Postprandial Cardiometabolic Parameters in Older Adults with Normal-Weight Obesity: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Initial Assessment
2.3. Fat Tolerance Test
2.4. Body Composition Assessment
2.5. Biochemical Analyses
2.6. Physical Activity and Diet Assessments
2.7. Statistical Analyses
3. Results
3.1. Participant Characteristics
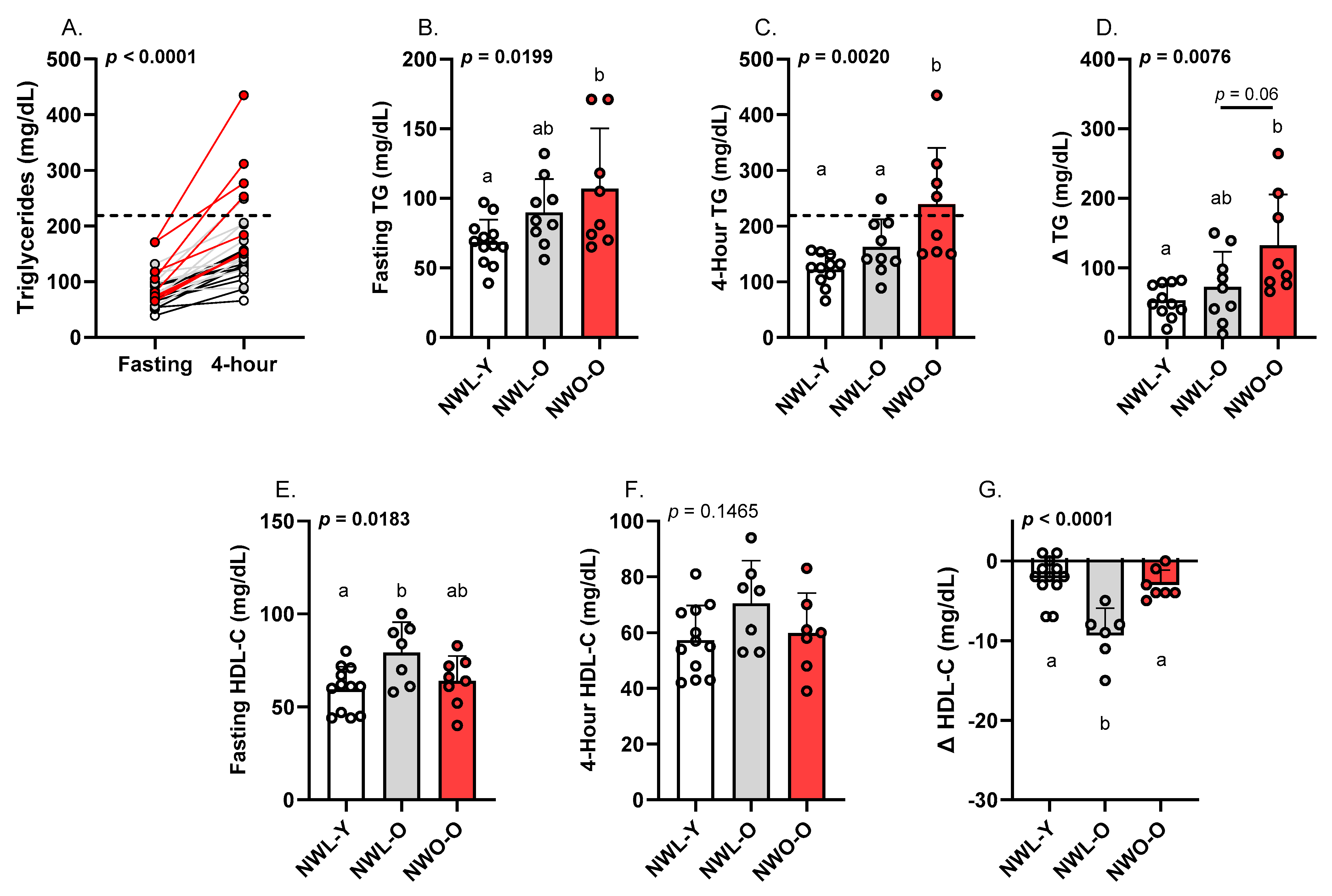
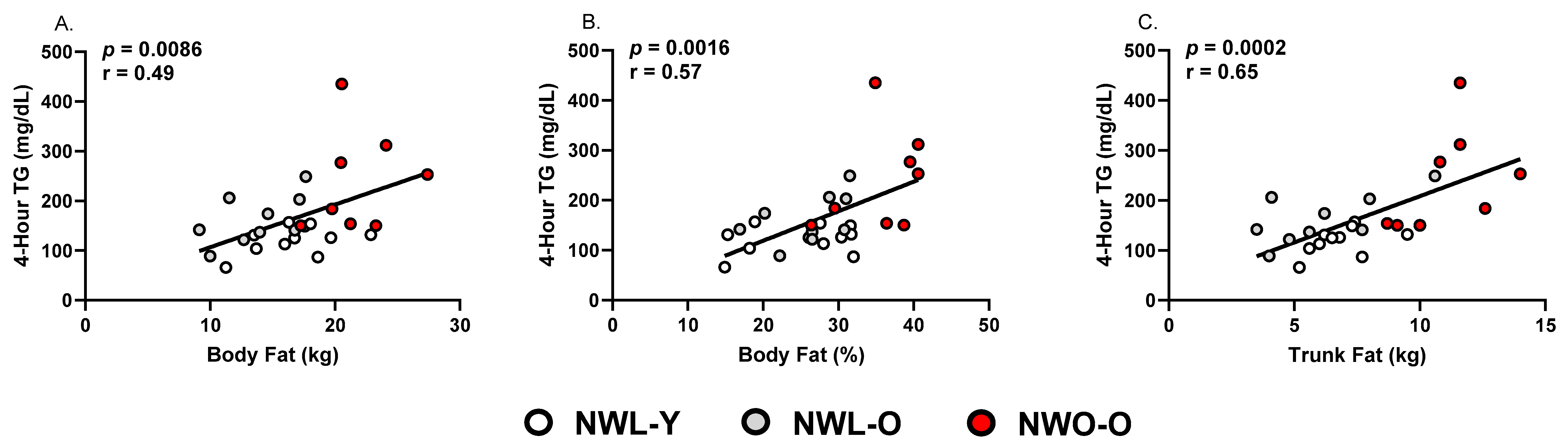
3.2. Fasting and Postprandial Triglycerides and HDL-C
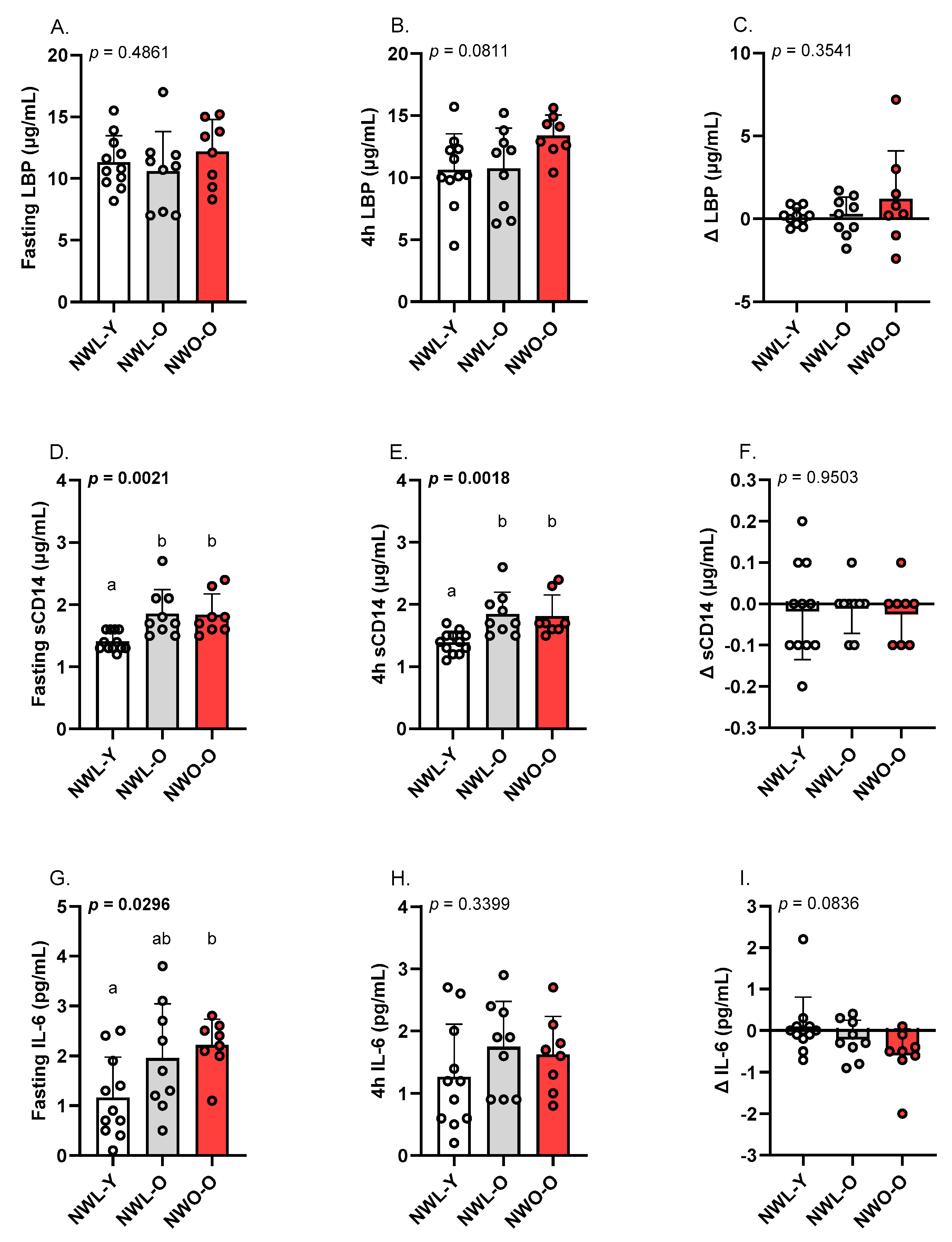
3.3. Fasting and Postprandial LBP, sCD14, and IL-6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joynt Maddox, K.E.; Elkind, M.S.