Dual Impact of Iron Deficiency and Antibiotics on Host Metabolism: A Tissue-Level Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglet Housing, Diet, and Antibiotic Treatment
2.2. Polar Metabolite Extraction
2.3. NMR Data Acquisition and Spectral Analysis
2.4. Hippocampal Protein Extraction and Quantification
2.5. Protein Expression Using Western Blot
2.6. Statistical Analysis
3. Results
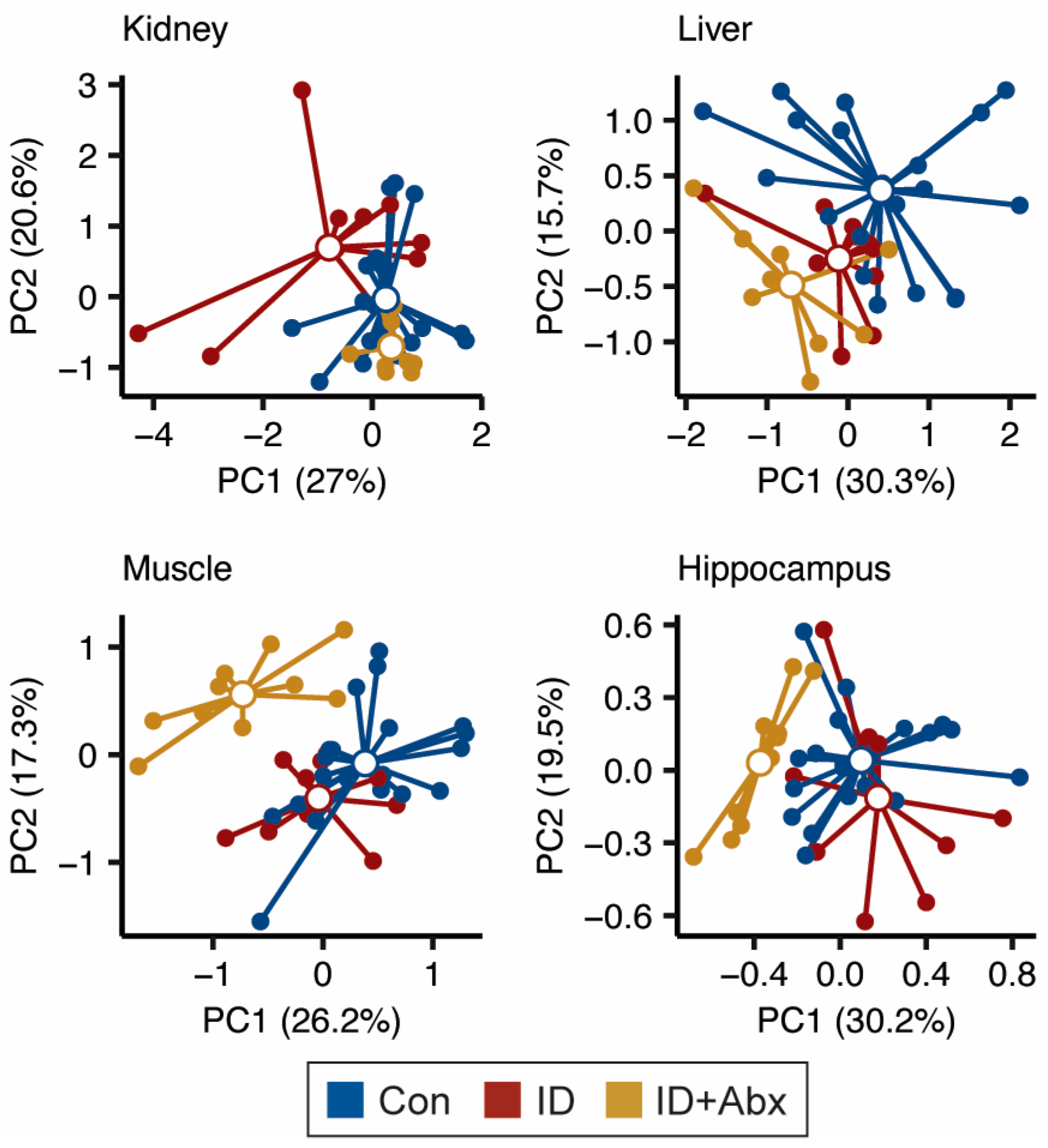
3.1. Impact of ID and ID + Abx on the Liver, Muscle, Kidney, and Hippocampal Metabolomes
3.2. Antibiotic Administration to Otherwise Healthy Piglets Has Minimal Impact
4. Discussion
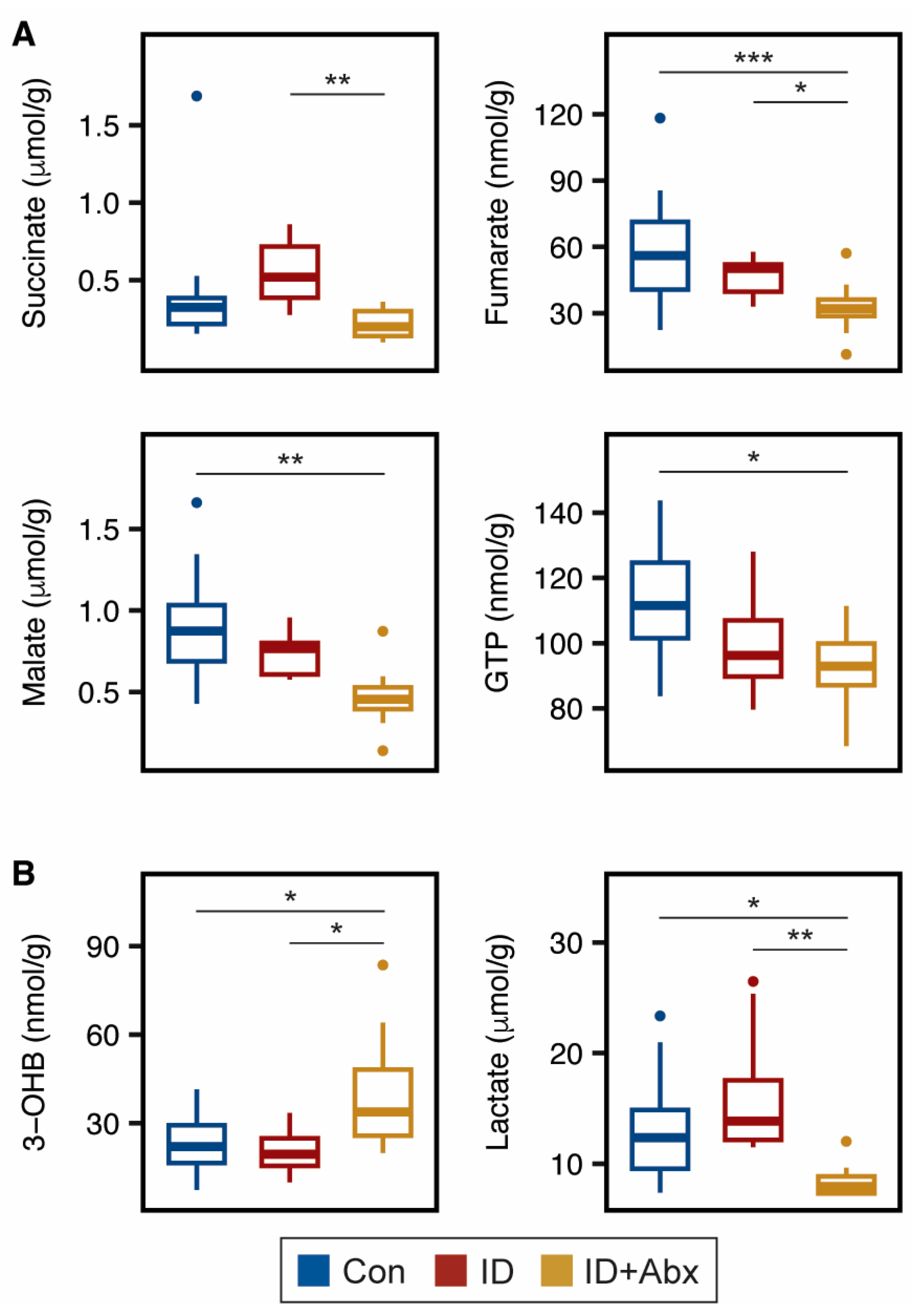
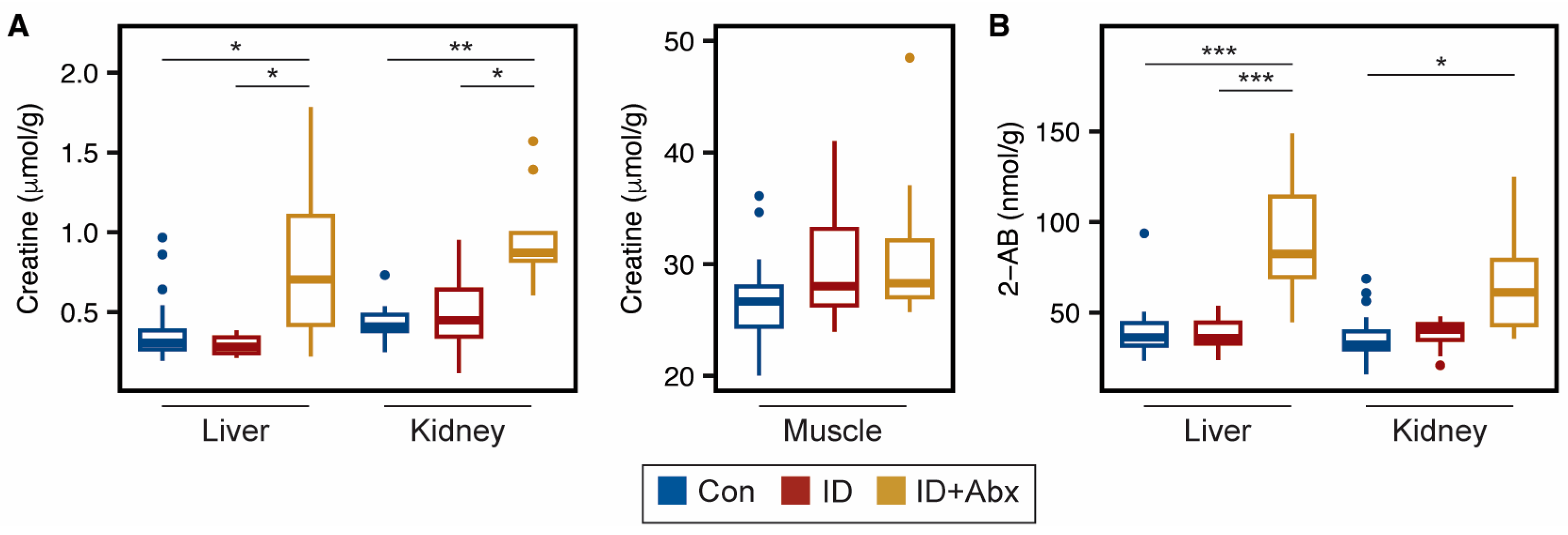
4.1. ID + Abx Negatively Influences Energy Metabolism and Oxidative Stress in Kidney, Liver, and Muscle
4.2. Hippocampal Energy Metabolism Is Impacted Differently in ID and ID + Abx
4.3. ID + Abx Has Greater Impact on Neurotransmitters in the Hippocampus than ID Alone
4.4. Hippocampal mTOR Signaling Is Upregulated in ID + Abx
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSS-d6 | (3-(trimethylsilyl)-1-propanesulfonic acid-d6 |
| FDR | False discovery rate |
| GABA | γ-Aminobutyric acid |
| Hb | Hemoglobin |
| ID | Iron deficiency |
| LMIC | Low- and middle-income countries |
| PCA | Principal components analysis |
| PD | Postnatal day |
References
- Gedfie, S.; Getawa, S.; Melku, M. Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis. Glob. Pediatr. Health 2022, 9, 2333794X221110860. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-Dependent Changes in Cellular Energy Metabolism: Influence on Citric Acid Cycle and Oxidative Phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- de Ungria, M.; Rao, R.; Wobken, J.D.; Luciana, M.; Nelson, C.A.; Georgieff, M.K. Perinatal Iron Deficiency Decreases Cytochrome c Oxidase (CytOx) Activity in Selected Regions of Neonatal Rat Brain. Pediatr. Res. 2000, 48, 169–176. [Google Scholar] [CrossRef]
- Carlson, E.S.; Stead, J.D.H.; Neal, C.R.; Petryk, A.; Georgieff, M.K. Perinatal Iron Deficiency Results in Altered Developmental Expression of Genes Mediating Energy Metabolism and Neuronal Morphogenesis in Hippocampus. Hippocampus 2007, 17, 679–691. [Google Scholar] [CrossRef]
- Klempa, K.L.; Willis, W.T.; Chengson, R.; Dallman, P.R.; Brooks, G.A. Iron Deficiency Decreases Gluconeogenesis in Isolated Rat Hepatocytes. J. Appl. Physiol. 1989, 67, 1868–1872. [Google Scholar] [CrossRef]
