Metabolomic Profiling Reveals the Effects of Cu-Ag Nanoparticles on Tomato Bacterial Wilt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Culture and Treatment
2.3. Metabolite Extraction
2.4. Metabolomic Analysis
3. Results
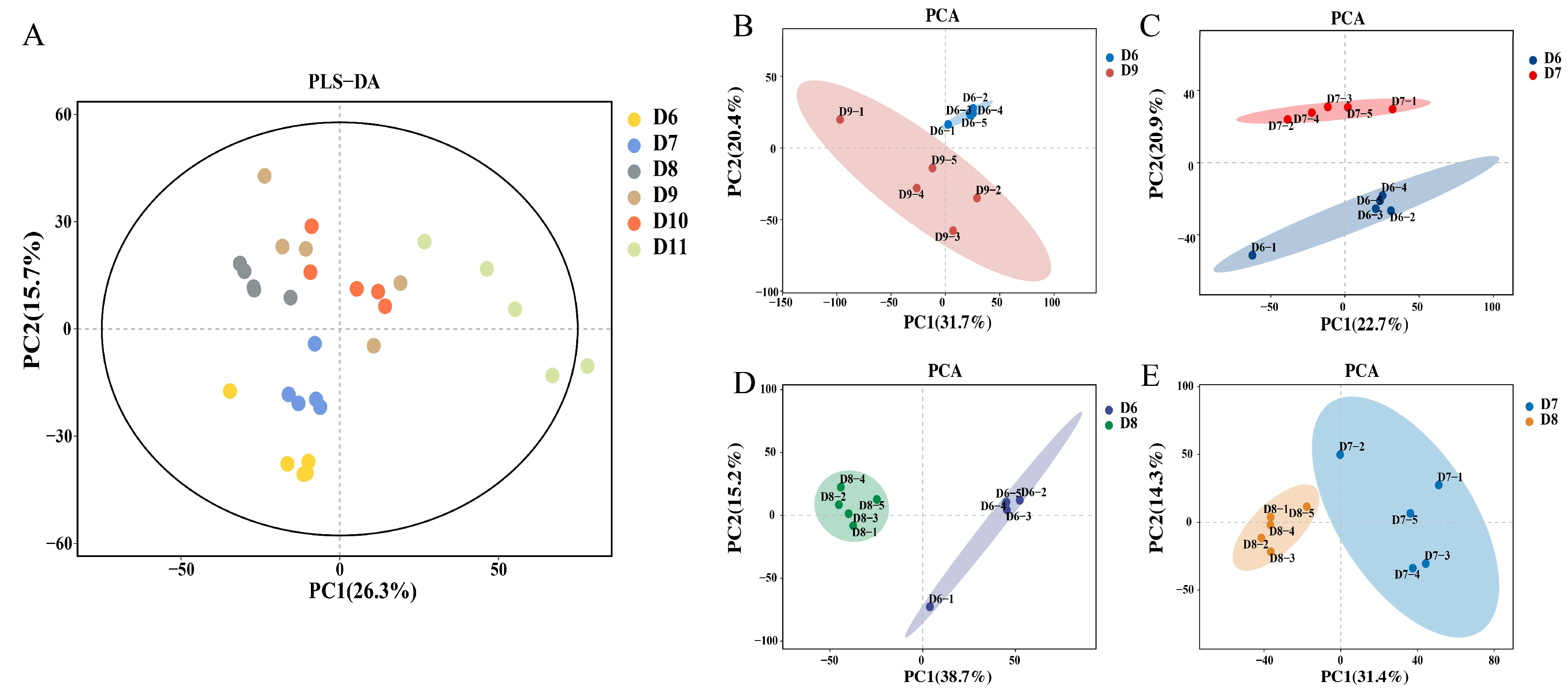
3.1. Effect of Nanoparticles on the Overall Metabolic Profile of Tomato Root
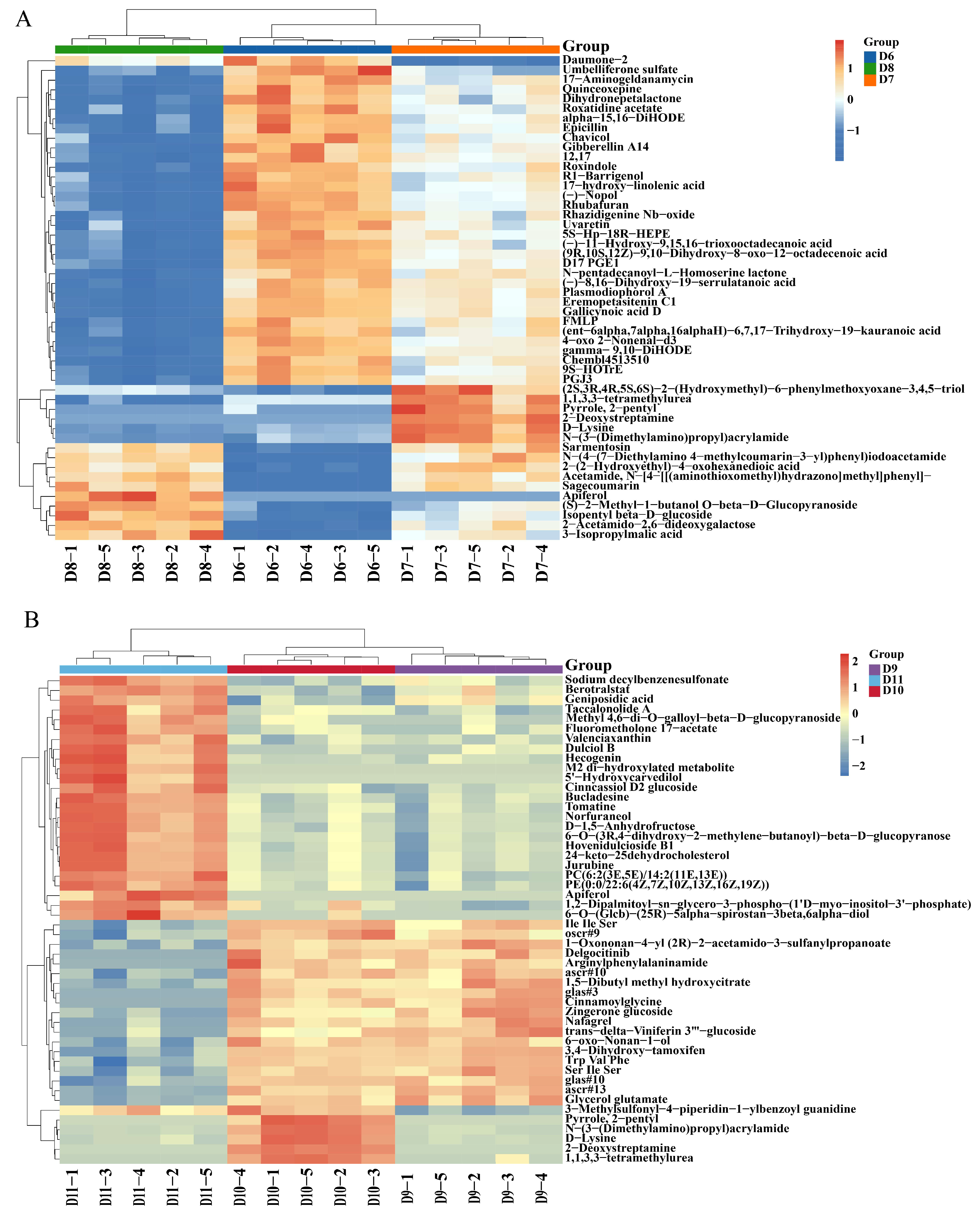
