Phenolic-Rich Indian Almond (Terminalia catappa Linn) Leaf Extract Ameliorates Lipid Metabolism and Inflammation in High-Fat Diet (HFD)-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Purification and UPLC-Based Quantification of Phenolic Compounds
2.3. Mouse Experiment Methods
2.4. Serum Biochemical Analysis
2.5. Western Blot Analysis
2.6. Histopathological Analysis
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Statistical Analysis
3. Results
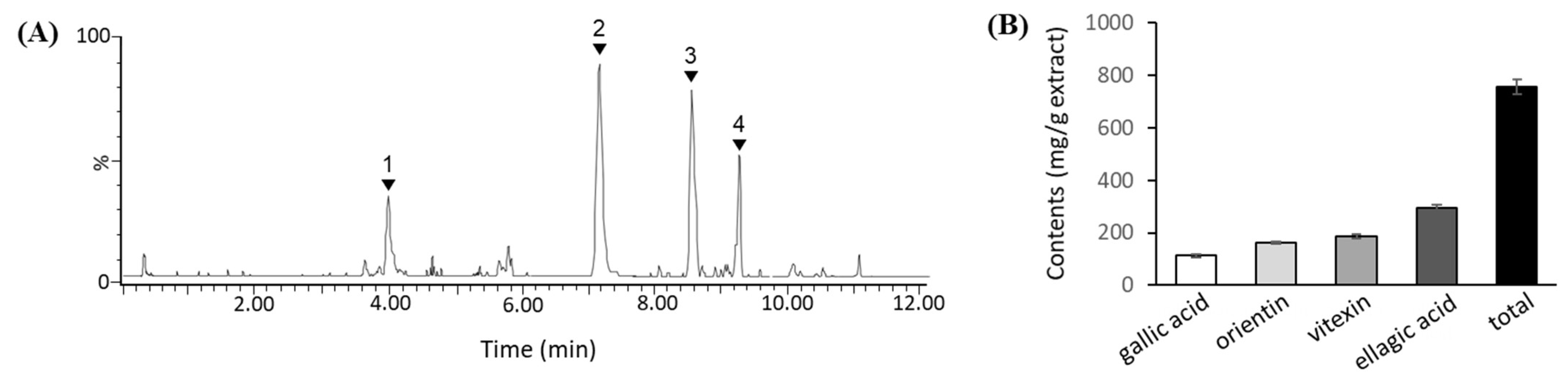
