 1 Diabetes Following Low Carbohydrate Diet: Post-hoc Analysis of a Randomized Crossover Trial. Endocrinol. Diabetes Metab. 2021, 4, e00213. [Google Scholar] [CrossRef]
- Coelho, M.O.C.; Monteyne, A.J.; Dirks, M.L.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Daily Mycoprotein Consumption for 1 Week Does Not Affect Insulin Sensitivity or Glycaemic Control but Modulates the Plasma Lipidome in Healthy Adults: A Randomised Controlled Trial. Br. J. Nutr. 2021, 125, 147–160. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Zhou, H.; Cazenave-Gassiot, A.; Choi, H.; Burla, B.; Bendt, A.K.; Wenk, M.R.; Ling, L.H.; Kim, J.E. Effects of Wolfberry (Lycium Barbarum) Consumption on the Human Plasma Lipidome and Its Association with Cardiovascular Disease Risk Factors: A Randomized Controlled Trial of Middle-Aged and Older Adults. Front. Nutr. 2024, 11, 1258570. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Álvarez, R.; Muñoz-Durango, K. Changes in the Plasma Lipidome of Healthy Subjects after Coffee Consumption Reveal Potential Cardiovascular Benefits: A Randomized Controlled Trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef]
- Ryan, M.J.; Grant-St James, A.; Lawler, N.G.; Fear, M.W.; Raby, E.; Wood, F.M.; Maker, G.L.; Wist, J.; Holmes, E.; Nicholson, J.K.; et al. Comprehensive Lipidomic Workflow for Multicohort Population Phenotyping Using Stable Isotope Dilution Targeted Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2023, 22, 1419–1433. [Google Scholar] [CrossRef]
- Huynh, K.; Duong, T.; Mellett, N.A.; Cinel, M.; Giles, C.; Meikle, P.J. Comprehensive Targeted Lipidomic Profiling for Research and Clinical Applications. In Serum/Plasma Proteomics: Methods and Protocols; Springer: New York, NY, USA, 2023; Volume 2628, pp. 489–504. [Google Scholar] [CrossRef]
- Medina, J.; Borreggine, R.; Teav, T.; Gao, L.; Ji, S.; Carrard, J.; Jones, C.; Blomberg, N.; Jech, M.; Atkins, A.; et al. Omic-Scale High-Throughput Quantitative LC–MS/MS Approach for Circulatory Lipid Phenotyping in Clinical Research. Anal. Chem. 2023, 95, 3168–3179. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Lai, K.; Muirhead, R.P.; Chung, L.H.; Huang, Y.; James, E.; Liu, X.T.; Wu, J.; Atkinson, F.S.; Yan, S.; et al. Deep Serum Lipidomics Identifies Evaluative and Predictive Biomarkers for Individualized Glycemic Responses Following Low-Energy Diet-Induced Weight Loss: A PREVention of Diabetes through Lifestyle Intervention and Population Studies in Europe and around the World (PREVIEW) Substudy. Am. J. Clin. Nutr. 2024, 120, 864–878. [Google Scholar] [CrossRef]
- Zhang, T.; Naudin, S.; Hong, H.G.; Albanes, D.; Männistö, S.; Weinstein, S.J.; Moore, S.C.; Stolzenberg-Solomon, R.Z. Dietary Quality and Circulating Lipidomic Profiles in 2 Cohorts of Middle-Aged and Older Male Finnish Smokers and American Populations. J. Nutr. 2023, 153, 2389–2400. [Google Scholar] [CrossRef]
- Rojo-López, M.I.; Barranco-Altirriba, M.; Rossell, J.; Antentas, M.; Castelblanco, E.; Yanes, O.; Weber, R.J.M.; Lloyd, G.R.; Winder, C.; Dunn, W.B.; et al. The Lipidomic Profile Is Associated with the Dietary Pattern in Subjects with and without Diabetes Mellitus from a Mediterranean Area. Nutrients 2024, 16, 1805. [Google Scholar] [CrossRef]
- Lindqvist, H.M.; Bärebring, L.; Gjertsson, I.; Jylhä, A.; Laaksonen, R.; Winkvist, A.; Hilvo, M. A Randomized Controlled Dietary Intervention Improved the Serum Lipid Signature towards a Less Atherogenic Profile in Patients with Rheumatoid Arthritis. Metabolites 2021, 11, 632. [Google Scholar] [CrossRef]
- Wu, M.; Hu, C.; Shen, L. Effects of Dietary Olive Oil, Camellia Seed Oil and Soybean Oil on Serum Lipid Composition in Women with a High Risk of Cardiovascular Disease: A Lipidomic Analysis. Food Sci. Hum. Wellness 2024, 13, 3193–3201. [Google Scholar] [CrossRef]
