Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Silencing CERS2 and CERS5/6
2.4. Total RNA Isolation and RT-qPCR
2.5. Cell Viability
2.6. Library Preparation and mRNA-Sequencing
2.7. Bioinformatics and Pathway Analysis
2.7.1. Gene Expression Analysis
2.7.2. Gene Set Enrichment Analysis (GSEA)
3. Results
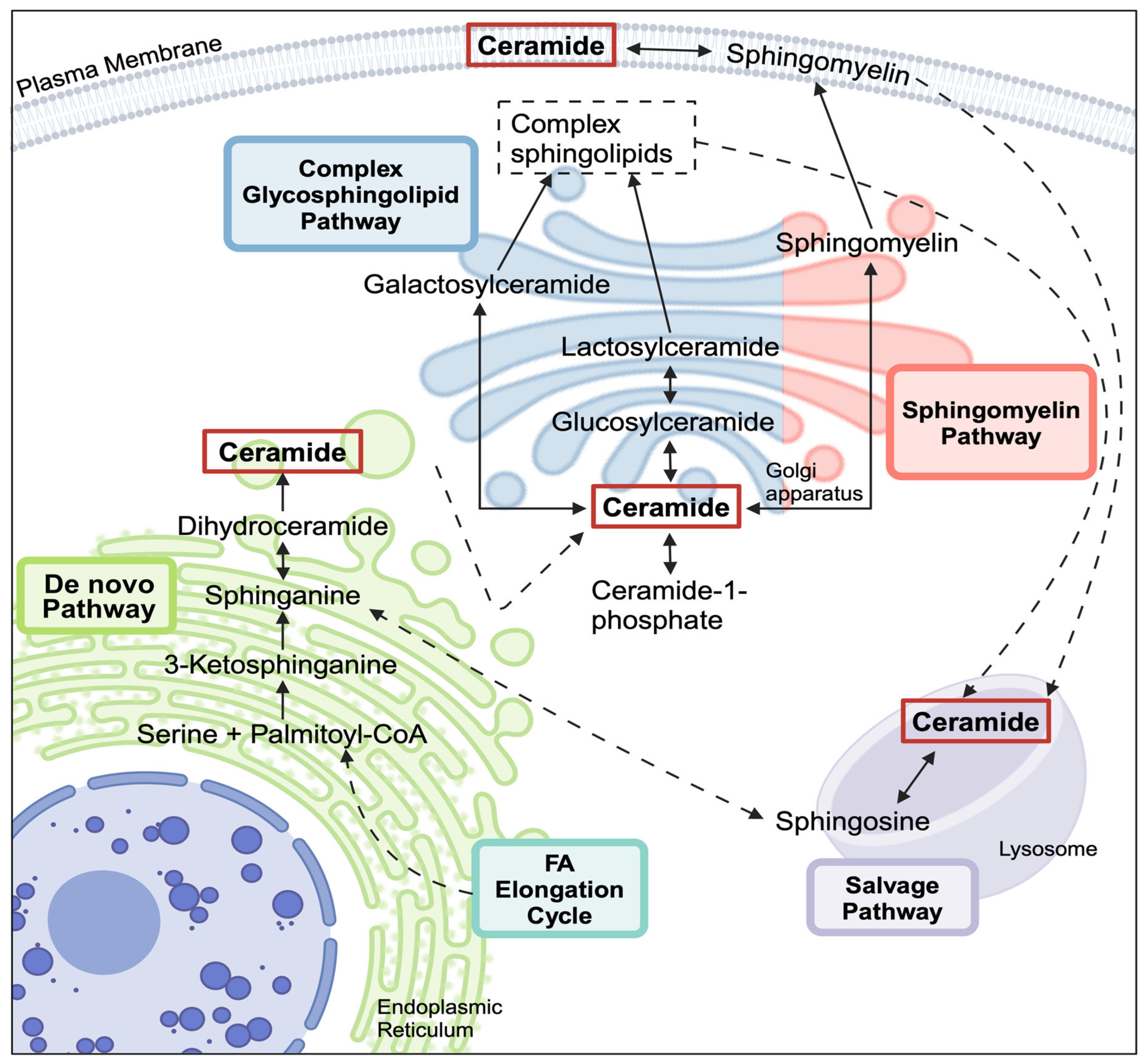
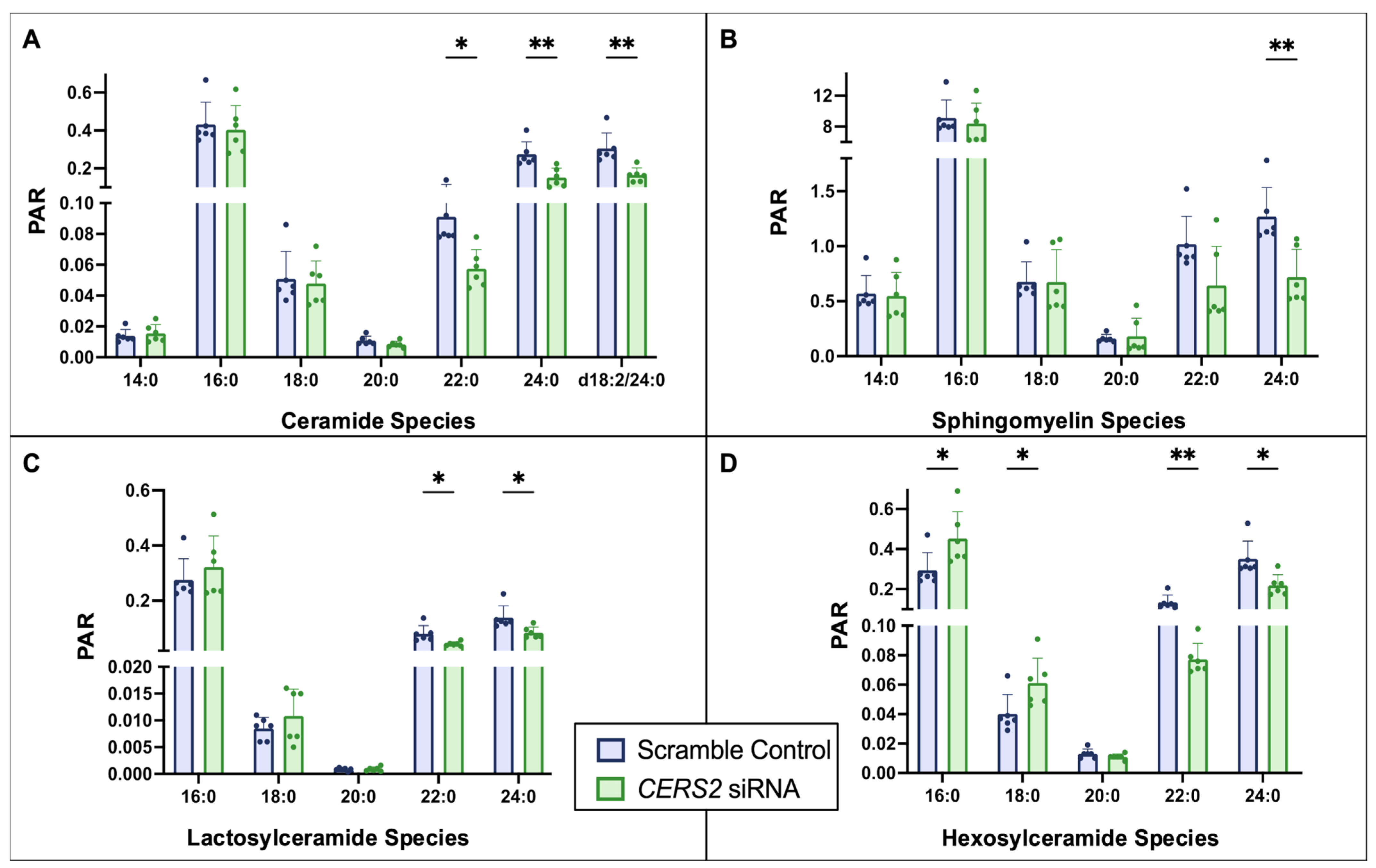
3.1. Ceramide Synthase 2 (CERS2) Knockdown
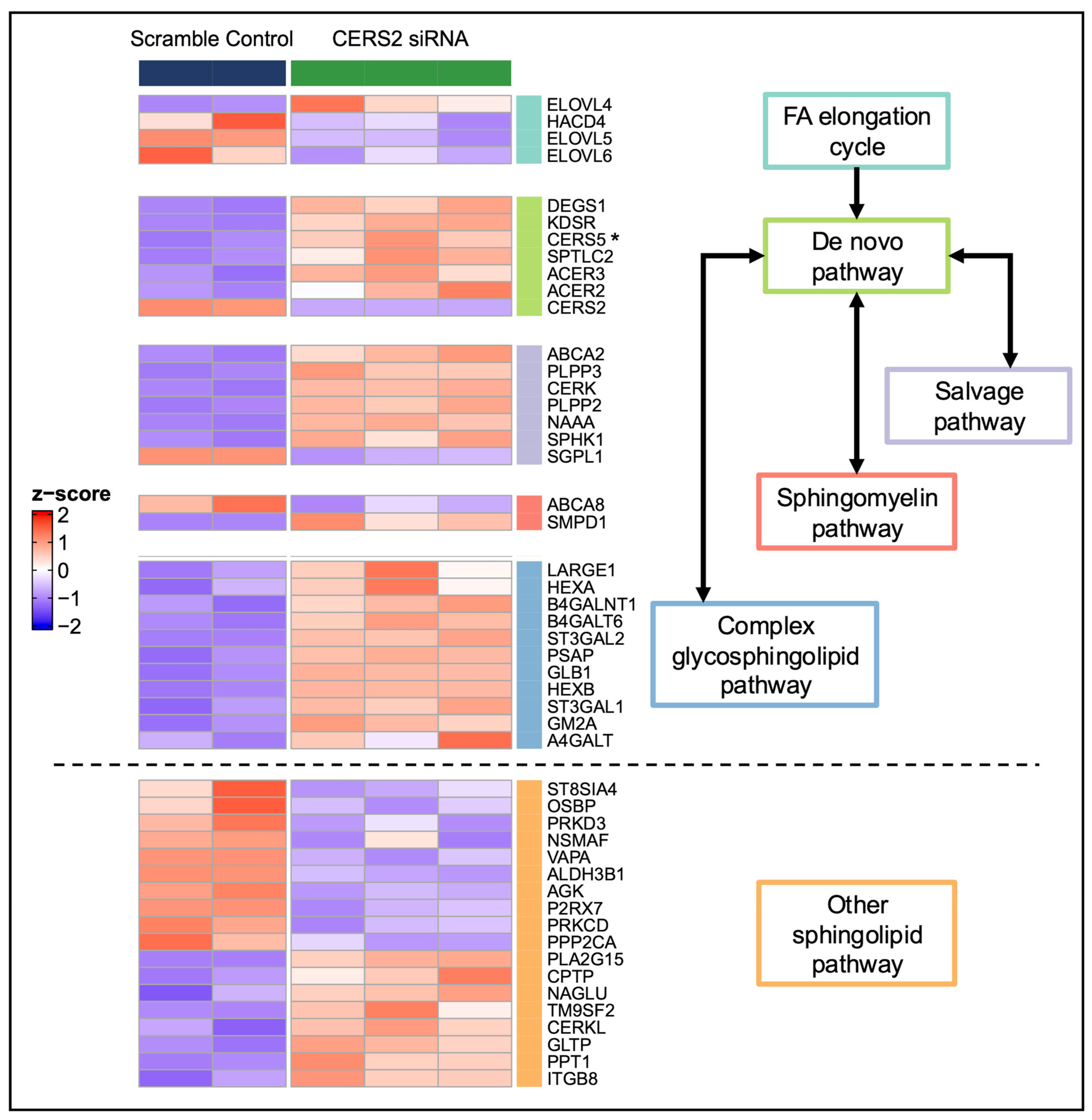
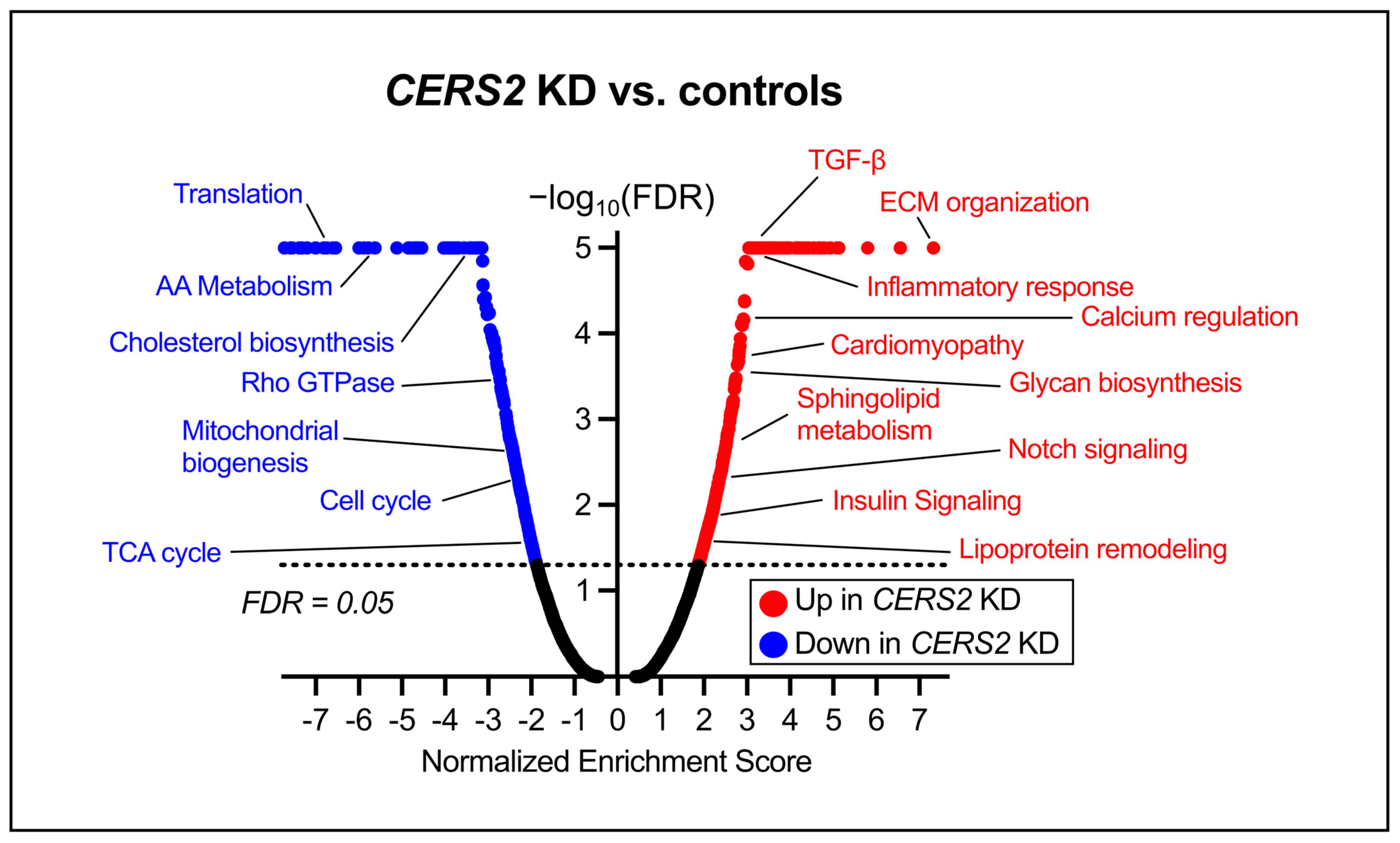
3.2. Global Transcriptomic Changes Following CERS2 Knockdown
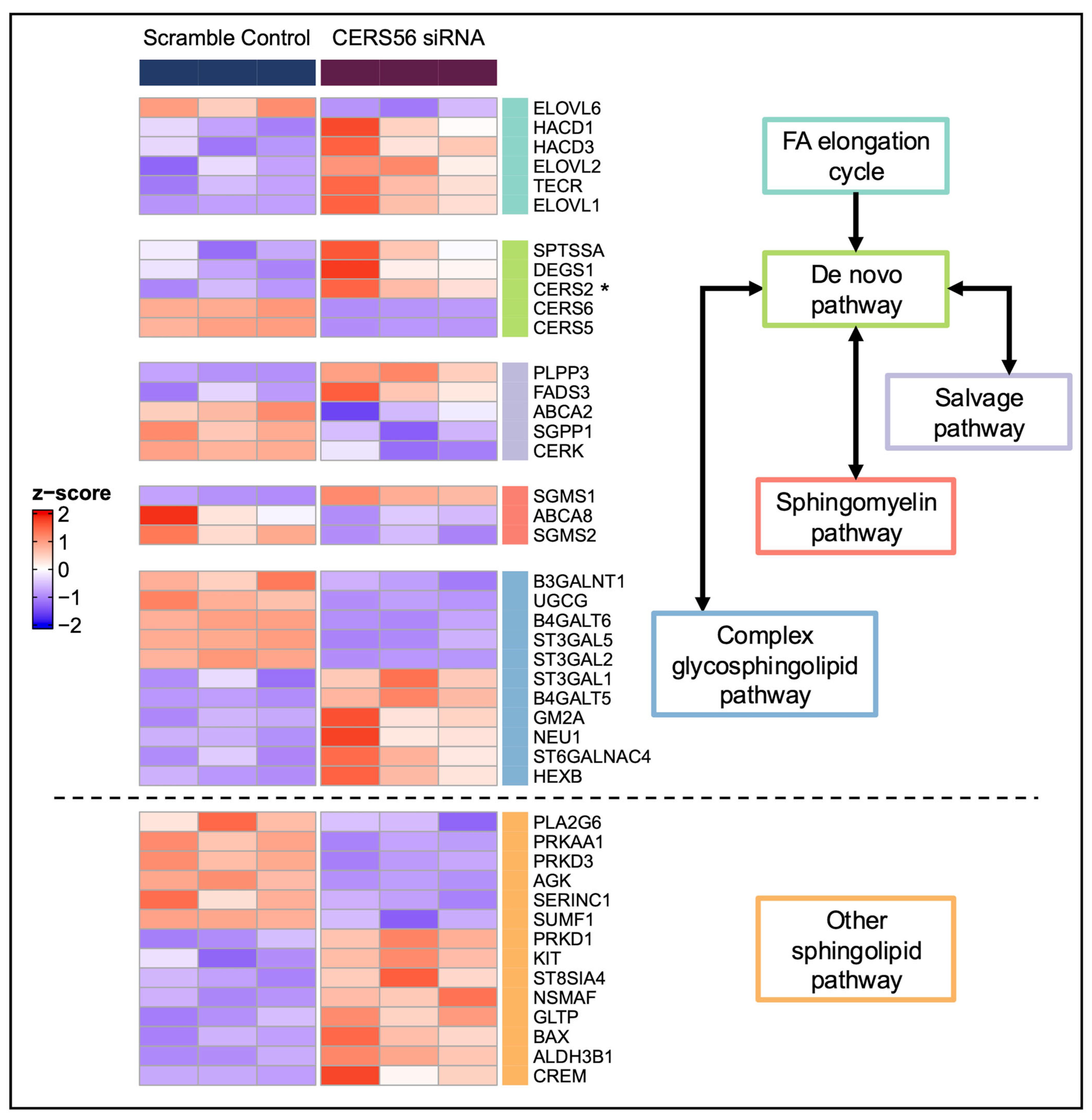
3.3. Ceramide Synthase 5 and 6 (CERS5/6) Knockdown
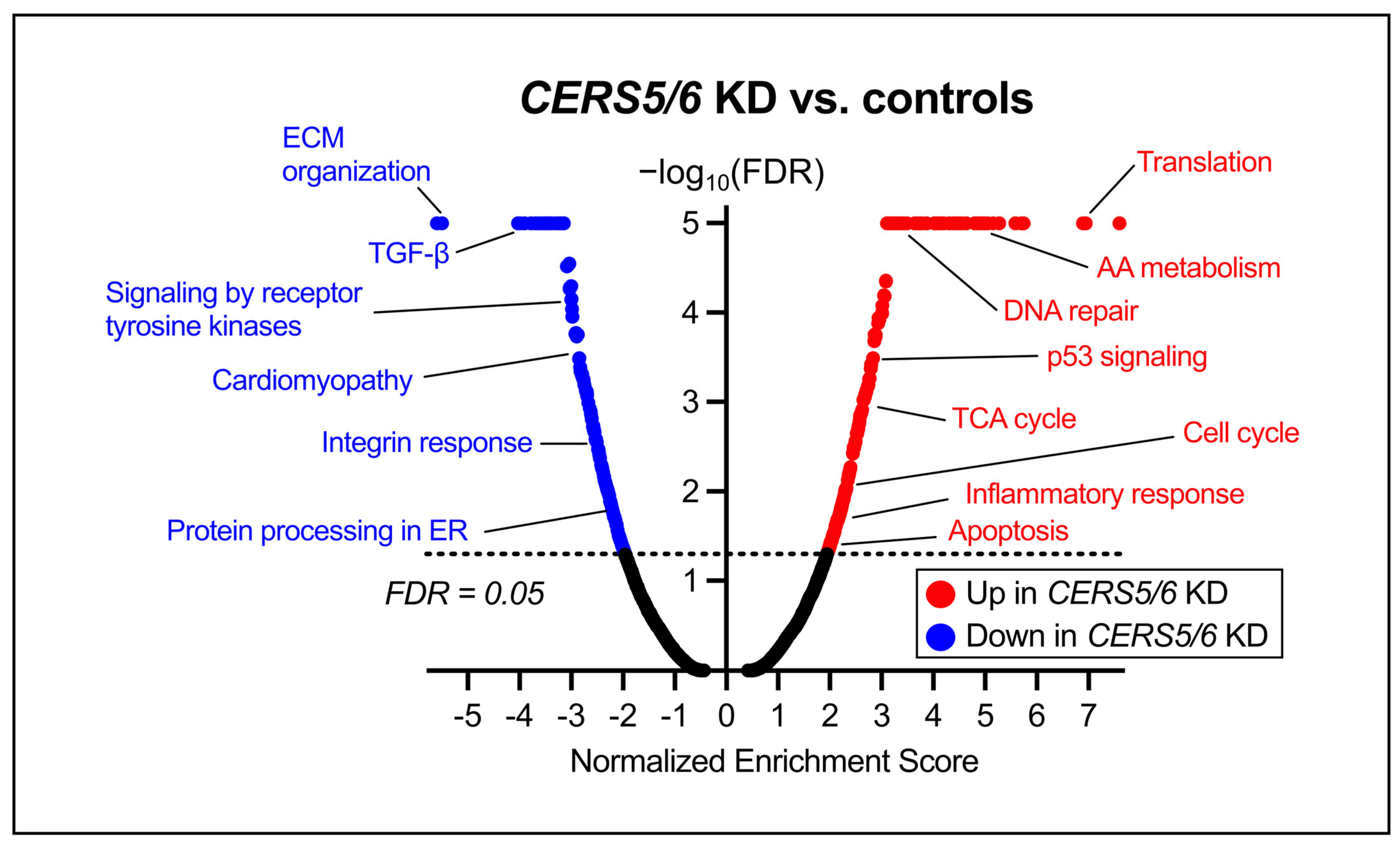
3.4. Global Transcriptomic Changes Following CERS5/6 Knockdown
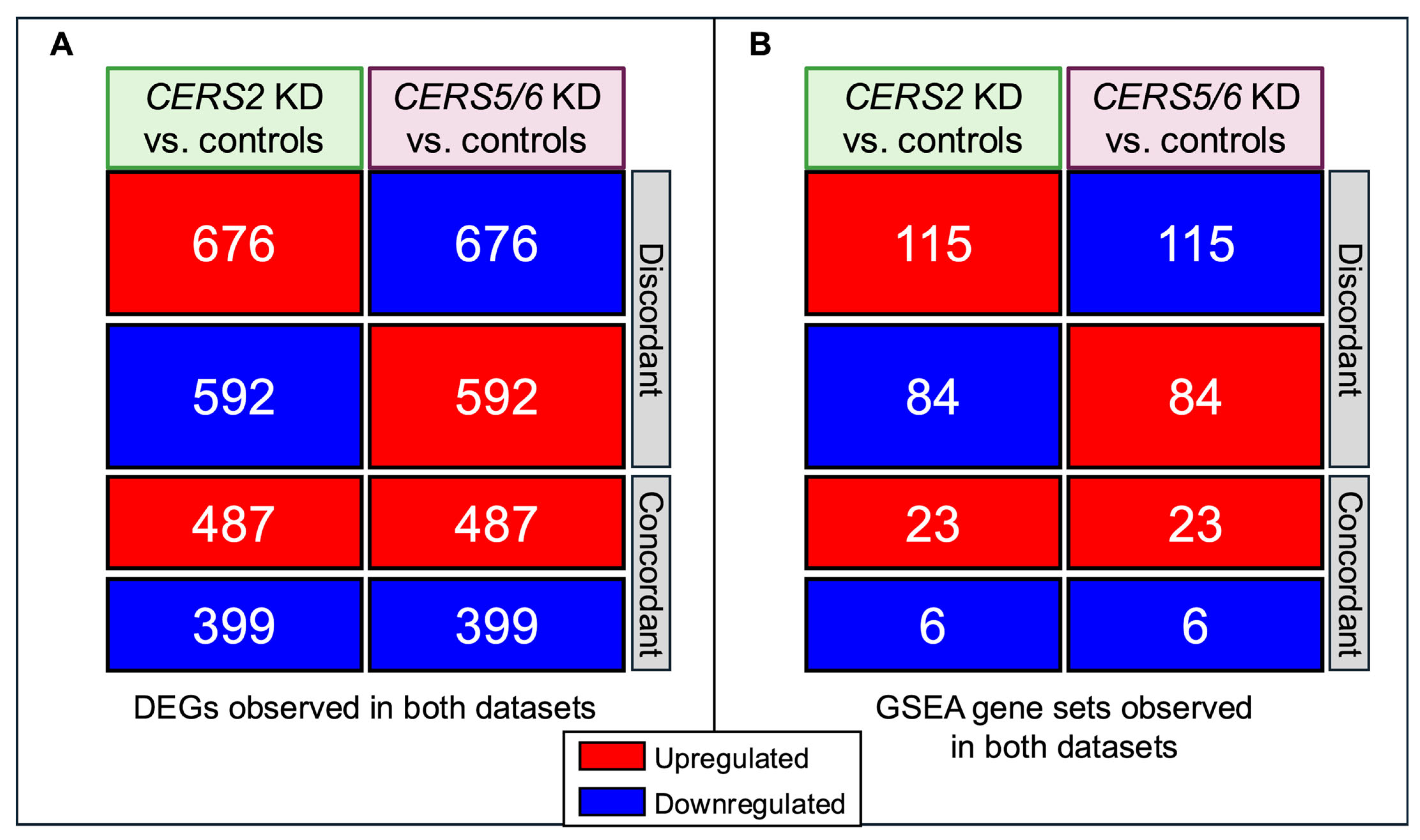
3.5. Discordant Transcriptional Patterns Between Different CERS Knockdowns
4. Discussion
4.1. CERS Knockdown Leads to Intracellular Ceramide Reductions
4.2. Pathway Changes Within the HCMs Due to CERS Knockdown
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| LC | long-chain |
| VLC | very long-chain |
| CerS | ceramide synthase |
| HCM | Ventricular human cardiomyocyte |