3.1. Phenolic Compounds in TCE
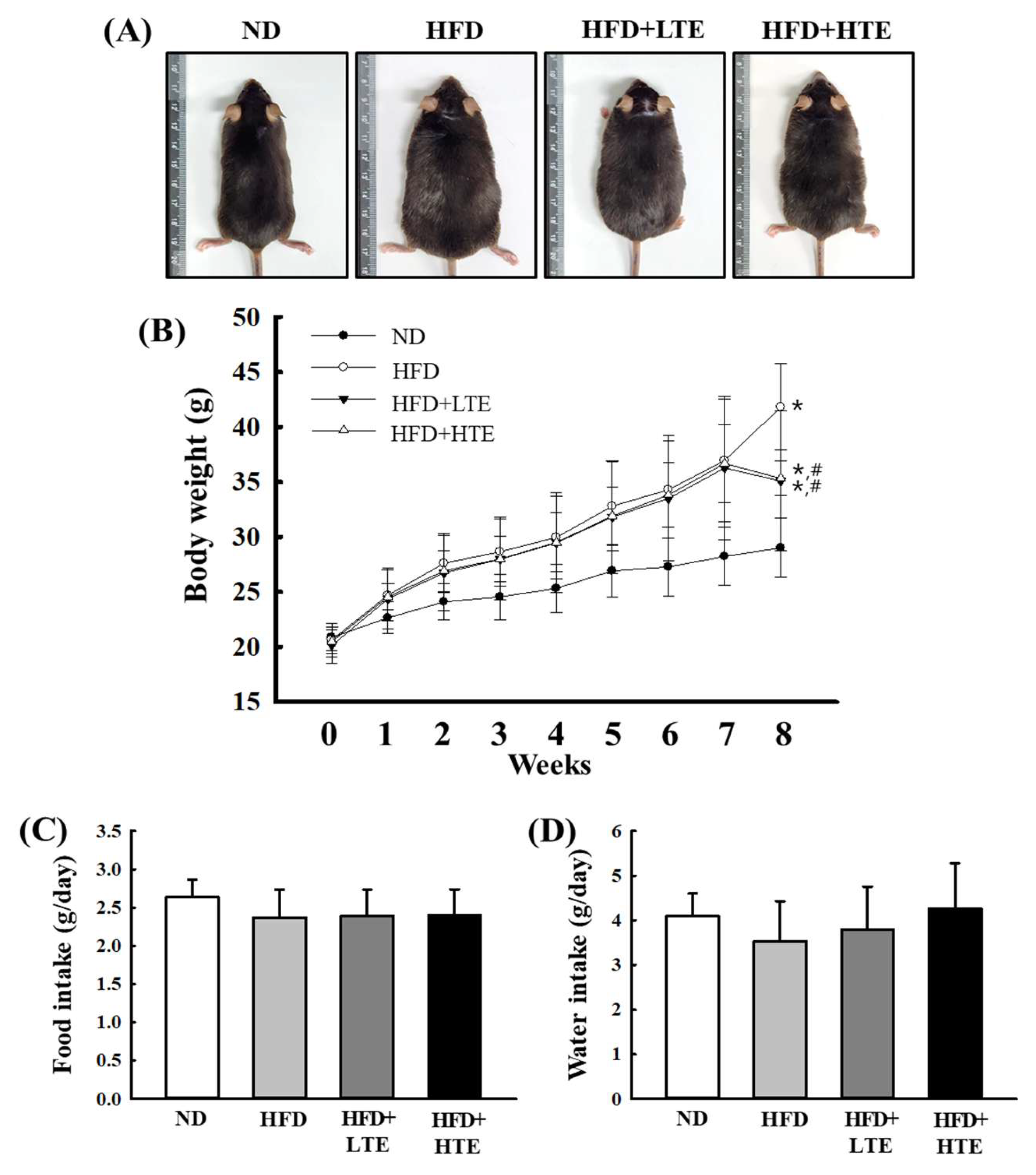
3.2. Effects of TCE on Body Weight in HFD-Fed Mice
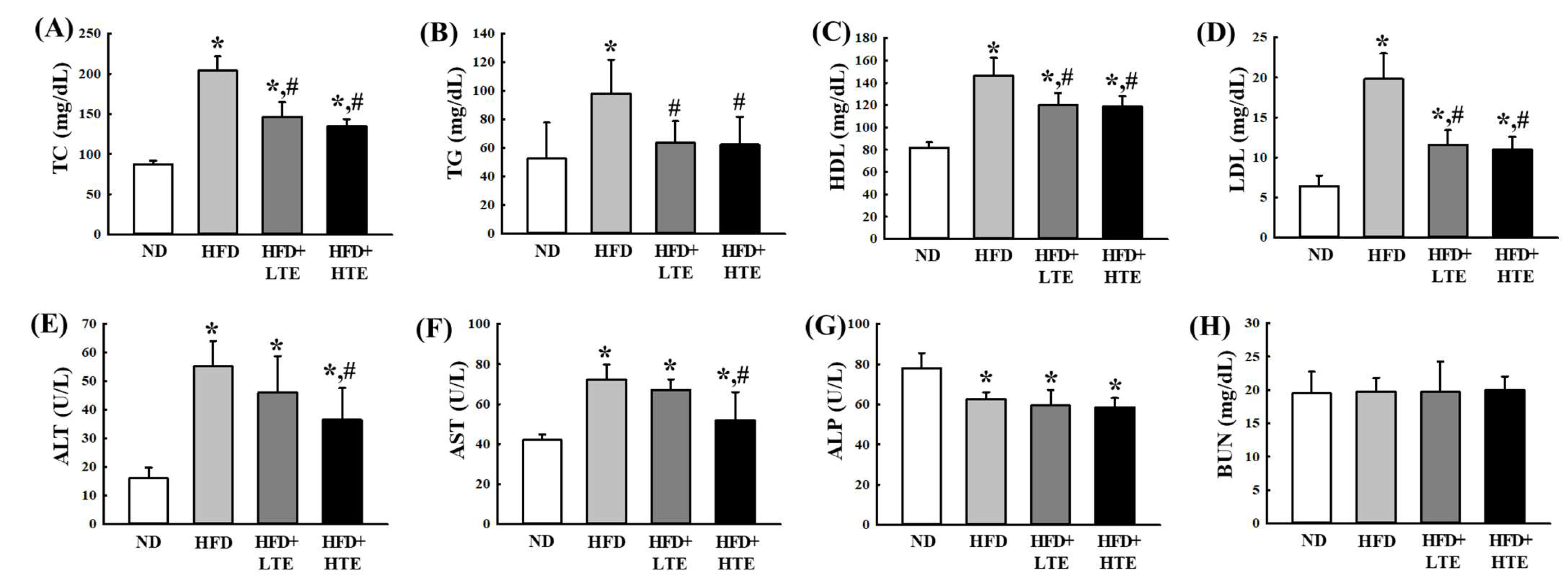
3.3. Effects of TCE on Serum Lipid and Liver Enzyme Profiles in HFD-Fed Mice
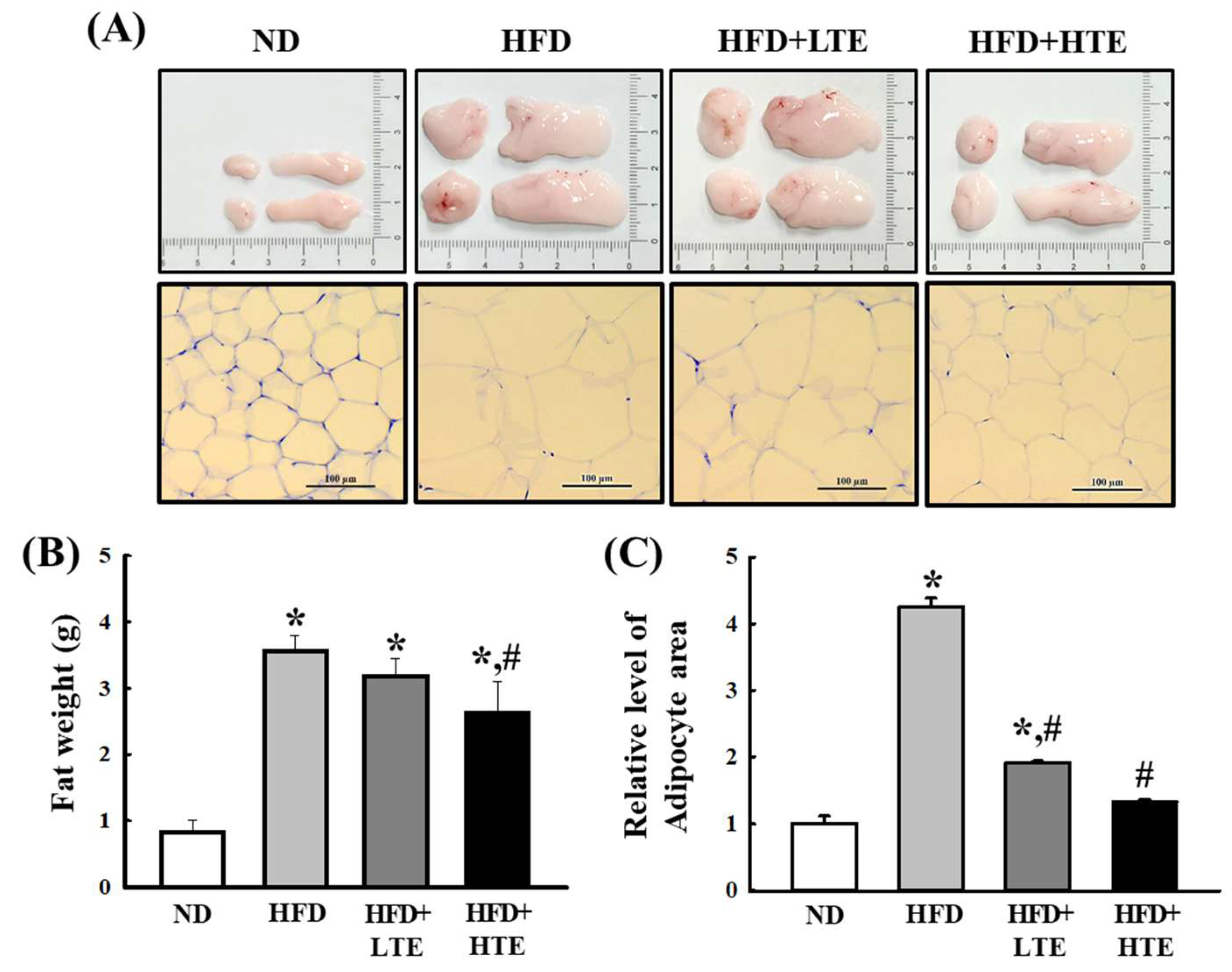
3.4. TCE Reduces Abdominal Fat Accumulation and Adipocyte Hypertrophy in HFD-Fed Mice
3.5. TCE Reduces Liver Weight and Hepatic Lipid Accumulation in HFD-Fed Mice
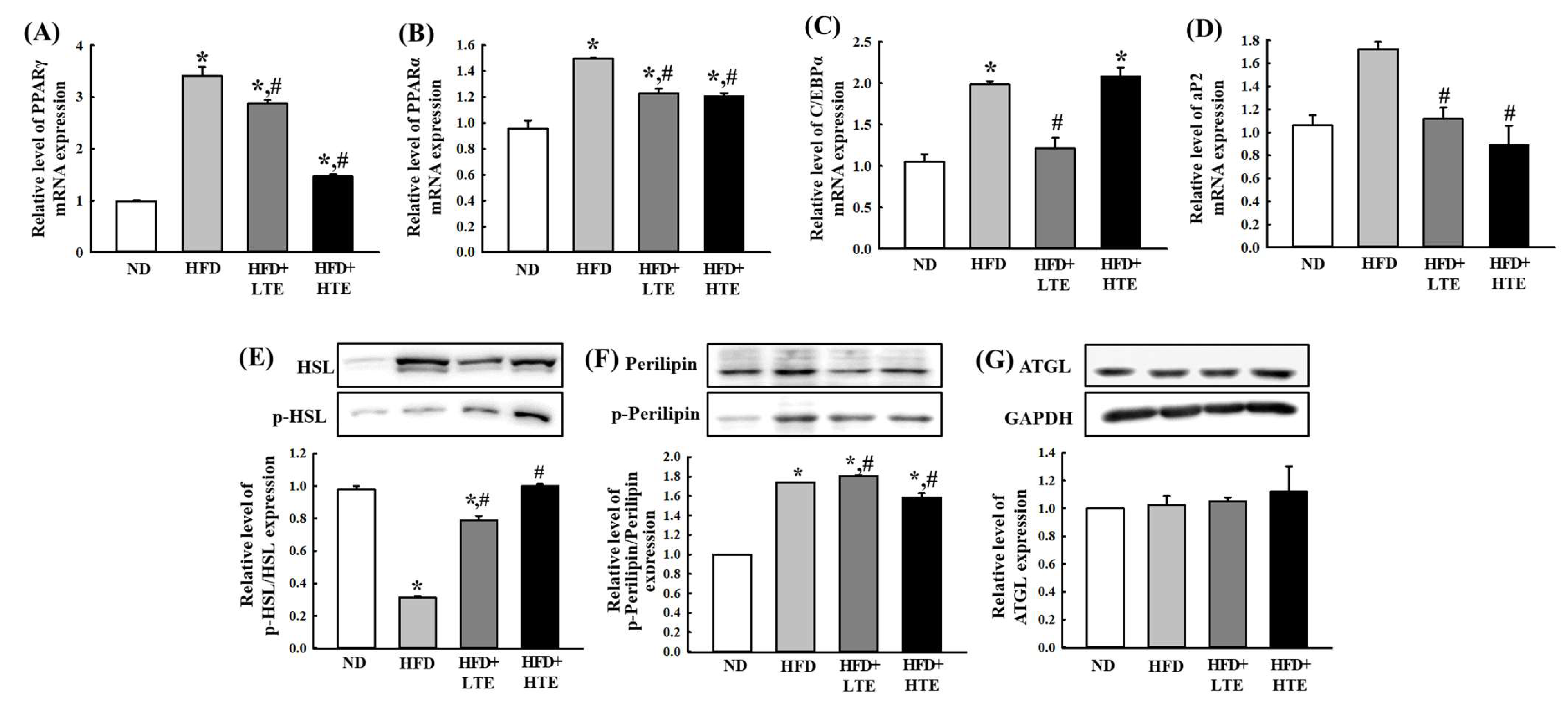