- Borel, M.J.; Beard, J.L.; Farrell, P.A. Hepatic Glucose Production and Insulin Sensitivity and Responsiveness in Iron-Deficient Anemic Rats. Am. J. Physiol. 1993, 264, E380–E390. [Google Scholar] [CrossRef] [PubMed]
- Masini, A.; Salvioli, G.; Cremonesi, P.; Botti, B.; Gallesi, D.; Ceccarelli, D. Dietary Iron Deficiency in the Rat. I. Abnormalities in Energy Metabolism of the Hepatic Tissue. Biochim. Biophys. Acta 1994, 1188, 46–52. [Google Scholar] [CrossRef]
- Knutson, M.D.; Walter, P.B.; Ames, B.N.; Viteri, F.E. Both Iron Deficiency and Daily Iron Supplements Increase Lipid Peroxidation in Rats. J. Nutr. 2000, 130, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, T.; Weil, S.; Weis, S.; Vollmar, C.; Heiss, D.; Egger, J.; Scheck, R.; Hahn, K. Normative Volumetric Data of the Developing Hippocampus in Children Based on Magnetic Resonance Imaging. Epilepsia 1999, 40, 414–423. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Takano, K.; Okazaki, M.; Mitsudome, A. Development of the Temporal Lobe in Infants and Children: Analysis by MR-Based Volumetry. AJNR Am. J. Neuroradiol. 1999, 20, 717–723. [Google Scholar] [PubMed]
- Fretham, S.J.B.; Carlson, E.S.; Georgieff, M.K. The Role of Iron in Learning and Memory. Adv. Nutr. 2011, 2, 112–121. [Google Scholar] [CrossRef]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-Lasting Neural and Behavioral Effects of Iron Deficiency in Infancy. Nutr. Rev. 2006, 64, 34–43. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and Mechanisms of Emotional Behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Bastian, T.W.; Rao, R.; Tran, P.V.; Georgieff, M.K. The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. Neurosci. Insights 2020, 15, 2633105520935104. [Google Scholar] [CrossRef]
- Jorgenson, L.A.; Wobken, J.D.; Georgieff, M.K. Perinatal Iron Deficiency Alters Apical Dendritic Growth in Hippocampal CA1 Pyramidal Neurons. Dev. Neurosci. 2004, 25, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Tkac, I.; Schmidt, A.T.; Georgieff, M.K. Fetal and Neonatal Iron Deficiency Causes Volume Loss and Alters the Neurochemical Profile of the Adult Rat Hippocampus. Nutr. Neurosci. 2013, 14, 59–65. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and Immunity: Immunological Consequences of Iron Deficiency and Overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Choudhry, V.P. Iron Deficiency and Infection. Indian J. Pediatr. 2010, 77, 789–793. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Drakesmith, H. Effects of Iron Status on Adaptive Immunity and Vaccine Efficacy: A Review. Adv. Nutr. 2024, 15, 100238. [Google Scholar] [CrossRef]