| GSEA | gene set enrichment analysis |
| DEG | differentially expressed gene |
| FDR | false discovery rate |
| PCA | principal component analysis |
| LacCer | lactosylceramide |
| HexCer | hexosylceramide |
| GlcCer | glucosylceramide |
| GalCer | galactosylceramide |
| ECM | extracellular matrix |
References
- Pralhada Rao, R.; Vaidyanathan, N.; Rengasamy, M.; Mammen Oommen, A.; Somaiya, N.; Jagannath, M.R. Sphingolipid Metabolic Pathway: An Overview of Major Roles Played in Human Diseases. J. Lipids 2013, 2013, 178910. [Google Scholar] [CrossRef]
- Kraft, M.L. Sphingolipid Organization in the Plasma Membrane and the Mechanisms That Influence It. Front. Cell Dev. Biol. 2017, 4, 154. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Hla, T. Regulation of cellular and systemic sphingolipid homeostasis. Nat. Rev. Mol. Cell Biol. 2024, 25, 802–821. [Google Scholar] [CrossRef]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in apoptosis: An overview and current perspectives. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Patwardhan, G.A.; Beverly, L.J.; Siskind, L.J. Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 2016, 48, 153–168. [Google Scholar] [CrossRef]
- Zhu, Q.-Y.; Wang, Z.; Ji, C.; Cheng, L.; Yang, Y.-L.; Ren, J.; Jin, Y.-H.; Wang, Q.-J.; Gu, X.-J.; Bi, Z.-G.; et al. C6-ceramide synergistically potentiates the anti-tumor effects of histone deacetylase inhibitors via AKT dephosphorylation and α-tubulin hyperacetylation both in vitro and in vivo. Cell Death Dis. 2011, 2, e117. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Goñi, F.M. The Physical Properties of Ceramides in Membranes. Annu. Rev. Biophys. 2018, 47, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Milhas, D.; Clarke, C.J.; Hannun, Y.A. Sphingomyelin metabolism at the plasma membrane: Implications for bioactive sphingolipids. FEBS Lett. 2010, 584, 1887–1894. [Google Scholar] [CrossRef]
- Aureli, M.; Loberto, N.; Chigorno, V.; Prinetti, A.; Sonnino, S. Remodeling of Sphingolipids by Plasma Membrane Associated Enzymes. Neurochem. Res. 2011, 36, 1636–1644. [Google Scholar] [CrossRef]
- Hammad, S.M. Blood Sphingolipids in Homeostasis and Pathobiology. In Sphingolipids and Metabolic Disease; Advances in Experimental Medicine and Biology; Cowart, L.A., Ed.; Springer: New York, NY, USA, 2011; Volume 721, pp. 57–66. [Google Scholar] [CrossRef]