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the burden of cardiovascular disease and stroke in the United States through 2050—Prevalence of risk factors and disease: A presidential advisory from the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Wijayatunga, N.N.; Dhurandhar, E.J. Normal weight obesity and unaddressed cardiometabolic health risk—A narrative review. Int. J. Obes. 2021, 45, 2141–2155. [Google Scholar] [CrossRef]
- Mohammadian Khonsari, N.; Khashayar, P.; Shahrestanaki, E.; Kelishadi, R.; Mohammadpoor Nami, S.; Heidari-Beni, M.; Esmaeili Abdar, Z.; Tabatabaei-Malazy, O.; Qorbani, M. Normal weight obesity and cardiometabolic risk factors: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 857930. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef]
- Di Renzo, L.; Galvano, F.; Orlandi, C.; Bianchi, A.; Di Giacomo, C.; La Fauci, L.; Acquaviva, R.; De Lorenzo, A. Oxidative stress in normal-weight obese syndrome. Obesity 2010, 18, 2125–2130. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Lopez-Jimenez, F.; Bartels, S.J. Normal-weight obesity and disability in older adults: Data from the National Health and Nutrition Examination study 1999–2004. J. Am. Geriatr. Soc. 2016, 64, 1367–1368. [Google Scholar] [CrossRef]
- Kruger, J. How Active Are Older Americans? 2007. Available online: https://www.cdc.gov/pcd//issues/2007/jul/06_0094.htm (accessed on 15 May 2025).
- United States Census-Quick Facts. 2024. Available online: https://data.census.gov/ (accessed on 15 May 2025).
