Tentative Identification of Chemical Constituents in Liuwei Dihuang Pills Based on UPLC-Orbitrap-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
2.2. Preparation of Liuwei Dihuang Pills Solution
2.3. Chromatographic Conditions
2.4. Mass Spectrometric Conditions
2.5. Data Processing
3. Results and Discussion
3.1. Structure Identification of Triterpenoids
3.2. Structural Identification of Iridoid Compounds
3.3. Structure Identification of Monoterpenoids
3.4. Structure Identification of Flavonoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (Part I); China Medical Science and Technology Press: Beijing, Chian, 2020; p. 705. [Google Scholar]
- Xie, B.; Zhang, Z.; Gong, T.; Zhang, N.; Wang, H.; Zou, H. Application of metabonomic strategy to discover an unreported active ingredient in LiuWeiDiHuang pills suppressing beta-glucuronidase. Anal. Bioanal. Chem. 2015, 407, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lv, H.T.; Zhang, A.H.; Sun, H.; Yan, G.L.; Han, Y.; Wu, X.H.; Wang, X.J. Improved ultra-performance liquid chromatography with electrospray ionization quadrupole-time-of-flight high-definition mass spectrometry method for the rapid analysis of the chemical constituents of a typical medical formula: Liuwei Dihuang Wan. J. Sep. Sci. 2013, 36, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, H.; Zhang, J.; Zhang, P.; Li, M.; Qi, F.; Wang, Y.; Kou, S.; Zheng, Q.; Wang, L. The regulatory effect of liuwei dihuang pills on cytokines in mice with experimental autoimmune encephalomyelitis. Am. J. Chin. Med. 2012, 40, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Advancement in treating osteoporosis with traditional Chinese medicine Liuwei Dihuang pill. Front. Musculoskelet. Disord. 2025, 3, 1575864. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Li, R.; Gao, S.; Cao, S.M.; Feng, L.; Jia, X.B. Structure characteristics and quality control of Liuwei Dihuang concentrted pills from different manufacturers. Chin. J. Chin. Mater Med. 2020, 45, 3369. [Google Scholar]
- Hong, Y.I.; Zhang, W.W.; Yu, Y.A.N.; Qin, S.I. Discussion for quality control of chinese patent drugs from quality difference among different dosage forms of Liuwei Dihuang preparations. Chin. J. Exp. Tradit. Med. Form 2021, 27, 138. [Google Scholar]
- Wang, R.; Wang, X.; Ou, J. Network pharmacology-based approach reveals that Fructus mume exerts therapeutic effects against ulcerative colitis via the AGE/RAGE signaling pathway. Arab. J. Chem. 2024, 17, 105534. [Google Scholar] [CrossRef]
- Hu, S.; Guo, H.; Miao, R.; Yu, J.; Wang, X.; Zhao, H. Screening and Identification of Anti-Gout Components From Herb Pair Combining Ultrafiltration-HPLC-MS, On-Line HPLC-2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid)-MS and In Silico Analysis: Case Study by Smilacis Glabrae Rhizoma-Dioscoreae Spongiosae Rhizoma. J. Sep. Sci. 2025, 48, e70209. [Google Scholar]