- Calzada, C.; Vors, C.; Penhoat, A.; Cheillan, D.; Michalski, M.C. Role of circulating sphingolipids in lipid metabolism: Why dietary lipids matter. Front. Nutr. 2023, 9, 1108098. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef]
- Venkataraman, K.; Riebeling, C.; Bodennec, J.; Riezman, H.; Allegood, J.C.; Sullards, M.C.; Merrill, A.H.; Futerman, A.H. Upstream of Growth and Differentiation Factor 1 (uog1), a Mammalian Homolog of the Yeast Longevity Assurance Gene 1 (LAG1), RegulatesN-Stearoyl-sphinganine (C18-(Dihydro)ceramide) Synthesis in a Fumonisin B1-independent Manner in Mammalian Cells. J. Biol. Chem. 2002, 277, 35642–35649. [Google Scholar] [CrossRef] [PubMed]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H.; Futerman, A.H. Characterization of Ceramide Synthase 2. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 2006, 398, 531–538. [Google Scholar] [CrossRef]
- Lahiri, S.; Futerman, A.H. LASS5 Is a Bona Fide Dihydroceramide Synthase That Selectively Utilizes Palmitoyl-CoA as Acyl Donor. J. Biol. Chem. 2005, 280, 33735–33738. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 2005, 390, 263–271. [Google Scholar] [CrossRef]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network With High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef]