- Oppenheimer, S.J. Iron and Its Relation to Immunity and Infectious Disease. J. Nutr. 2001, 131, 616S–635S. [Google Scholar] [CrossRef]
- Fink, G.; D’Acremont, V.; Leslie, H.H.; Cohen, J. Antibiotic Exposure among Children Younger than 5 Years in Low-Income and Middle-Income Countries: A Cross-Sectional Study of Nationally Representative Facility-Based and Household-Based Surveys. Lancet Infect. Dis. 2020, 20, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, C.; Moretti, R.; Rebuzzi, L.; Albergati, I.V.; Somma, A.D.; Decorti, G.; Bella, S.D.; Crocè, L.S.; Giuffrè, M. Antibiotics and Liver Cirrhosis: What the Physicians Need to Know. Antibiotics 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Saban, J.A.; Pizzi, M.; Caldwell, J.; Palijan, A.; Zappitelli, M. Previous Aminoglycoside Use and Acute Kidney Injury Risk in Non-Critically Ill Children. Pediatr. Nephrol. 2017, 32, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.S.; Johnson, R.W. The Domestic Piglet: An Important Model for Investigating the Neurodevelopmental Consequences of Early Life Insults. Annu. Rev. Anim. Biosci. 2015, 3, 245–264. [Google Scholar] [CrossRef]
- Lunney, J.K.; Goor, A.V.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- McClorry, S.; Ji, P.; Parenti, M.G.; Slupsky, C.M. Antibiotics Augment the Impact of Iron Deficiency on Metabolism in a Piglet Model. J. Nutr. Biochem. 2023, 119, 109405. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Otoki, Y.; McClorry, S.; Coates, L.C.; Lombardi, R.L.; Taha, A.Y.; Slupsky, C.M. Optimization of a Method for the Simultaneous Extraction of Polar and Non-Polar Oxylipin Metabolites, DNA, RNA, Small RNA, and Protein from a Single Small Tissue Sample. Methods Protoc. 2020, 3, 61. [Google Scholar] [CrossRef]
- Perng, V.; Li, C.; Klocke, C.R.; Navazesh, S.E.; Pinneles, D.K.; Lein, P.J.; Ji, P. Iron Deficiency and Iron Excess Differently Affect Dendritic Architecture of Pyramidal Neurons in the Hippocampus of Piglets. J. Nutr. 2021, 151, 235–244. [Google Scholar] [CrossRef]
- Geis, L.; Kurtz, A. Oxygen Sensing in the Kidney. Nephrol. Dial. Transplant. 2024, 40, 446–454. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef]
- Lichter-Konecki, U.; Hipke, C.M.; Konecki, D.S. Human Phenylalanine Hydroxylase Gene Expression in Kidney and Other Nonhepatic Tissues. Mol. Genet. Metab. 1999, 67, 308–316. [Google Scholar] [CrossRef]
- Beinert, H.; Kennedy, M.C. Aconitase, a Two-Faced Protein: Enzyme and Iron Regulatory Factor. FASEB J. 1993, 7, 1442–1449. [Google Scholar] [CrossRef]