- Kim, M.J.; Son, S.Y.; Jeon, S.G.; Kim, J.G.; Lee, C.H. Metabolite Profiling of Dioscorea (Yam) Leaves to Identify Bioactive Compounds Reveals Their Potential as Renewable Resources. Plants 2021, 10, 1751. [Google Scholar] [CrossRef]
- Wu, L.R.; Shi, Z.F.; Jin, X.B.; Gao, W.D.; Zhu, J.Y. Sample Description of Diabetic Rats Treated with the Drug Pair of Astragalus and Chinese Yam by Using Multivariated Data Mapping Methods of R. Adv. Mater. Res. 2013, 791, 3–6. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Wu, Z.; Ding, L. ESI-QqTOF-MS/MS and APCI-IT-MS/MS analysis of steroid saponins from the rhizomes of Dioscorea panthaica. J. Mass Spectrom. 2006, 41, 1–22. [Google Scholar] [CrossRef]
- Liu, B.; Shao, T.; Xiao, D.; Yang, S.; Lin, W.; Sun, L.; Zhang, W.; Luo, M.; Zhao, J.; Yang, L. Aqueous extract of Cornus officinalis alleviate NAFLD via protecting hepatocytes proliferation through regulation of the tricarboxylic acid cycle. J. Ethnopharmacol. 2025, 341, 119330. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Liu, H.; Wang, Y.; Lin, Z.; Sun, L. The rapid profiling and simultaneous determination of 12 major alkaloids in Nauclea officinalis by UPLC-Q-TOF-MS and HPLC-ESI-MS/MS. Anal. Methods Adv. Methods Appl. 2021, 13, 5787–5803. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H.; Zhao, M.; Jiang, S.; Zhang, W.; Xu, B.H.; Duan, J.A. UPLC-Q-TOF/MS-Based Metabolic Profiling Comparison of Two Major Bioactive Components and Their Metabolites in Normal and CKD Rat Plasma, Urine and Feces Following Oral Administration of Fructus Corni Extract. J. Chromatogr. Sci. 2017, 55, 857–865. [Google Scholar]
- Cao, M.; Wang, X.; Yue, J.; Li, N.; Wang, R. Dissection of the Potential Activity Materials and Mechanism of Cortex Moutan Based on In Vivo Substances Profiling. Sep. Sci. Plus 2025, 8, e70023. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.C.; Seo, D.; Kim, K.T.; Lee, S.H.; Hong, S.B. Genome diversity, population structure and MALDI-TOF MS profiling of Aspergillus oryzae/flavus strains from fermentation and wild environments. BMC Genom. 2025, 26, 429. [Google Scholar] [CrossRef]
- Yan, L.; Ou, Z.; Cheng, Y.; Tong, Y.; Iqbal, J.; Wang, J.; Liu, D. Comparative study on the material basis and mechanism of improvement of kidney yin deficiency edema syndrome before and after preparation of Alisma in UPLC-Q-TOF-MS and transcriptomics. Sci. Tradit. Chin. Med. 2024, 2, 169–179. [Google Scholar] [CrossRef]