- Batsis, J.A.; Sahakyan, K.R.; Rodriguez-Escudero, J.P.; Bartels, S.J.; Somers, V.K.; Lopez-Jimenez, F. Normal weight obesity and mortality in United States subjects ≥ 60 years of age (from the Third National Health and Nutrition Examination Survey). Am. J. Cardiol. 2013, 112, 1592–1598. [Google Scholar] [CrossRef]
- Keirns, B.H.; Hart, S.M.; Sciarrillo, C.M.; Poindexter, K.L.; Clarke, S.L.; Emerson, S.R. Postprandial triglycerides, endothelial function, and inflammatory cytokines as potential candidates for early risk detection in normal-weight obesity. Obes. Res. Clin. Pract. 2022, 16, 386–392. [Google Scholar] [CrossRef]
- Ji, T.; Zhang, L.; Tang, Z.; Sun, F.; Li, Y.; Ma, L. Prevalence of Normal-weight obesity in community-dwelling Chinese older adults: Results from the Beijing longitudinal study of aging. Diabetes Metab. Syndr. Obes. 2020, 13, 1611–1617. [Google Scholar] [CrossRef]
- Batsis, J.A.; Sahakyan, K.R.; Rodriguez-Escudero, J.P.; Bartels, S.J.; Lopez-Jimenez, F. Normal weight obesity and functional outcomes in older adults. Eur. J. Intern. Med. 2014, 25, 517–522. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef]
- Keirns, B.H.; Sciarrillo, C.M.; Koemel, N.A.; Emerson, S.R. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci. 2021, 10, e75. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Jia, A.; Xu, S.; Xing, Y.; Zhang, W.; Yu, X.; Zhao, Y.; Ming, J.; Ji, Q. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: A nationwide study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1045–1053. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Watts, G.F.; Mikhailidis, D.P.; Pérez-Martínez, P.; Mora, S.; Bilianou, H.; Panotopoulos, G.; Katsiki, N.; Ooi, T.C.; Lopez-Miranda, J.; et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profiles: Executive summary of a 2019 expert panel statement. Curr. Vasc. Pharmacol. 2019, 17, 538–540. [Google Scholar] [CrossRef]
- Sciarrillo, C.M.; Koemel, N.A.; Kurti, S.P.; Emerson, S.R. Validity of an abbreviated, clinically feasible test for postprandial lipemia in healthy adults: A randomized cross-over study. Nutrients 2019, 11, 180. [Google Scholar] [CrossRef]
- Ross, R.; Berentzen, T.; Bradshaw, A.J.; Janssen, I.; Kahn, H.S.; Katzmarzyk, P.T.; Kuk, J.L.; Seidell, J.C.; Snijder, M.B.; Sørensen, T.I.A.; et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes. Rev. 2008, 9, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef]
- Teeman, C.S.; Kurti, S.P.; Cull, B.J.; Emerson, S.R.; Haub, M.D.; Rosenkranz, S.K. Postprandial lipemic and inflammatory responses to high-fat meals: A review of the roles of acute and chronic exercise. Nutr. Metab. 2016, 13, 80. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Camargo, A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Garcia-Carpintero, S.; Lopez-Moreno, J.; Blanco-Rojo, R.; Delgado-Lista, J.; Perez-Martinez, P.; van Ommen, B.; et al. Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: From the CORDIOPREV study. Clin. Nutr. 2019, 38, 529–538. [Google Scholar] [CrossRef]