- Dallman, P.R. Biochemical Basis for the Manifestations of Iron Deficiency. Annu. Rev. Nutr. 1986, 6, 13–40. [Google Scholar] [CrossRef]
- Balakumar, P.; Rohilla, A.; Thangathirupathi, A. Gentamicin-Induced Nephrotoxicity: Do We Have a Promising Therapeutic Approach to Blunt It? Pharmacol. Res. 2010, 62, 179–186. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Sajid, M.I.; Nunez, F.J.; Amirrad, F.; Roosan, M.R.; Vojtko, T.; McCulloch, S.; Alachkar, A.; Nauli, S.M. Untargeted Metabolomics Analysis on Kidney Tissues from Mice Reveals Potential Hypoxia Biomarkers. Sci. Rep. 2023, 13, 17516. [Google Scholar] [CrossRef]
- Irino, Y.; Toh, R.; Nagao, M.; Mori, T.; Honjo, T.; Shinohara, M.; Tsuda, S.; Nakajima, H.; Satomi-Kobayashi, S.; Shinke, T.; et al. 2-Aminobutyric Acid Modulates Glutathione Homeostasis in the Myocardium. Sci. Rep. 2016, 6, 36749. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential Metabolomics Reveals Ophthalmic Acid as an Oxidative Stress Biomarker Indicating Hepatic Glutathione Consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef] [PubMed]
- Schomakers, B.V.; Jillings, S.L.; van Weeghel, M.; Vaz, F.M.; Salomons, G.S.; Janssens, G.E.; Houtkooper, R.H. Ophthalmic Acid Is a Glutathione Regulating Tripeptide. FEBS J. 2024, 291, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Henderson, S.A.; Dallman, P.R. Increased Glucose Dependence in Resting, Iron-Deficient Rats. Am. J. Physiol. 1987, 253, E461–E466. [Google Scholar] [CrossRef] [PubMed]
- Isasi, E.; Olivera-Bravo, S. Neurovascular Unit Impairment in Iron Deficiency Anemia. Neuroscience 2025, 567, 56–66. [Google Scholar] [CrossRef]
- Segal, J.A.; Harris, B.D.; Kustova, Y.; Basile, A.; Skolnick, P. Aminoglycoside Neurotoxicity Involves NMDA Receptor Activation. Brain Res. 1999, 815, 270–277. [Google Scholar] [CrossRef]
- Raman, L.; Tkac, I.; Ennis, K.; Georgieff, M.K.; Gruetter, R.; Rao, R. In Vivo Effect of Chronic Hypoxia on the Neurochemical Profile of the Developing Rat Hippocampus. Dev. Brain Res. 2005, 156, 202–209. [Google Scholar] [CrossRef]
- Rao, R.; Tkac, I.; Townsend, E.L.; Gruetter, R.; Georgieff, M.K. Perinatal Iron Deficiency Alters the Neurochemical Profile of the Developing Rat Hippocampus. J. Nutr. 2003, 133, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.L.; Tkac, I.; Jing, Y.; Felt, B.; Beard, J.; Connor, J.; Schallert, T.; Georgieff, M.K.; Rao, R. Gestational and Lactational Iron Deficiency Alters the Developing Striatal Metabolome and Associated Behaviors in Young Rats1. J. Nutr. 2007, 137, 1043–1049. [Google Scholar] [CrossRef]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer Behavioral and Developmental Outcome More Than 10 Years After Treatment for Iron Deficiency in Infancy. Pediatrics 2000, 105, e51. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early Exposure to Antibiotic Drugs and Risk for Psychiatric Disorders: A Population-Based Study. Transl. Psychiatry 2019, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B. Early Iron Deficiency Has Brain and Behavior Effects Consistent with Dopaminergic Dysfunction1–3. J. Nutr. 2011, 141, S740–S746. [Google Scholar] [CrossRef]
- Ganesan, A.; Mohammadi, N.; Wang, F. From Building Blocks of Proteins to Drugs: A Quantum Chemical Study on Structure–Property Relationships of Phenylalanine, Tyrosine and Dopa. RSC Adv. 2014, 4, 8617–8626. [Google Scholar] [CrossRef]
- Petty, F. GABA and Mood Disorders: A Brief Review and Hypothesis. J. Affect. Disord. 1995, 34, 275–281. [Google Scholar] [CrossRef]
- Curt, M.J.-C.; Voicu, P.-M.; Fontaine, M.; Dessein, A.-F.; Porchet, N.; Mention-Mulliez, K.; Dobbelaere, D.; Soto-Ares, G.; Cheillan, D.; Vamecq, J. Creatine Biosynthesis and Transport in Health and Disease. Biochimie 2015, 119, 146–165. [Google Scholar] [CrossRef]
- Huang, Q.; Liao, C.; Ge, F.; Ao, J.; Liu, T. Acetylcholine Bidirectionally Regulates Learning and Memory. J. Neurorestoratology 2022, 10, 100002. [Google Scholar] [CrossRef]
- Antonides, A.; Schoonderwoerd, A.C.; Scholz, G.; Berg, B.M.; Nordquist, R.E.; van der Staay, F.J. Pre-Weaning Dietary Iron Deficiency Impairs Spatial Learning and Memory in the Cognitive Holeboard Task in Piglets. Front. Behav. Neurosci. 2015, 9, 291. [Google Scholar] [CrossRef]
- Rytych, J.L.; Elmore, M.R.P.; Burton, M.D.; Conrad, M.S.; Donovan, S.M.; Dilger, R.N.; Johnson, R.W. Early Life Iron Deficiency Impairs Spatial Cognition in Neonatal Piglets. J. Nutr. 2012, 142, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.C.; Dimova, J.G.; Siddappa, A.J.M.; Tran, P.V.; Gewirtz, J.C.; Georgieff, M.K. Prenatal Choline Supplementation Ameliorates the Long-Term Neurobehavioral Effects of Fetal-Neonatal Iron Deficiency in Rats. J. Nutr. 2014, 144, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Kennedy, B.C.; Pisansky, M.T.; Won, K.-J.; Gewirtz, J.C.; Simmons, R.A.; Georgieff, M.K. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency–Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus. J. Nutr. 2016, 146, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.C.; Tran, P.V.; Kohli, M.; Maertens, J.J.; Gewirtz, J.C.; Georgieff, M.K. Beneficial Effects of Postnatal Choline Supplementation on Long-Term Neurocognitive Deficit Resulting from Fetal-Neonatal Iron Deficiency. Behav. Brain Res. 2018, 336, 40–43. [Google Scholar] [CrossRef]