- Hu, N.; Da, J.; Chen, X.; Li, S.R.; Wang, Q.R.; Wu, T.T.; Yang, L.; Wu, W.Y.; Guo, D.A. A precise and specific method for quick determination of sulfur fumigation for moutan cortex. World J. Tradit. Chin. Med. 2017, 3, 16–21. [Google Scholar] [CrossRef]
- Xi, H.; Yuan, M.; Xie, J.; Wang, Y. Cyclocarysaponins A–J, dammarane-type triterpenoid glycosides from the leaves of Cyclocarya paliurus. Chin. J. Nat. Med. 2024, 22, 955–964. [Google Scholar] [CrossRef]
- Liu, H.; Bi, L.; Chen, Q.; He, X.; Yan, H.; Ni, W.; Wu, W.; He, L.; Liu, H. Enrichment process, structural prediction, isolation, in vitro cytotoxic and anti-inflammatory effects of triterpenoid saponins in Camellia japonica L. leaves water extract through UPLC-Q-TOF based mass spectrometry similarity networking. Food Chem. 2024, 441, 138360. [Google Scholar] [CrossRef]
- Song, S.; Guo, H.L.; Kang, J.P. Simultaneous determination of 9 active components in Bazhen Yimu Pills by HPLC-ESI-MS/MS. Chin. Tradit. Herb. Drugs 2019, 50, 402–407. [Google Scholar]
- Zhang, J.; Guo, H.; Yan, F.; Yuan, S.; Li, S.; Zhu, P.; Chen, W.; Peng, C.; Peng, D. An UPLC-Q-Orbitrap method for pharmacokinetics and tissue distribution of four triterpenoids in rats after oral administration of Poria cocos ethanol extracts. J. Pharm. Biomed. Anal. 2021, 203, 114237. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, S.; Zhang, F.; Ruan, H.; Xue, H.; Wang, J.; Li, Z.; Jin, W.; Wang, W.; Xia, J.; et al. Qualitative Analysis of Drug-Containing Plasma and its Application to Quantitative Analysis and Pharmacokinetic Study of Zexie Decoction Using UPLC-MS/MS. Front. Chem. 2022, 10, 815886. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Zhang, N.; Wu, P.; Feng, X.; Gao, X.; Shen, J.; Liu, W.; Feng, W.; Sun, J. Screening of ESR2-targeted anti-postmenopausal osteoporosis chemistry from Rehmanniae Radix Preparata based on affinity ultrafiltration with UPLC-QE-Orbitrap-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2024, 1251, 124419. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Xia, M.; Yang, L.; Cheng, W.; Song, Q. Combining network pharmacology with chromatographic fingerprinting and multicomponent quantitative analysis for the quality evaluation of Moutan Cortex. Biomed. Chromatogr. 2022, 36, e5434. [Google Scholar] [CrossRef]
- Ai, Y.; Wu, Y.; Wang, F.; Ma, W.; Bian, Q.; Lee, D.Y.W.; Dai, R. A UPLC-MS/MS method for simultaneous quantitation of three monoterpene glycosides and four alkaloids in rat plasma: Application to a comparative pharmacokinetic study of Huo Luo Xiao Ling Dan and single herb extract. J. Mass Spectrom. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Xiao, H.; Yao, R.; Liu, B.; Duan, L.; Liu, J.; Lin, F.; Wang, S.; Gao, J. Comparative evaluation of moutan pods and moutan barks by HPLC-DAD-ESI-MS/MS technique. Sci. Rep. 2025, 15, 21739. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, X.; Shi, P.; Niu, G.; Zhang, S.; Gu, Z.; Guo, Q. Tissue-specific chemical expression and quantitative analysis of bioactive components of Moutan Cortex by laser-microdissection combined with UPLC-Q-Orbitrap-MS technique. J. Pharm. Biomed. Anal. 2025, 253, 116537. [Google Scholar] [CrossRef]