- Nicholson, R.J.; Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hopkins, P.N.; Hunt, S.C.; Playdon, M.C.; Holland, W.L.; Summers, S.A. Characterizing a Common CERS2 Polymorphism in a Mouse Model of Metabolic Disease and in Subjects from the Utah CAD Study. J. Clin. Endocrinol. Metab. 2021, 106, e3098-109. [Google Scholar] [CrossRef]
- De La Monte, S.M. Triangulated Mal-Signaling in Alzheimer’s Disease: Roles of Neurotoxic Ceramides, ER Stress, and Insulin Resistance Reviewed. J. Alzheimers Dis. 2012, 30, S231–S249. [Google Scholar] [CrossRef]
- Gosejacob, D.; Jäger, P.S.; Dorp, K.V.; Frejno, M.; Carstensen, A.C.; Köhnke, M.; Degen, J.; Dörmann, P.; Hoch, M. Ceramide Synthase 5 Is Essential to Maintain C16:0-Ceramide Pools and Contributes to the Development of Diet-induced Obesity. J. Biol. Chem. 2016, 291, 6989–7003. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-Induced CerS6-Dependent C16:0 Ceramide Production Promotes Weight Gain and Glucose Intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, J.R.; Hu, Y.; Khan, R.; Kim, S.-J.; Bharadwaj, K.G.; Davidson, M.M.; Choi, C.-S.; Shin, K.-O.; Lee, Y.-M.; et al. Cardiomyocyte Specific Deficiency of Serine Palmitoyltransferase Subunit 2 Reduces Ceramide but Leads to Cardiac Dysfunction. J. Biol. Chem. 2012, 287, 18429–18439. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk: The Cardiovascular Health Study. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Yu, C.; Hoofnagle, A.; Hari, N.; Jensen, P.N.; Fretts, A.M.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; et al. Circulating Sphingolipids, Insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 2018, 67, 1663–1672. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]
- Mullen, T.D.; Spassieva, S.; Jenkins, R.W.; Kitatani, K.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J. Lipid Res. 2011, 52, 68–77. [Google Scholar] [CrossRef]
- Andersson, L.; Cinato, M.; Mardani, I.; Miljanovic, A.; Arif, M.; Koh, A.; Lindbom, M.; Laudette, M.; Bollano, E.; Omerovic, E.; et al. Glucosylceramide synthase deficiency in the heart compromises b1-adrenergic receptor trafficking. Eur. Heart J. 2021, 42, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Dorrance, A. Increased membrane sphingomyelin and arachidonic acid in stroke-prone spontaneously hypertensive rats. Am. J. Hypertens. 2001, 14, 1149–1153. [Google Scholar] [CrossRef]