- Quirk, A.R.; Schifferer, J.K.; Maki, K.A.; Robinson, A.T.; Keirns, B.H. Biomarkers of intestinal permeability are linked to incident cardiovascular diseases and cardiovascular events: A review of prospective studies. Am. J. Physiol.-Gastrointest. Liver Physiol. 2025, 329, G79–G87. [Google Scholar] [CrossRef]
- Lundman, P.; Boquist, S.; Samnegård, A.; Bennermo, M.; Held, C.; Ericsson, C.G.; Silveira, A.; Hamsten, A.; Tornvall, P. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 195–202. [Google Scholar] [CrossRef]
- Schwander, F.; Kopf-Bolanz, K.A.; Buri, C.; Portmann, R.; Egger, L.; Chollet, M.; McTernan, P.G.; Piya, M.K.; Gijs, M.A.; Vionnet, N.; et al. A Dose-Response Strategy Reveals Differences between Normal-Weight and Obese Men in Their Metabolic and Inflammatory Responses to a High-Fat Meal. J. Nutr. 2014, 144, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup; Teng, K.-T.; Raff, M. Dietary cocoa butter or refined olive oil does not alter postprandial hsCRP and IL-6 concentrations in healthy women. Lipids 2011, 46, 365–370. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: A systematic review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Wong, X.; Madrid, A.M.; Tralma, K.; Castillo, R.; Carrasco-Pozo, C.; Navarrete, P.; Beltrán, C.; Pastene, E.; Gotteland, M. Polyphenol extracts interfere with bacterial lipopolysaccharide in vitro and decrease postprandial endotoxemia in human volunteers. J. Funct. Foods 2016, 26, 406–417. [Google Scholar] [CrossRef]
- Reiner, A.P.; Lange, E.M.; Jenny, N.S.; Chaves, P.H.; Ellis, J.; Li, J.; Walston, J.; Lange, L.A.; Cushman, M.; Tracy, R.P. Soluble CD14: Genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 158–164. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | NWL-Y (n = 12) | NWL-O (n = 9) | NWO-O (n = 8) | p-Value |
|---|---|---|---|---|
| General/Body Composition | ||||
| Age (years) | 21.0 ± 3.0 a | 71.0 ± 6.0 b | 67.0 ± 7.0 b | <0.0001 |
| Sex (M/F) | 4/8 | 2/7 | 2/6 | 0.5514 |
| Ethnicity (White/People of Color) | 11/1 | 9/0 | 7/1 | 0.2950 |
| Systolic BP (mmHg) | 112.0 ± 13.0 | 124.0 ± 17.0 | 128.0 ± 17.0 | 0.0695 |
| Diastolic BP (mmHg) | 72.0 ± 8.0 | 75.0 ± 8.0 | 78.0 ± 5.0 | 0.2384 |
| BMI (kg/m2) | 22.5 ± 1.6 | 20.9 ± 2.1 | 22.8 ± 1.5 | 0.0671 |
| WC (cm) | 75.9 ± 6.1 (65.7–83.5) | 77.5 ± 10.4 (67.9–97.8) | 84.2 ± 7.4 (71.5–92.5) | 0.0955 |
| WHtR | 0.43 ± 0.03 a (0.39–0.48) | 0.48 ± 0.03 ab (0.42–0.58) | 0.51 ± 0.04 b (0.45–0.56) | 0.0023 |
| Body Fat (%) | 25.4 ± 6.6 a | 26.0 ± 5.2 a | 35.8 ± 5.3 b | 0.0013 |
| Body Fat (kg) | 17.0 ± 3.2 a | 13.7 ± 3.1 a | 21.8 ± 3.1 b | <0.0001 |
| Trunk fat (kg) | 7.2 ± 1.4 b | 6.1 ± 2.3 b | 11.1 ± 1.8 a | <0.0001 |
| Lean Mass (%) | 70.7 ± 6.5 a | 70.0 ± 4.9 a | 60.5 ± 5.3 b | 0.0013 |