- Tao, C.; Ren, W.; Zhai, Y.; Qiu, F. Iceberg pharmacokinetic strategy for simultaneous evaluation of five flavonoids from Viticis fructus extract in rats using UHPLC-ESI-MS/MS. J. Pharm. Biomed. Anal. 2025, 262, 116898. [Google Scholar] [CrossRef]
- An, L.; Liu, H.; Li, M.; Ma, J.; Zheng, L.; Zhou, J.; Zhang, J.; Yuan, Y.; Wu, X. Unveiling the impact of harvest time on Dioscorea opposita Thunb. cv. Tiegun maturity by NMR-Based metabolomics and LC-MS/MS analysis. J. Sci. Food Agric. 2024, 104, 6342–6349. [Google Scholar] [CrossRef]
- Fu, S.; Du, L.; Wu, Z.; Hu, P.; Huang, C.; Zhang, S.; He, X. Development and application of a rapid and sensitive LC-MS/MS method for simultaneous quantification of six diterpene lactones in rats after oral administration of Rhizoma Dioscoreae Bulbiferae extract. J. Chromatogr. B 2019, 1128, 121781. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Liu, H.; Tian, X.; Wang, Y.; Xu, H.; Chen, S.; Chen, M.; Hu, P.; Huang, C. Analysis of Herbal Constituents and In Vivo Pharmacokinetics of Gegen-Huangqi Decoction in Rat Plasma Using HPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Biomed. Chromatogr. 2024, 39, e6046. [Google Scholar] [CrossRef]
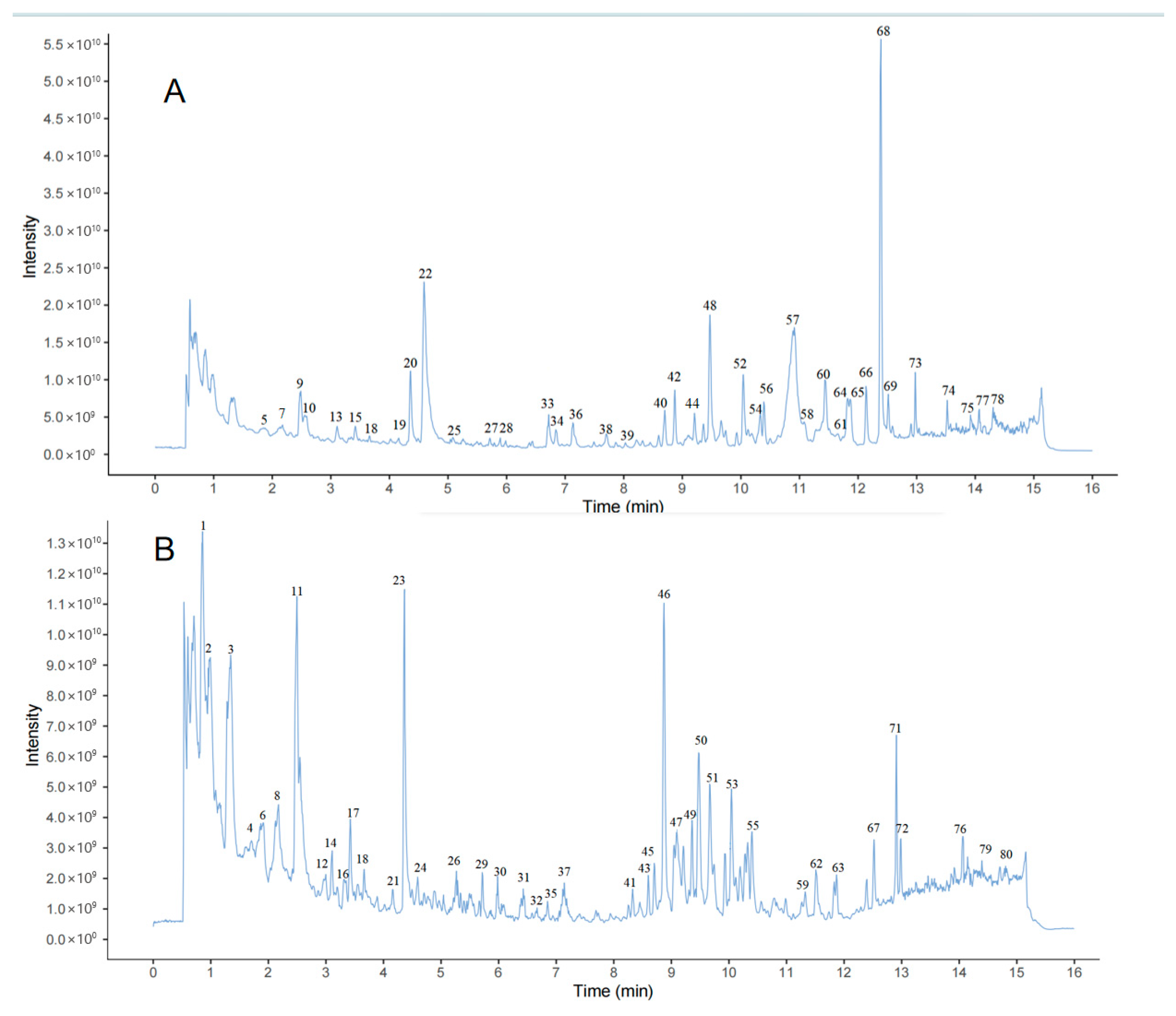

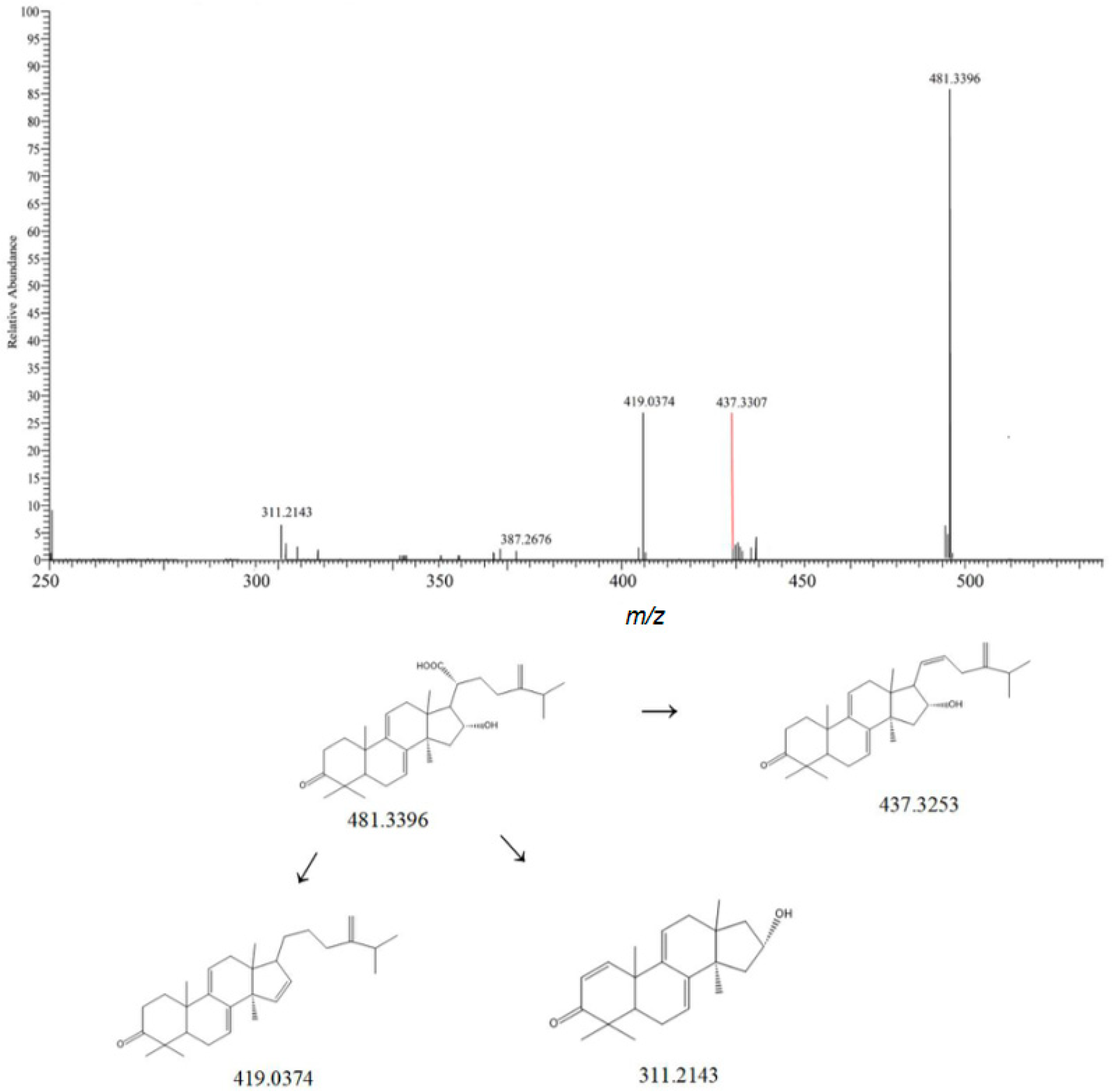

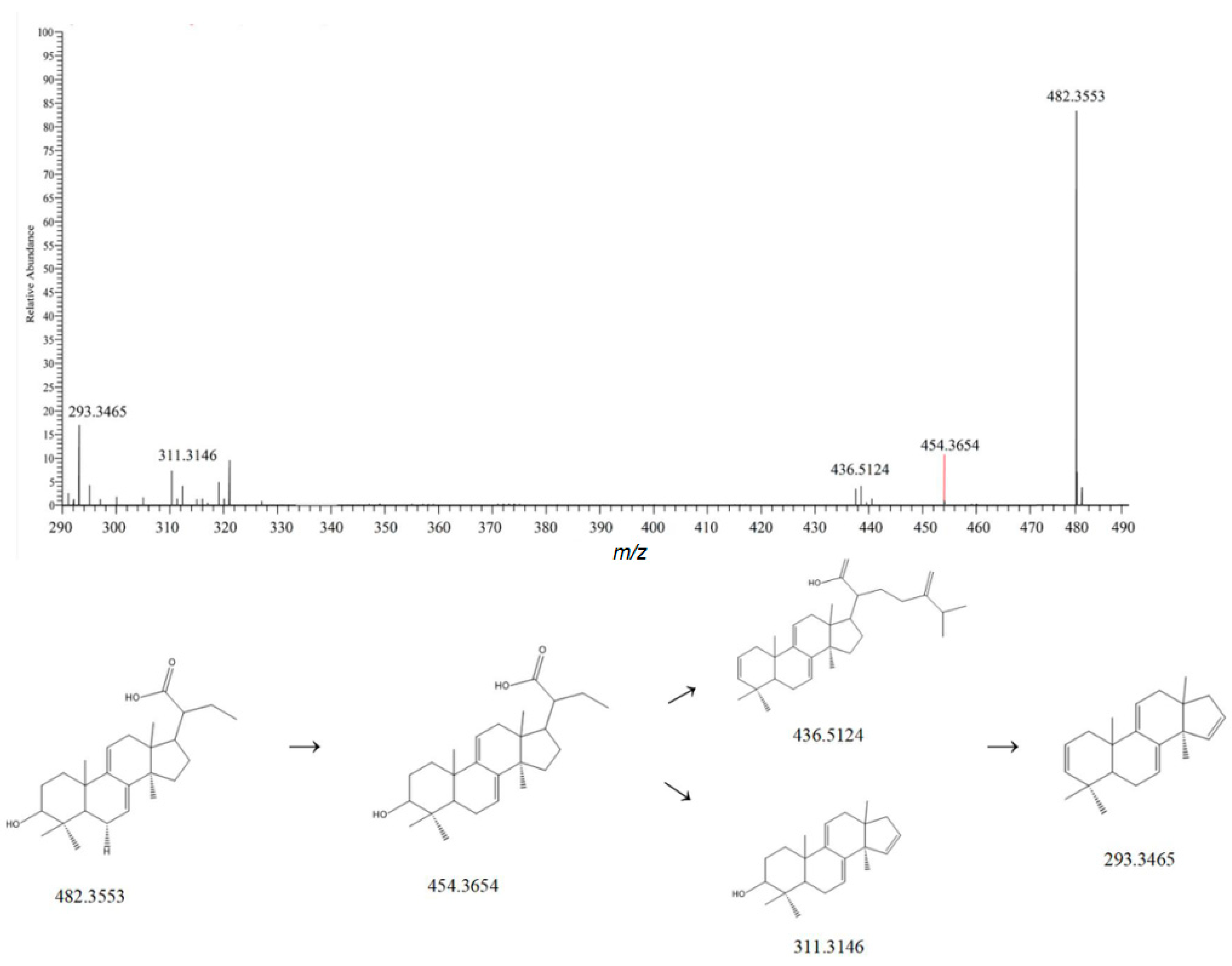


| NO. | Rt | Measured Mass | Theoretical Mass | ppm | Frag Mention | Formula | Ion Mode | Compound | Classify | Crude Drugs | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | 498.3345 | 497.8362 | 1.1 | 497.3254, 171.9834 | C31H46O5 | [M+H]+ | 6α-Hydroxyporia acid C | Triterpenoids | Poria cocos | [9] |
| 2 | 0.99 | 472.3396 | 471.8673 | 2.1 | 469.3310, 448.4997 | C30H48O4 | [M+H]+ | 16α-Hydroxypsilocine | Triterpenoids | Poria cocos | [9] |
| 3 | 1.33 | 454.3451 | 453.8907 | 0.8 | 423.2926, 371.2584 | C30H46O3 | [M-H]− | 3-Dehydrotrametenolic acid | Triterpenoids | Poria cocos | [9] |
| 4 | 1.72 | 486.3709 | 485.8845 | −1.1 | 423.3619, 339.5969 | C31H50O4 | [M+H]+ | Tumulosic acid | Triterpenoids | Poria cocos | [9] |
| 5 | 1.81 | 481.3396 | 482.8573 | −0.9 | 437.3307, 419.0374 | C31H46O4 | [M-H]− | Polyporenic acid C | Triterpenoids | Poria cocos | [9] |
| 6 | 1.94 | 482.3553 | 483.8709 | 1.2 | 293.3465, 311.3146 | C31H48O4 | [M-H]− | Dehydrotumulosic acid | Triterpenoids | Poria cocos | [9] |