3.6. TCE Regulates Hepatic Lipid Metabolism by Modulating Lipogenesis and Lipolysis in HFD-Fed Mice
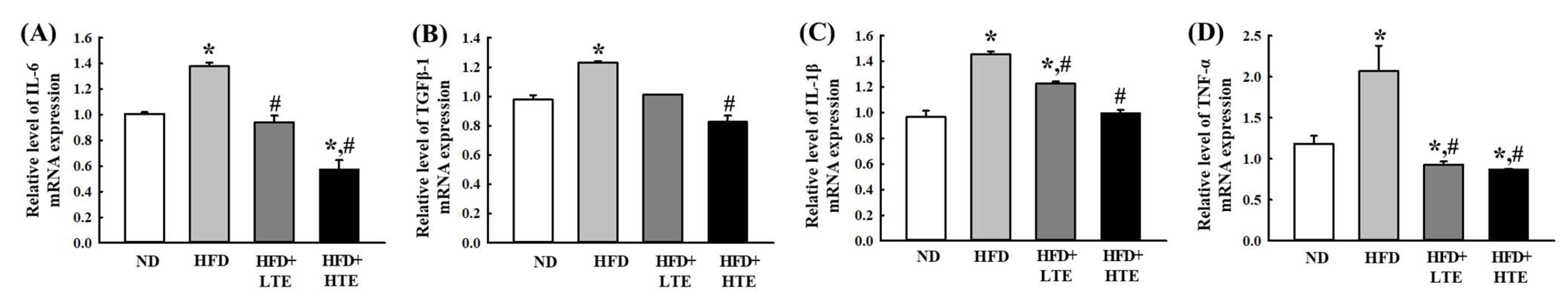
3.7. TCE Mitigates Inflammation by Modulating Pro-Inflammatory Cytokines in HFD-Fed Mice
3.8. TCE Restores Leptin and Adiponectin Expression in HFD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AST | Aspartate amino transferase |
| ATGL | Adipose triglyceride lipase |
| BMI | Body mass index |
| BUN | Blood Urea Nitrogen |
| C/EβPα | CCAAT-enhancer-binding proteins |
| DMSO | Dimethyl sulfoxide |
| HDL | High-density lipoprotein |
| HPLC | High performance liquid chromatography |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin 6 |
| LDL | Low Density Lipoprotein |
| PPAR | Peroxisome proliferator–activated receptor |
| RT-PCR | Real time reverse transcription–polymerase chain reaction |
| TC | Total cholesterol |
| TGF-β | Transforming growth factor beta |
| TG | Total triglyceride |
| TNF-α | Tumor necrosis factor-alpha |
| VLDL | Very low-density Lipoprotein |
References
- Bhutani, S.; VanDellen, M.R.; Cooper, J.A. Longitudinal Weight Gain and Related Risk Behaviors During the COVID-19 Pandemic in Adults in the US. Nutrients 2021, 13, 671. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund. Protect the Promise: Equal Access and Opportunity for Every Woman, Child and Adolescent. 2022 Progress Report on Every Woman Every Child Global Strategy for Women’s, Children’s and Adolescents’ Health (2016–2030). 2022. Available online: https://www.who.int/publications/i/item/9789240060104 (accessed on 18 October 2022).