- Fiekers, J.F. Effects of the Aminoglycoside Antibiotics, Streptomycin and Neomycin, on Neuromuscular Transmission. I. Presynaptic Considerations. J. Pharmacol. Exp. Ther. 1983, 225, 487–495. [Google Scholar] [CrossRef]
- Tyler, W.A.; Gangoli, N.; Gokina, P.; Kim, H.A.; Covey, M.; Levison, S.W.; Wood, T.L. Activation of the Mammalian Target of Rapamycin (MTOR) Is Essential for Oligodendrocyte Differentiation. J. Neurosci. 2009, 29, 6367–6378. [Google Scholar] [CrossRef]
- Tyler, W.A.; Jain, M.R.; Cifelli, S.E.; Li, Q.; Ku, L.; Feng, Y.; Li, H.; Wood, T.L. Proteomic Identification of Novel Targets Regulated by the Mammalian Target of Rapamycin Pathway during Oligodendrocyte Differentiation. Glia 2011, 59, 1754–1769. [Google Scholar] [CrossRef]
- Bercury, K.K.; Dai, J.; Sachs, H.H.; Ahrendsen, J.T.; Wood, T.L.; Macklin, W.B. Conditional Ablation of Raptor or Rictor Has Differential Impact on Oligodendrocyte Differentiation and CNS Myelination. J. Neurosci. 2014, 34, 4466–4480. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.E.; McLane, L.E.; Bercury, K.K.; Macklin, W.B.; Wood, T.L. Mammalian Target of Rapamycin Promotes Oligodendrocyte Differentiation, Initiation and Extent of CNS Myelination. J. Neurosci. 2014, 34, 4453–4465. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Flores, A.I.; Wang, F.; Macklin, W.B. AKT Signals through the Mammalian Target of Rapamycin Pathway to Regulate CNS Myelination. J. Neurosci. 2009, 29, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Julien, F.; Bachmann, L.; Norrmen, C.; Trotzmuller, M.; Kofeler, H.; Ruegg, M.A.; Hall, M.N.; Suter, U. Balanced MTORC1 Activity in Oligodendrocytes Is Required for Accurate CNS Myelination. J. Neurosci. 2014, 34, 8432–8448. [Google Scholar] [CrossRef]
- Ehninger, D.; Han, S.; Shilyansky, C.; Zhou, Y.; Li, W.; Kwiatkowski, D.J.; Ramesh, V.; Silva, A.J. Reversal of Learning Deficits in a Tsc2+/− Mouse Model of Tuberous Sclerosis. Nat. Med. 2008, 14, 843–848. [Google Scholar] [CrossRef]
- Norrmén, C.; Suter, U. Akt/MTOR Signalling in Myelination. Biochem. Soc. Trans. 2013, 41, 944–950. [Google Scholar] [CrossRef]