| 7 | 2.18 | 514.7361 | 514.2213 | −0.3 | 513.3565, 251.8673 | C33H50O6 | [M-H]− | 3-O-Acetyl-16α-hydroxydehydrotrametenolic acid | Triterpenoids | Poria cocos | [9] |
| 8 | 2.21 | 526.3658 | 525.8394 | 2.2 | 525.3566, 505.2605 | C33H50O5 | [M+H]+ | Dehydropachymic acid | Triterpenoids | Poria cocos | [9] |
| 9 | 2.42 | 529.3815 | 528.1691 | −0.6 | 451.3571, 355.1951 | C33H52O5 | [M+H]+ | Pachymic acid | Triterpenoids | Poria cocos | [9] |
| 10 | 2.45 | 456.3604 | 455.9040 | 0.9 | 455.3517, 390.7080 | C30H48O3 | [M+H]+ | Trametenolic acid | Triterpenoids | Poria cocos | [9] |
| 11 | 2.62 | 287.055 | 287.0548 | 0.9 | 259.5467, 153.4792 | C15H10O6 | [M-H]− | Luteolin | Flavonoids | Dioscoreae Rhizoma | [10] |
| 12 | 2.99 | 579.1708 | 579.1700 | 2.1 | 271.1449, 197.0597 | C15H10O5 | [M+H]+ | Yuankanin | Flavonoids | Dioscoreae Rhizoma | [10] |
| 14 | 3.10 | 415.1032 | 415.3687 | 1.9 | 253.0506, 225.0543 | C21H20O9 | [M-H]− | Puerarin | Flavonoids | Dioscoreae Rhizoma | [10] |
| 13 | 3.11 | 470.3762 | 469.9056 | −0.8 | 469.3673, 112.9855 | C31H50O3 | [M+H]+ | Eburicoic acid | Triterpenoids | Dioscoreae Rhizoma | [10] |
| 15 | 3.23 | 607.1668 | 607.1673 | 2.1 | 284.6423, 240.2348 | C28H32O15 | [M-H]− | Spinosin | Flavonoids | Dioscoreae Rhizoma | [11] |
| 16 | 3.31 | 473.1089 | 473.1099 | 1.8 | 283.0606, 269.0459 | C23H22O11 | [M-H]− | 6"-0-acetylgenistin | Flavonoids | Dioscoreae Rhizoma | [11] |
| 17 | 3.45 | 299.0914 | 299.0922 | 0.6 | 445.1132, 299.0559 | C27H30O15 | [M-H]− | Mosloflavone | Flavonoids | Dioscoreae Rhizoma | [12] |
| 18 | 3.65 | 271.0601 | 271.0601 | 1 | 153.0181, 135.0804 | C15H10O6 | [M+H]+ | Apigenin | Flavonoids | Dioscoreae Rhizoma | [12] |
| 19 | 3.71 | 415.1035 | 415.1032 | 2.1 | 253.0506, 225.0543 | C21H20O9 | [M-H]− | Puerarin | Flavonoids | Dioscoreae Rhizoma | [12] |
| 24 | 4.65 | 148.1145 | 147.032 1 | −2.4 | 147.5896, 129.1459 | C7H6O5 | [M+H]+ | Malicacid4-Meester | Organic acids | fructus corni | [13] |
| 25 | 4.75 | 345.0969 | 345.0997 | 0.6 | 271.0623, 240.7008 | C28H32O15 | [M-H]− | Eupatilin | Flavonoids | Dioscoreae Rhizoma | [11] |
| 26 | 5.08 | 283.1464 | 284.30700 | 2.3 | 219.0919, 178.2154 | C17H16O4 | [M-H]− | Batatasin I | Flavonoids | Dioscoreae Rhizoma | [11] |
| 27 | 5.28 | 164.1584 | 163.047 8 | 1.4 | 105.6283, 119.3586 | C8H8O3 | [M+H]+ | ρ-coumaric acid | Phenolic acids | fructus corni | [14] |
| 28 | 5.75 | 454.3471 | 453.8904 | 0.5 | 453.3358, 112.9850 | C30H46O3 | [M+H]+ | Dehydrotrametenolic acid | Triterpenoids | fructus corni | [15] |
| 29 | 5.82 | 568.4567 | 567.147 8 | 2.1 | 567.1986, 521.1786 | C25H38O16 | [M+H]+ | Cormusglucoside F | Iridoid glycosides | fructus corni | [15] |
| 30 | 5.91 | 635.2186 | 635.3114 | 1.3 | 465.5152, 300.1463 | C27H14O18 | [M+H]+ | Trigalloylglucose | Phenolic acids | Moutan Cortex | [16] |