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. Obes. Obstet. 2020, 2, 3–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Gao, L.; Pan, A.; Xue, H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 446–461. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef]
- Kalligeros, M.; Shehadeh, F.; Mylona, E.K.; Benitez, G.; Beckwith, C.G.; Chan, P.A.; Mylonakis, E. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity 2020, 28, 1200–1204. [Google Scholar] [CrossRef]
- Wiggins, T.; Guidozzi, N.; Welbourn, R.; Ahmed, A.R.; Markar, S.R. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003206. [Google Scholar] [CrossRef]
- Pamuk, F.; Kantarci, A. Inflammation as a link between periodontal disease and obesity. Periodontology 2022, 90, 186–196. [Google Scholar] [CrossRef]
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ferrie, J.E. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef]
- Kansra, A.R.; Lakkunarajah, S.; Jay, M.S. Childhood and Adolescent Obesity: A Review. Front. Pediatr. 2021, 8, 581461. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-mediated metabolic regulation in adipose tissue. Obes. Rev. 2024, 25, e13724. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Cho, M.; Choe, S.; Jang, M. Indian Almond (Terminalia catappa Linn.) Leaf Extract Extends Lifespan by Improving Lipid Metabolism and Antioxidant Activity Dependent on AMPK Signaling Pathway in Caenorhabditis elegans Under High-Glucose-Diet Conditions. Antioxidants 2023, 13, 14. [Google Scholar] [CrossRef]
- Chukwuma, E.C.; Ossai, F.N.; Nworah, V.O.; Apeh, E.O.; Abiaziem, F.N.; Iheagwam, M. Korzeniowska Changes in nutritional, health benefits, and pharmaceutical potential of raw and roasted tropical almond (Terminalia catappa Linn.) nuts from Nigeria. PLoS ONE 2024, 19, e0287840. [Google Scholar] [CrossRef]
- De Oliveira, N.D.; Martins, A.C.S.; Cirino, J.A.; Dutra, L.M.G.; da Silva, E.F.; do Nascimento, Y.M.; Soares, J.K.B. Exploring the potential of the tropical almond (Terminalia catappa L.): Analysis of bioactive compounds, morphology and metabolites. Ind. Crops Prod. 2024, 221, 119378. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Iheagwam, O.T.; Onuoha, M.K.; Ogunlana, O.O.; Chinedu, S.N. Terminalia catappa aqueous leaf extract reverses insulin resistance, improves glucose transport and activates PI3K/AKT signalling in high fat/streptozotocin-induced diabetic rats. Sci. Rep. 2022, 12, 10711. [Google Scholar] [CrossRef]
- Amelia, F.; Parbuntari, H.; Sudji, I.R.; Rahmayani, S.; Ramadhani, A.N.; Ananda, S. Phytoconstituents of Terminalia catappa linn fruits extract exhibit promising antidiabetic activities against α-amylase and α-glucosidase in vitro and in silico. Inform. Med. Unlocked 2023, 47, 101509. [Google Scholar] [CrossRef]
- Roh, Y.J.; Lee, S.J.; Kim, J.E.; Jin, Y.J.; Seol, A.; Song, H.J.; Park, J.; Park, S.H.; Douangdeuane, B.; Souliya, O.; et al. Dipterocarpus tuberculatus as a promising anti-obesity treatment in Lep knockout mice. Front. Endocrinol. 2023, 14, 1167285. [Google Scholar] [CrossRef]
- Mencin, M.; Abramovič, H.; Ulrih, N.P.; Vidrih, R. Development and optimization of solid-phase extraction technique for isolation and concentration of extractable and bound phenolic acids from germinated spelt (Triticum spelta L.) seeds. Foods 2021, 10, 2755. [Google Scholar] [CrossRef]
- Deflaoui, L.; Boudebbouze, S.; Kaci, M.; Amarouche, H.; Guérin, F.; Meneguzzi, M.; Dabbou, S.; Allaf, K.; Tomao, V.; Aït-Kaci, A. Phenolic Compounds in Olive Oil by Solid Phase Extraction: Optimization of Extraction Conditions and Liquid Chromatography Characterization. Processes 2021, 9, 2053. [Google Scholar] [CrossRef]