- Woods, M.N.; Wanke, C.A.; Ling, P.-R.; Hendricks, K.M.; Tang, A.M.; Knox, T.A.; Andersson, C.E.; Dong, K.R.; Skinner, S.C.; Bistrian, B.R. Effect of a Dietary Intervention and n–3 Fatty Acid Supplementation on Measures of Serum Lipid and Insulin Sensitivity in Persons with HIV. Am. J. Clin. Nutr. 2009, 90, 1566–1578. [Google Scholar] [CrossRef]
- Lu, J.; Liu, R.; Ren, H.; Wang, S.; Hu, C.; Shi, Z.; Li, M.; Liu, W.; Wan, Q.; Su, Q.; et al. Impact of Omega-3 Fatty Acids on Hypertriglyceridemia, Lipidomics, and Gut Microbiome in Patients with Type 2 Diabetes. Med 2024, 6, 100496. [Google Scholar] [CrossRef]
- Szymańska, E.; van Dorsten, F.A.; Troost, J.; Paliukhovich, I.; van Velzen, E.J.J.; Hendriks, M.M.W.B.; Trautwein, E.A.; van Duynhoven, J.P.M.; Vreeken, R.J.; Smilde, A.K. A Lipidomic Analysis Approach to Evaluate the Response to Cholesterol-Lowering Food Intake. Metabolomics 2011, 8, 894–906. [Google Scholar] [CrossRef][Green Version]
- Altmaier, E.; Kastenmüller, G.; Römisch-Margl, W.; Thorand, B.; Weinberger, K.M.; Adamski, J.; Illig, T.; Döring, A.; Suhre, K. Variation in the Human Lipidome Associated with Coffee Consumption as Revealed by Quantitative Targeted Metabolomics. Mol. Nutr. Food Res. 2009, 53, 1357–1365. [Google Scholar] [CrossRef]
- Zhang, F.; Lim, W.L.F.; Huang, Y.; Lam, S.M.; Wang, Y. Lipidomics and Metabolomics Investigation into the Effect of DAG Dietary Intervention on Hyperuricemia in Athletes. J. Lipid Res. 2024, 65, 100605. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the Complexities of the HDL Lipidome1. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef]
- Mueller, P.A.; Bergstrom, P.; Rosario, S.; Heard, M.; Pamir, N. Fish Oil Supplementation Modifies the Proteome, Lipidome, and Function of High-Density Lipoprotein: Findings from a Trial in Young Healthy Adults. J. Nutr. 2024, 154, 1130–1140. [Google Scholar] [CrossRef]
- Sawrey-Kubicek, L.; Zhu, C.; Bardagjy, A.S.; Rhodes, C.H.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole Egg Consumption Compared with Yolk-Free Egg Increases the Cholesterol Efflux Capacity of High-Density Lipoproteins in Overweight, Postmenopausal Women. Am. J. Clin. Nutr. 2019, 110, 617–627. [Google Scholar] [CrossRef]
- Zhu, C.; Sawrey-Kubicek, L.; Beals, E.; Hughes, R.L.; Rhodes, C.H.; Sacchi, R.; Zivkovic, A.M. The HDL Lipidome Is Widely Remodeled by Fast Food versus Mediterranean Diet in 4 Days. Metabolomics 2019, 15, 114. [Google Scholar] [CrossRef]
- Hevonoja, T.; Pentikäinen, M.O.; Hyvönen, M.T.; Kovanen, P.T.; Ala-Korpela, M. Structure of Low Density Lipoprotein (LDL) Particles: Basis for Understanding Molecular Changes in Modified LDL. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1488, 189–210. [Google Scholar] [CrossRef]
- März, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL Cholesterol: Reappraisal of Its Clinical Relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological Activities of HDL Subpopulations and Their Relevance to Cardiovascular Disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary α-Linolenic Acid, EPA, and DHA Have Differential Effects on LDL Fatty Acid Composition but Similar Effects on Serum Lipid Profiles in Normolipidemic Humans12. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef]