- Fretham, S.J.B.; Carlson, E.S.; Georgieff, M.K. Neuronal-Specific Iron Deficiency Dysregulates Mammalian Target of Rapamycin Signaling during Hippocampal Development in Nonanemic Genetic Mouse Models. J. Nutr. 2013, 143, 260–266. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. A Complex Interplay between Akt, TSC2 and the Two MTOR Complexes. Biochem. Soc. Trans. 2009, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Kobayashi, C.; Hamamura, R.; Okabe, S.; Tauchi, T.; Ohyashiki, K. The Oral Iron Chelator Deferasirox Represses Signaling through the MTOR in Myeloid Leukemia Cells by Enhancing Expression of REDD1. Cancer Sci. 2009, 100, 970–977. [Google Scholar] [CrossRef]
- Ndong, M.; Kazami, M.; Suzuki, T.; Uehara, M.; Katsumata, S.; Inoue, H.; Kobayashi, K.-I.; Tadokoro, T.; Suzuki, K.; Yamamoto, Y. Iron Deficiency Down-Regulates the Akt/TSC1-TSC2/Mammalian Target of Rapamycin Signaling Pathway in Rats and in COS-1 Cells. Nutr. Res. 2009, 29, 640–647. [Google Scholar] [CrossRef]
- Wallin, D.J.; Zamora, T.G.; Alexander, M.; Ennis, K.M.; Tran, P.V.; Georgieff, M.K. Neonatal Mouse Hippocampus: Phlebotomy-Induced Anemia Diminishes and Treatment with Erythropoietin Partially Rescues Mammalian Target of Rapamycin Signaling. Pediatr. Res. 2017, 82, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Fretham, S.J.B.; Wobken, J.; Miller, B.S.; Georgieff, M.K. Gestational-Neonatal Iron Deficiency Suppresses and Iron Treatment Reactivates IGF Signaling in Developing Rat Hippocampus. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E316–E324. [Google Scholar] [CrossRef]
- Ye, Y.; Tong, H.Y.K.; Chong, W.H.; Li, Z.; Tam, P.K.H.; Baptista-Hon, D.T.; Monteiro, O. A Systematic Review and Meta-Analysis of the Effects of Long-Term Antibiotic Use on Cognitive Outcomes. Sci. Rep. 2024, 14, 4026. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Activating Akt and the Brain’s Resources to Drive Cellular Survival and Prevent Inflammatory Injury. Histol. Histopathol. 2005, 20, 299–315. [Google Scholar] [CrossRef]
- Shekhar, S.; Petersen, F.C. The Dark Side of Antibiotics: Adverse Effects on the Infant Immune Defense Against Infection. Front. Pediatr. 2020, 8, 544460. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Liang, X.; Zhu, L.; Fang, Y.; Dong, L.; Zheng, Y.; Xu, X.; Li, M.; Cai, T.; et al. A Decrease in Flavonifractor Plautii and Its Product, Phytosphingosine, Predisposes Individuals with Phlegm-Dampness Constitution to Metabolic Disorders. Cell Discov. 2025, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Champagne-Jorgensen, K.; Kunze, W.A.; Forsythe, P.; Bienenstock, J.; Neufeld, K.-A.M. Antibiotics and the Nervous System: More than Just the Microbes? Brain Behav. Immun. 2019, 77, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.F.; Maganti, R.K. Neurotoxic Effects Associated with Antibiotic Use: Management Considerations. Br. J. Clin. Pharmacol. 2011, 72, 381–393. [Google Scholar] [CrossRef]