- Androutsos, O.; Perperidi, M.; Georgiou, C.; Chouliaras, G. Lifestyle Changes and Determinants of Children’s and Adolescents’ Body Weight Increase During the First COVID-19 Lockdown in Greece: The COV-EAT Study. Nutrients 2021, 13, 3. [Google Scholar] [CrossRef]
- Crimarco, A.; Landry, M.J.; Gardner, C.D. Ultra-processed Foods, Weight Gain, and Co-morbidity Risk. Curr. Obes. Rep. 2022, 11, 80–92. [Google Scholar] [CrossRef]
- Zachary, Z.; Brianna, F.; Brianna, L.; Garrett, P.; Jade, W.; Alyssa, D.; Mikayla, K. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes. Res. Clin. Pract. 2020, 14, 210–216. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S.; Yang, D.; Li, L.; Li, B.; Wang, L.; Li, M.; Wang, G.; Li, J.; Xu, Y.; et al. Increased Variation in Body Weight and Food Intake Is Related to Increased Dietary Fat but Not Increased Carbohydrate or Protein in Mice. Front. Nutr. 2022, 8, 9. [Google Scholar] [CrossRef]
- Auger, C.; Kajimura, S. Adipose tissue remodeling in pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 71–93. [Google Scholar] [CrossRef]
- Worm, N. Beyond Body Weight-Loss: Dietary Strategies Targeting Intrahepatic Fat in NAFLD. Nutrients 2020, 12, 1316. [Google Scholar] [CrossRef]
- Singha, S.; Kumar, S.; Nayak, S.K.; Kumar, P.; Banik, A.; Srivastava, P.P. Blue lotus (Nymphaea nouchali) flower and leaf supplements enhance growth and hemato-immunological parameters in Indian major carp (Labeo rohita) juveniles. Anim. Feed. Sci. Technol. 2023, 304, 115741. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Chang, J.; Chen, J.; Han, X.; Zhang, T.; Meng, F. The liver steatosis severity and lipid characteristics in primary biliary cholangitis. BMC Gastroenterol. 2021, 21, 395. [Google Scholar] [CrossRef]
- Le, T.N.; Choi, H.; Jun, H. Ethanol Extract of Liriope platyphylla Root Attenuates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice via Regulation of Lipogenesis and Lipid Uptake. Nutrients 2021, 13, 3338. [Google Scholar] [CrossRef]
- Baldini, F.; Portincasa, P.; Grasselli, E.; Damonte, G.; Salis, A.; Bonomo, M.; Florio, M.; Serale, N.; Voci, A.; Gena, P. Aquaporin-9 is involved in the lipid-lowering activity of the nutraceutical silybin on hepatocytes through modulation of autophagy and lipid droplets composition. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158586. [Google Scholar] [CrossRef]
- Sadeghi, E.; Hosseini, S.M.; Vossoughi, M.; Aminorroaya, A.; Amini, M. Association of Lipid Profile with Type 2 Diabetes in First-Degree Relatives: A 14-Year Follow-Up Study in Iran. Diabetes. Metab. Syndr. Obes. 2020, 13, 2743–2750. [Google Scholar] [CrossRef]
- Tijjani, H.; Banbilbwa Joel, E.; Luka, C.D. Modulatory effects of some Fruit juices on lipid profile in rats fed with high lipid diet. Asian J. Biochem. Genet. Mol. Biol. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of liver diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef]
- A’laa, H.J.; Hussein, A.L. Correlation between lipid profile and liver function in patients with non-alcoholic fatty liver. Diyala J. Med. 2024, 27, 29–39. Available online: http://148.72.244.84/xmlui/handle/xmlui/15445 (accessed on 25 December 2024). [CrossRef]
- Zhang, D.; Luo, W.; Liu, C.; Wang, X.; Li, Y.; Zheng, P.; Li, Y.; Cao, S. Curcumin Improves glycolipid metabolism through regulating PPARγ signaling pathway and upregulating the expression of GLUT4 in 3T3-L1 Adipocytes and HFD-induced obese mice. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, H.; Zhang, C.; Li, Z.; Wang, Y.; Yu, H.; Wang, T.; Yuan, H. Curcumin ameliorates liver steatosis and insulin resistance in high-fat diet-induced obese mice via activating AMPK pathway and upregulating PPARα expression. Nutrients 2020, 12, 144. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2013, 92, 260–269. [Google Scholar] [CrossRef]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 95. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Huang, Y.; Chen, J.; Fu, H.; Yang, N.; et al. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 2016, 9, 76. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, J. Role of phytoconstituents as PPAR agonists: Implications for neurodegenerative disorders. Biomedicines 2021, 9, 1914. [Google Scholar] [CrossRef]