| 31 | 5.95 | 139.0397 | 140.4301 | 1.4 | 136.1463, 121.2544; | C7H6O3 | [M+H]+ | p-hydroxybenzoic acid | Phenolic acids | Moutan Cortex | [16] |
| 32 | 6.45 | 180.1574 | 179.034 0 | −0.9 | 179.0304, 149.0081 | C9H8O4 | [M+H]+ | Caffeic acid | Phenolic acids | fructus corni | [14] |
| 33 | 6.62 | 391.4725 | 390.0291 | 2.1 | 341.5409, 221.1036 | C17H26O10 | [M+H]+ | Loganin | Iridoid glycosides | fructus corni | [14] |
| 34 | 6.75 | 388.4512 | 387.1425 | −1.1 | 375.1286, 327.0721 | C19H30O9 | [M+H]+ | Cornin | Iridoid glycosides | fructus corni | [14] |
| 35 | 6.88 | 505.1558 | 510.2074 | −3.1 | 205.0356, 167.0704 | C20H28O12 | [M+H]+ | Paeonolide | Monoterpenoids | Moutan Cortex | [16] |
| 36 | 6.91 | 488.1477 | 487.9792 | −2 | 209.0472, 165.0523 | C15H20O8 | [M+H]+ | Apiopaeonoside | Monoterpenoids | Moutan Cortex | [16] |
| 37 | 7.15 | 388.3745 | 387.129 0 | 1.6 | 383.0439, 117.0354 | C19H30O9 | [M+H]+ | Ketologanin | Monoterpenoids | fructus corni | [15] |
| 38 | 7.18 | 523.1663 | 523.1663 | 0 | 323.0977, 199.0606 | C21H32O15 | [M+H]+ | Rehmannioside A | Iridoid glycosides | Rehmanniae Radix | [17] |
| 39 | 7.84 | 495.1519 | 495.1508 | −2.3 | 281.0662, 195.0654 | C23H28O12 | [M-H]− | Oxypaeoniflorin | Monoterpals | Moutan Cortex | [16] |
| 40 | 8.05 | 420.4125 | 419.1553 | −1.2 | 373.1494, 358.1269 | C18H28O11 | [M-H]− | 7-O-Methylmorroniside | Iridoid glycosides | fructus corni | [14] |
| 41 | 8.34 | 404.3642 | 403.1236 | −2.4 | 403.1240, 357.1191 | C17H24O11 | [M-H]− | Hastatoside | Iridoid glycosides | fructus corni | [14] |
| 42 | 8.72 | 404.3662 | 403.1246 | −2.4 | 225.0760, 179.0558 | C17H24O11 | [M-H]− | Secoxyloganin | Iridoid glycosides | fructus corni | [14] |
| 43 | 8.77 | 358.3412 | 357.1186 | 1.27 | 195.0656, 173.0449 | C16H22O9 | [M-H]− | Sweroside | Iridoid glycosides | fructus corni | [14] |
| 44 | 8.81 | 406.3824 | 405.1394 | −1.9 | 373.1133, 243.0863 | C17H26O11 | [M-H]− | Morroniside | Iridoid glycosides | fructus corni | [14] |
| 45 | 8.91 | 505.3531 | 505.4783 | 1.6 | 487.3445, 469.3329 | C30H48O6 | [M-H]− | Alismanol | Triterpenoids | Alismatis Rhizoma | [18] |
| 46 | 8.93 | 507.3591 | 507.6980 | 1.3 | 453.3214, 397.2099 | C30H50O6 | [M-H]− | 13,17-epoxyalisol A | Triterpenoids | Alismatis Rhizoma | [18] |
| 47 | 9.15 | 505.3510 | 504.6950 | −1.1 | 487.3408, 397.3415 | C30H48O6 | [M+H]+ | 16-oxo-alisol A | Triterpenoids | Alismatis Rhizoma | [18] |
| 48 | 9.21 | 685.2207 | 685.2191 | 2.3 | 505.1564, 179.0556 | C27H42O20 | [M+H]+ | Rehmannia D | Iridoid glycosides | Rehmanniae Radix | [17] |
| 49 | 9.31 | 390.3475 | 389.1083 | −1.3 | 389.1082, 345.1180 | C16H22O11 | [M-H]− | Secoxyloganic acid | Iridoid glycosides | fructus corni | [14] |