- Sirén, A.-L.; Fratelli, M.; Brines, M.; Goemans, C.; Casagrande, S.; Lewczuk, P.; Keenan, S.; Gleiter, C.; Pasquali, C.; Capobianco, A.; et al. Erythropoietin Prevents Neuronal Apoptosis after Cerebral Ischemia and Metabolic Stress. Proc. Natl. Acad. Sci. USA 2001, 98, 4044–4049. [Google Scholar] [CrossRef]
- Mehrjerdi, F.Z.; Aboutaleb, N.; Habibey, R.; Ajami, M.; Soleimani, M.; Arabian, M.; Niknazar, S.; Davoodi, S.H.; Pazoki-Toroudi, H. Increased Phosphorylation of MTOR Is Involved in Remote Ischemic Preconditioning of Hippocampus in Mice. Brain Res. 2013, 1526, 94–101. [Google Scholar] [CrossRef]
- Khaksari, M.; Mehrjerdi, F.Z.; Rezvani, M.E.; Safari, F.; Mirgalili, A.; Niknazar, S. The Role of Erythropoietin in Remote Renal Preconditioning on Hippocampus Ischemia/Reperfusion Injury. J. Physiol. Sci. 2017, 67, 163–171. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, S.J.; Antoine, D.J.; Smyth, R.L.; Pirmohamed, M. Aminoglycoside-Induced Nephrotoxicity in Children. Pediatr. Nephrol. 2017, 32, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Web Annex A. World Health Organization Model List of Essential Medicines–23rd List, 2023. In The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoff, S.; Thomas, S.; Ji, P.; Parenti, M.; Slupsky, C.M. Dual Impact of Iron Deficiency and Antibiotics on Host Metabolism: A Tissue-Level Analysis. Metabolites 2025, 15, 549. https://doi.org/10.3390/metabo15080549
Shoff S, Thomas S, Ji P, Parenti M, Slupsky CM. Dual Impact of Iron Deficiency and Antibiotics on Host Metabolism: A Tissue-Level Analysis. Metabolites. 2025; 15(8):549. https://doi.org/10.3390/metabo15080549
Chicago/Turabian StyleShoff, Shannon, Sydney Thomas, Peng Ji, Mariana Parenti, and Carolyn M. Slupsky. 2025. "Dual Impact of Iron Deficiency and Antibiotics on Host Metabolism: A Tissue-Level Analysis" Metabolites 15, no. 8: 549. https://doi.org/10.3390/metabo15080549
APA StyleShoff, S., Thomas, S., Ji, P., Parenti, M., & Slupsky, C. M. (2025). Dual Impact of Iron Deficiency and Antibiotics on Host Metabolism: A Tissue-Level Analysis. Metabolites, 15(8), 549. https://doi.org/10.3390/metabo15080549