- Erkkilä, A.T.; Manninen, S.; Fredrikson, L.; Bhalke, M.; Holopainen, M.; Ruuth, M.; Lankinen, M.; Käkelä, R.; Öörni, K.; Schwab, U.S. Lipidomic Changes of LDL after Consumption of Camelina Sativa Oil, Fatty Fish and Lean Fish in Subjects with Impaired Glucose Metabolism—A Randomized Controlled Trial. J. Clin. Lipidol. 2021, 15, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Padro, T.; Vilahur, G.; Sánchez-Hernández, J.; Hernández, M.; Antonijoan, R.M.; Perez, A.; Badimon, L. Lipidomic Changes of LDL in Overweight and Moderately Hypercholesterolemic Subjects Taking Phytosterol-and Omega-3-Supplemented Milk. J. Lipid Res. 2015, 56, 1043–1056. [Google Scholar] [CrossRef]
- Zhu, D.; Vernon, S.T.; D’Agostino, Z.; Wu, J.; Giles, C.; Chan, A.S.; Kott, K.A.; Gray, M.P.; Gholipour, A.; Tang, O.; et al. Lipidomics Profiling and Risk of Coronary Artery Disease in the BioHEART-CT Discovery Cohort. Biomolecules 2023, 13, 917. [Google Scholar] [CrossRef]
- Sigruener, A.; Kleber, M.E.; Heimerl, S.; Liebisch, G.; Schmitz, G.; Maerz, W.; Calabresi, L. Glycerophospholipid and Sphingolipid Species and Mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. PLoS ONE 2014, 9, e85724. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Zheng, Y.; Toledo, E.; Razquin, C.; Ruiz-Canela, M.; Guasch-Ferre, M.; Yu, E.; Corella, D.; Gomez-Gracia, E.; Fiol, M.; et al. Lipid Metabolic Networks, Mediterranean Diet and Cardiovascular Disease in the PREDIMED Trial. Leuk. Res. 2018, 47, 1830–1845. [Google Scholar] [CrossRef] [PubMed]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-Secreted Ceramides Regulate Vascular Redox State and Influence Outcomes in Patients with Cardiovascular Disease. JACC 2021, 77, 2494–2513. [Google Scholar] [CrossRef]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arter. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and Other Sphingolipids as Drivers of Cardiovascular Disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Boren, J.; Taskinen, M.-R.; Björnson, E.; Packard, C.J. Metabolism of Triglyceride-Rich Lipoproteins in Health and Dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C–Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma Sphingomyelin Level as a Risk Factor for Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef]
- Nelson, J.C.; Jiang, X.-C.; Tabas, I.; Tall, A.; Shea, S. Plasma Sphingomyelin and Subclinical Atherosclerosis: Findings from the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2006, 163, 903–912. [Google Scholar] [CrossRef] [PubMed]
| Lipid Category | Examples |
|---|---|
| Fatty acyls | FAs (e.g., palmitic acid, arachidonic acid), eicosanoids. |
| Glycerolipids | Mono-, di-, and triacylglycerols |
| Glycerophospholipids | PCs, PEs, LPCs |
| Sphingolipids | Cers, SMs and glycosphingolipids. |
| Sterol lipids | CEs |
| Prenol lipids | Isoprenoids such as ubiquinone (coenzyme Q), dolichol. |
| Saccharolipids | Lipid A (component of bacterial lipopolysaccharide). |
| Polyketides | Erythromycin, tetracycline (antibiotic polyketides). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas, R.; Sánchez-García, N.D.; Estruch, R.; López-Yerena, A. Impact of Dietary Interventions on the Human Plasma and Lipoprotein Lipidome. Metabolites 2025, 15, 602. https://doi.org/10.3390/metabo15090602
Casas R, Sánchez-García ND, Estruch R, López-Yerena A. Impact of Dietary Interventions on the Human Plasma and Lipoprotein Lipidome. Metabolites. 2025; 15(9):602. https://doi.org/10.3390/metabo15090602
Chicago/Turabian StyleCasas, Rosa, Nancy D. Sánchez-García, Ramon Estruch, and Anallely López-Yerena. 2025. "Impact of Dietary Interventions on the Human Plasma and Lipoprotein Lipidome" Metabolites 15, no. 9: 602. https://doi.org/10.3390/metabo15090602
APA StyleCasas, R., Sánchez-García, N. D., Estruch, R., & López-Yerena, A. (2025). Impact of Dietary Interventions on the Human Plasma and Lipoprotein Lipidome. Metabolites, 15(9), 602. https://doi.org/10.3390/metabo15090602