| 50 | 9.45 | 361.1125 | 361.1135 | −2.8 | 199.0603, 161.0441 | C15H22O10 | [M+H]+ | Monomelittoside | Iridoid glycosides | Rehmanniae Radix | [17] |
| 51 | 9.48 | 407.1187 | 407.7930 | 0.9 | 361.1046, 199.0909 | C30H46O6 | [M-H]− | Catalpol | Iridoid glycosides | Rehmanniae Radix | [17] |
| 52 | 9.61 | 523.1661 | 523.1663 | −0.4 | 463.1464, 343.1024 | C21H32O15 | [M+H]+ | Melittoside | Iridoid glycosides | Rehmanniae Radix | [17] |
| 53 | 10.11 | 510.5561 | 515.6617 | −1.6 | 509.1615, 479.1123 | C24H30O12 | [M+H]+ | Moudanpioside D | Monoterpals | Moutan Cortex | [16] |
| 54 | 10.15 | 221.1895 | 222.3460 | −2.2 | 203.1792, 161.1331 | C15H24O | [M+H]+ | Alismoxide | Triterpenoids | Alismatis Rhizoma | [18] |
| 55 | 10.31 | 611.1036 | 617.2146 | 1.5 | 445.0933, 343.1069 | C27H32O16 | [M-H]− | Suffruticoside D | Monoterpals | Moutan Cortex | [16] |
| 56 | 10.44 | 503.3362 | 503.5435 | −0.9 | 485.3254, 467.3106 | C30H46O6 | [M+H]+ | Dehydro-16-oxo-alisol A | Triterpenoids | Alismatis Rhizoma | [18] |
| 57 | 10.45 | 471.368 | 471.9631 | −0.9 | 471.3491, 453.3326 | C30H46O4 | [M-H]− | 24-deacetylalisol O | Triterpenoids | Alismatis Rhizoma | [18] |
| 58 | 10.95 | 515.6871 | 514.3679 | −0.9 | 453.3388, 337.2803 | C32H50O5 | [M+H]+ | 23-acetyl alisol B | Triterpenoids | Alismatis Rhizoma | [18] |
| 59 | 11.12 | 121.6761 | 122.8929 | 1.1 | 102.3516, 105.6456 | C7H6O2 | [M-H]− | Benzoic acid | Phenolic acids | Moutan Cortex | [16] |
| 60 | 11.28 | 509.1879 | 509.1870 | 1.8 | 449.1672, 179.0555 | C21H34O14 | [M+H]+ | Rehmannioside C | Iridoid glycosides | Rehmanniae Radix | [17] |
| 61 | 11.43 | 347.1335 | 347.1342 | −2 | 329.1227, 167.0704 | C15H24O9 | [M+H]+ | Leonuride | Phenolic glycosides | Rehmanniae Radix | [17] |
| 62 | 11.61 | 503.1623 | 508.1939 | −2.5 | 463.1592, 179.0691 | C23H28O11 | [M-H]− | Peoniflorin | Triterpenoids | Moutan Cortex | [16] |
| 63 | 11.65 | 461.1655 | 461.1659 | −0.9 | 161.0431, 135.0435 | C20H30O12 | [M+H]+ | decaffeoyl verbascoside | Phenolic glycosides | Rehmanniae Radix | [17] |
| 64 | 11.81 | 487.3416 | 488.2031 | −0.4 | 469.3312, 451.3205 | C30H46O5 | [M-H]− | Alisol C | Triterpenoids | Alismatis Rhizoma | [18] |
| 65 | 11.88 | 631.5481 | 637.8636 | −3.8 | 513.4863, 479.3541 | C30H32O15 | [M-H]− | Galonia paeoniflorin | Monoterpals | Moutan Cortex | [16] |
| 66 | 11.93 | 939.2481 | 948.6406 | −3.6 | 769.354, 617.6468 | C41H32O26 | [M-H]− | 5-Acetylglucose | Monoterpals | Moutan Cortex | [16] |
| 67 | 12.21 | 545.3466 | 545.3120 | −1.2 | 485.3242, 467.3166 | C33H48O7 | [M-H]− | Alisol M 23-acetate | Triterpenoids | Alismatis Rhizoma | [18] |
| 68 | 12.45 | 375.1281 | 375.1291 | −2.7 | 213.0756, 169.0857 | C16H24O10 | [M+H]+ | Loganic acid | Organic acids | Rehmanniae Radix | [17] |