- Arndt, L.; Hernandez-Resendiz, I.; Moos, D.; Dokas, J.; Müller, S.; Jeromin, F.; Burkhardt, R. Trib1 deficiency promotes hyperlipidemia, inflammation, and atherosclerosis in LDL receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 979–994. [Google Scholar] [CrossRef]
- Danielewski, M.; Rapak, A.; Kruszyńska, A.; Małodobra-Mazur, M.; Oleszkiewicz, P.; Dzimira, S.; Sozański, T. Cornelian cherry (Cornus mas L.) fruit extract lowers SREBP-1c and C/EBPα in liver and alters various PPAR-α, PPAR-γ, LXR-α target genes in cholesterol-rich diet rabbit model. Int. J. Mol. Sci. 2024, 25, 1199. [Google Scholar] [CrossRef] [PubMed]
- Griseti, E.; Bello, A.A.; Bieth, E.; Sabbagh, B.; Iacovoni, J.S.; Bigay, J.; Laurell, H.; Čopič, A. Molecular mechanisms of perilipin protein function in lipid droplet metabolism. FEBS Lett. 2024, 598, 1170–1198. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.N.; De Nardo, W.; Lou, J.; Schittenhelm, R.B.; Montgomery, M.K.; Granneman, J.G.; Watt, M.J. Perilipin 5 S155 phosphorylation by PKA is required for the control of hepatic lipid metabolism and glycemic control. J. Lipid Res. 2021, 62, 100016. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Geng, B.; Xu, G. Sulfhydration of perilipin 1 is involved in the inhibitory effects of cystathionine gamma lyase/hydrogen sulfide on adipocyte lipolysis. Biochem. Biophys. Res. Commun. 2020, 521, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jin, Y.; Jin, H.; Li, C.; Zhang, Y.; Liu, Y.; Sheng, L. New insights into the function of lipid droplet-related proteins and lipid metabolism of salt-stimulated porcine biceps femoris: Label-free quantitative phosphoproteomics, morphometry and bioinformatics. J. Sci. Food Agric. 2023, 103, 7517–7528. [Google Scholar] [CrossRef]
- Engin, A. Reappraisal of Adipose Tissue Inflammation in Obesity. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2024; pp. 297–327. [Google Scholar] [CrossRef]
- Deji-Oloruntoba, O.O.; Elufioye, T.O.; Adefegha, S.A.; Jang, M. Can caenorhabditis elegans serve as a reliable model for drug and nutraceutical discovery? Appl. Biosci. 2025, 4, 23. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deji-Oloruntoba, O.O.; Kim, J.E.; Song, H.J.; Seol, A.; Hwang, D.Y.; Jang, M. Phenolic-Rich Indian Almond (Terminalia catappa Linn) Leaf Extract Ameliorates Lipid Metabolism and Inflammation in High-Fat Diet (HFD)-Induced Obese Mice. Metabolites 2025, 15, 594. https://doi.org/10.3390/metabo15090594
Deji-Oloruntoba OO, Kim JE, Song HJ, Seol A, Hwang DY, Jang M. Phenolic-Rich Indian Almond (Terminalia catappa Linn) Leaf Extract Ameliorates Lipid Metabolism and Inflammation in High-Fat Diet (HFD)-Induced Obese Mice. Metabolites. 2025; 15(9):594. https://doi.org/10.3390/metabo15090594
Chicago/Turabian StyleDeji-Oloruntoba, Opeyemi O., Ji Eun Kim, Hee Jin Song, Ayun Seol, Dae Youn Hwang, and Miran Jang. 2025. "Phenolic-Rich Indian Almond (Terminalia catappa Linn) Leaf Extract Ameliorates Lipid Metabolism and Inflammation in High-Fat Diet (HFD)-Induced Obese Mice" Metabolites 15, no. 9: 594. https://doi.org/10.3390/metabo15090594
APA StyleDeji-Oloruntoba, O. O., Kim, J. E., Song, H. J., Seol, A., Hwang, D. Y., & Jang, M. (2025). Phenolic-Rich Indian Almond (Terminalia catappa Linn) Leaf Extract Ameliorates Lipid Metabolism and Inflammation in High-Fat Diet (HFD)-Induced Obese Mice. Metabolites, 15(9), 594. https://doi.org/10.3390/metabo15090594