- Shinitzky, M.; Barenholz, Y. Dynamics of the Hydrocarbon Layer in Liposomes of Lecithin and Sphingomyelin Containing Dicetylphosphate. J. Biol. Chem. 1974, 249, 2652–2657. [Google Scholar] [CrossRef]
- Kehat, I.; Molkentin, J.D. Molecular Pathways Underlying Cardiac Remodeling During Pathophysiological Stimulation. Circulation 2010, 122, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Gandoy-Fieiras, N.; Gonzalez-Juanatey, J.R.; Eiras, S. Myocardium Metabolism in Physiological and Pathophysiological States: Implications of Epicardial Adipose Tissue and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 2641. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid Metabolism and Toxicity in the Heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sano, M. Deranged Myocardial Fatty Acid Metabolism in Heart Failure. Int. J. Mol. Sci. 2022, 23, 996. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Janneh, A.H.; Ogretmen, B. Targeting Sphingolipid Metabolism as a Therapeutic Strategy in Cancer Treatment. Cancers 2022, 14, 2183. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiley, A.M.; Krueger, M.A.; Becker, J.O.; Karasu, M.; Sotoodehnia, N.; Umans, J.G.; Hoofnagle, A.N.; Gharib, S.A.; Totah, R.A.; Lemaitre, R.N. Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis. Metabolites 2025, 15, 584. https://doi.org/10.3390/metabo15090584
Wiley AM, Krueger MA, Becker JO, Karasu M, Sotoodehnia N, Umans JG, Hoofnagle AN, Gharib SA, Totah RA, Lemaitre RN. Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis. Metabolites. 2025; 15(9):584. https://doi.org/10.3390/metabo15090584
Chicago/Turabian StyleWiley, Alexandra M., Melissa A. Krueger, Jessica O. Becker, Matthew Karasu, Nona Sotoodehnia, Jason G. Umans, Andrew N. Hoofnagle, Sina A. Gharib, Rheem A. Totah, and Rozenn N. Lemaitre. 2025. "Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis" Metabolites 15, no. 9: 584. https://doi.org/10.3390/metabo15090584
APA StyleWiley, A. M., Krueger, M. A., Becker, J. O., Karasu, M., Sotoodehnia, N., Umans, J. G., Hoofnagle, A. N., Gharib, S. A., Totah, R. A., & Lemaitre, R. N. (2025). Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis. Metabolites, 15(9), 584. https://doi.org/10.3390/metabo15090584