| Lean Mass (kg) | 49.3 ± 11.9 a | 37.3 ± 7.8 b | 37.1 ± 5.5 b | 0.0072 |
| Fasting Metabolic Parameters | ||||
| Glucose (mg/dL) | 96.0 ± 4.0 | 102.0 ± 9.0 | 101.0 ± 11.0 | 0.1646 |
| Total-C (mg/dL) | 157.0 ± 24.0 a | 213.0 ± 22.0 b | 193.0 ± 27.0 b | <0.0001 |
| HDL-C (mg/dL) | 60.0 ± 12.0 a | 79.0 ± 16.0 b | 64.0 ± 13.0 ab | 0.0183 |
| LDL-C (mg/dL) | 84.0 ± 16.0 a | 115.0 ± 20.0 b | 106.0 ± 19.0 b | 0.0034 |
| Non-HDL-C (mg/dL) | 97.0 ± 17.0 a | 133.0 ± 17.0 b | 129.0 ± 21.0 b | 0.0004 |
| VLDL-C (mg/dL) | 14.0 ± 3.0 a | 18.0 ± 5.0 ab | 22.0 ± 9.0 b | 0.0129 |
| Triglycerides (mg/dL) | 69.0 ± 16.0 a | 90.0 ± 24.0 ab | 107.0 ± 43.0 b | 0.0199 |
| ALT (U/L) | 27.0 ± 7.0 | 25.0 ± 7.0 | 22.0 ± 4.0 | 0.1760 |
| AST (U/L) | 26.0 ± 4.0 | 29.0 ± 5.0 | 27.0 ± 3.0 | 0.3203 |
| Diet | ||||
| Energy (kcal) | 2292 ± 419 a | 1744 ± 327 b | 1773 ± 557.4 ab | 0.0289 |
| Carbohydrate (g) | 270.1 ± 68.0 a | 197.4 ± 42.9 b | 207.1 ± 60.2 ab | 0.0345 |
| Fiber (g) | 22.5 ± 8.2 | 23.3 ± 6.9 | 21.7 ± 6.7 | 0.9151 |
| Protein (g) | 93.1 ± 31.1 | 79.1 ± 10.8 | 75.4 ± 13.4 | 0.2103 |
| Fat (g) | 94.7 ± 19.9 | 70.1 ± 25.0 | 73.9 ± 38.6 | 0.1796 |
| Saturated fat (g) | 29.5 ± 8.4 | 23.3 ± 8.4 | 22.7 ± 11.3 | 0.2751 |
| Sodium (g) | 3.7 ± 1.2 | 3.1 ± 1.1 | 3.3 ± 0.7 | 0.4597 |
| HEI (0–100, a.u.) | 52.0 ± 13.7 a | 64.1 ± 6.8 a | 63.5 ± 10.3 a | 0.0497 |
| Physical Activity | ||||
| Steps/day | 9338.0 ± 3207.0 | 8268.0 ± 3054.0 | 8417.0 ± 3456.0 | 0.7396 |
| MVPA (min) | 26.2 ± 36.6 a | 5.3 ± 10.7 ab | 3.3 ± 9.2 b | 0.0186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathangi, D.O.; Quirk, A.R.; Schifferer, J.K.; Fruit, S.E.; Higgins, M.E.; Wolf, E.R.; Tsotsoros, C.E.; Emerson, S.R.; Keirns, B.H. Postprandial Cardiometabolic Parameters in Older Adults with Normal-Weight Obesity: A Cross-Sectional Pilot Study. Metabolites 2025, 15, 550. https://doi.org/10.3390/metabo15080550
Pathangi DO, Quirk AR, Schifferer JK, Fruit SE, Higgins ME, Wolf ER, Tsotsoros CE, Emerson SR, Keirns BH. Postprandial Cardiometabolic Parameters in Older Adults with Normal-Weight Obesity: A Cross-Sectional Pilot Study. Metabolites. 2025; 15(8):550. https://doi.org/10.3390/metabo15080550
Chicago/Turabian StylePathangi, Dhanya O., Alexis R. Quirk, Jenna K. Schifferer, Sarah E. Fruit, Morgan E. Higgins, Emily R. Wolf, Cindy E. Tsotsoros, Sam R. Emerson, and Bryant H. Keirns. 2025. "Postprandial Cardiometabolic Parameters in Older Adults with Normal-Weight Obesity: A Cross-Sectional Pilot Study" Metabolites 15, no. 8: 550. https://doi.org/10.3390/metabo15080550
APA StylePathangi, D. O., Quirk, A. R., Schifferer, J. K., Fruit, S. E., Higgins, M. E., Wolf, E. R., Tsotsoros, C. E., Emerson, S. R., & Keirns, B. H. (2025). Postprandial Cardiometabolic Parameters in Older Adults with Normal-Weight Obesity: A Cross-Sectional Pilot Study. Metabolites, 15(8), 550. https://doi.org/10.3390/metabo15080550