| 69 | 12.60 | 615.2791 | 621.4319 | −0.1 | 431.6489, 281.5146 | C30H32O14 | [M+H]+ | Moudanpioside H | Monoterpals | Moutan Cortex | [19] |
| 70 | 12.63 | 600.3211 | 606.3243 | −2.2 | 551.3947, 447.3923 | C30H32O13 | [M+H]+ | Moudanpioside C | Monoterpals | Moutan Cortex | [19] |
| 71 | 12.98 | 629.5461 | 635.8416 | −1.7 | 599.3651, 507.6423 | C31H34O14 | [M+H]+ | Moudanpioside J | Monoterpals | Moutan Cortex | [19] |
| 72 | 13.01 | 487.3422 | 488.3174 | 0.9 | 469.3338, 451.3322 | C30H46O5 | [M-H]− | 16-oxo-11-anhydro-alisol A | Triterpenoids | Alismatis Rhizoma | [18] |
| 73 | 13.02 | 547.3626 | 547.6535 | −0.6 | 529.3511, 415.2823 | C33H50O7 | [M+H]+ | 16-oxo-alisol A-23-acetate | Triterpenoids | Alismatis Rhizoma | [18] |
| 74 | 13.53 | 791.2391 | 799.1514 | 0.8 | 623.1978, 593.18640 | C36H42O17 | [M+H]+ | Paeoniflorin B | Monoterpals | Moutan Cortex | [19] |
| 75 | 13.91 | 529.3519 | 528.6345 | −0.8 | 511.3408, 469.3311 | C32H48O6 | [M+H]+ | 23-Acetyl alisol C | Triterpenoids | Alismatis Rhizoma | [18] |
| 76 | 14.11 | 785.2512 | 785.2504 | 1 | 623.2196, 161.024 | C35H46O20 | [M+H]+ | Purpureaside C | Cardiac glycosides | Rehmanniae Radix | [17] |
| 77 | 14.15 | 390.3512 | 394.2547 | 1.2 | 327.1018, 151.0394 | C16H24O8 | [M+H]+ | Moudanpioside G | Monoterpals | Moutan Cortex | [19] |
| 78 | 14.31 | 315.0507 | 318.2012 | −3.5 | 300.0239, 283.0768 | C16H12O7 | [M+H]+ | Isorhamnetin | Flavonoids | Moutan Cortex | [19] |
| 79 | 14.51 | 387.1294 | 387.1291 | 0.8 | 358.1248, 225.0764 | C17H24O10 | [M+H]+ | Geniposide | Iridoid glycosides | Rehmanniae Radix | [17] |
| 80 | 14.91 | 167.0326 | 166.7029 | −0.3 | 152.0264, 122.0154 | C9H10O3 | [M+H]+ | Paeonol | Phenolic acid | Moutan Cortex | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Tao, M.; Tao, R.; Cao, M.; Wang, R. Tentative Identification of Chemical Constituents in Liuwei Dihuang Pills Based on UPLC-Orbitrap-MS. Metabolites 2025, 15, 561. https://doi.org/10.3390/metabo15080561
Yang L, Tao M, Tao R, Cao M, Wang R. Tentative Identification of Chemical Constituents in Liuwei Dihuang Pills Based on UPLC-Orbitrap-MS. Metabolites. 2025; 15(8):561. https://doi.org/10.3390/metabo15080561
Chicago/Turabian StyleYang, Lanxiang, Min Tao, Rongping Tao, Mingzhu Cao, and Rui Wang. 2025. "Tentative Identification of Chemical Constituents in Liuwei Dihuang Pills Based on UPLC-Orbitrap-MS" Metabolites 15, no. 8: 561. https://doi.org/10.3390/metabo15080561
APA StyleYang, L., Tao, M., Tao, R., Cao, M., & Wang, R. (2025). Tentative Identification of Chemical Constituents in Liuwei Dihuang Pills Based on UPLC-Orbitrap-MS. Metabolites, 15(8), 561. https://doi.org/10.3390/metabo15080561