3.2. Number of Metabolite Changes Induced by Cu-Ag Nanoparticles
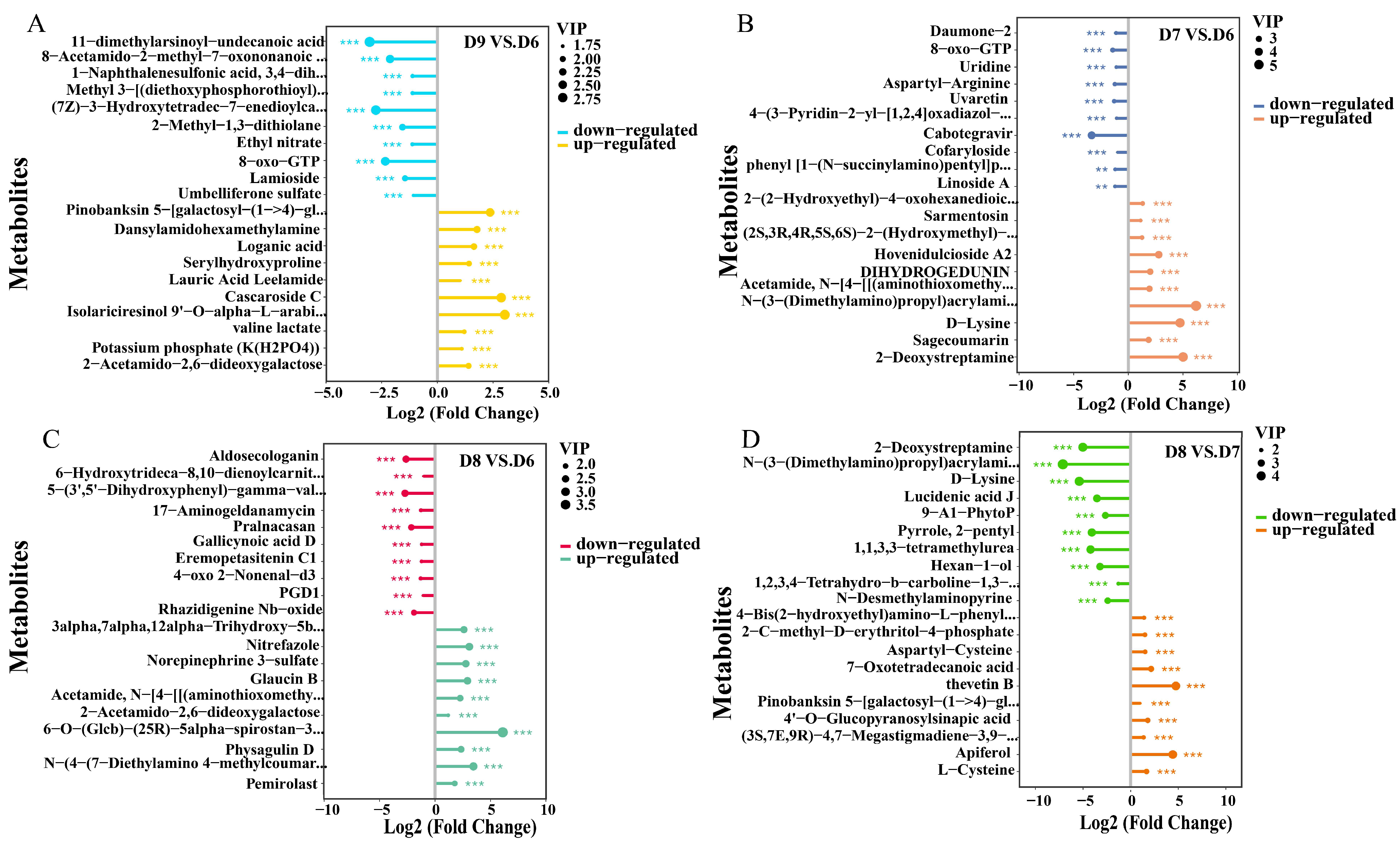
3.3. Analysis of Differential Root Metabolites Between Nanoparticle-Treated and Thiodiazole-Copper-Treated Plants
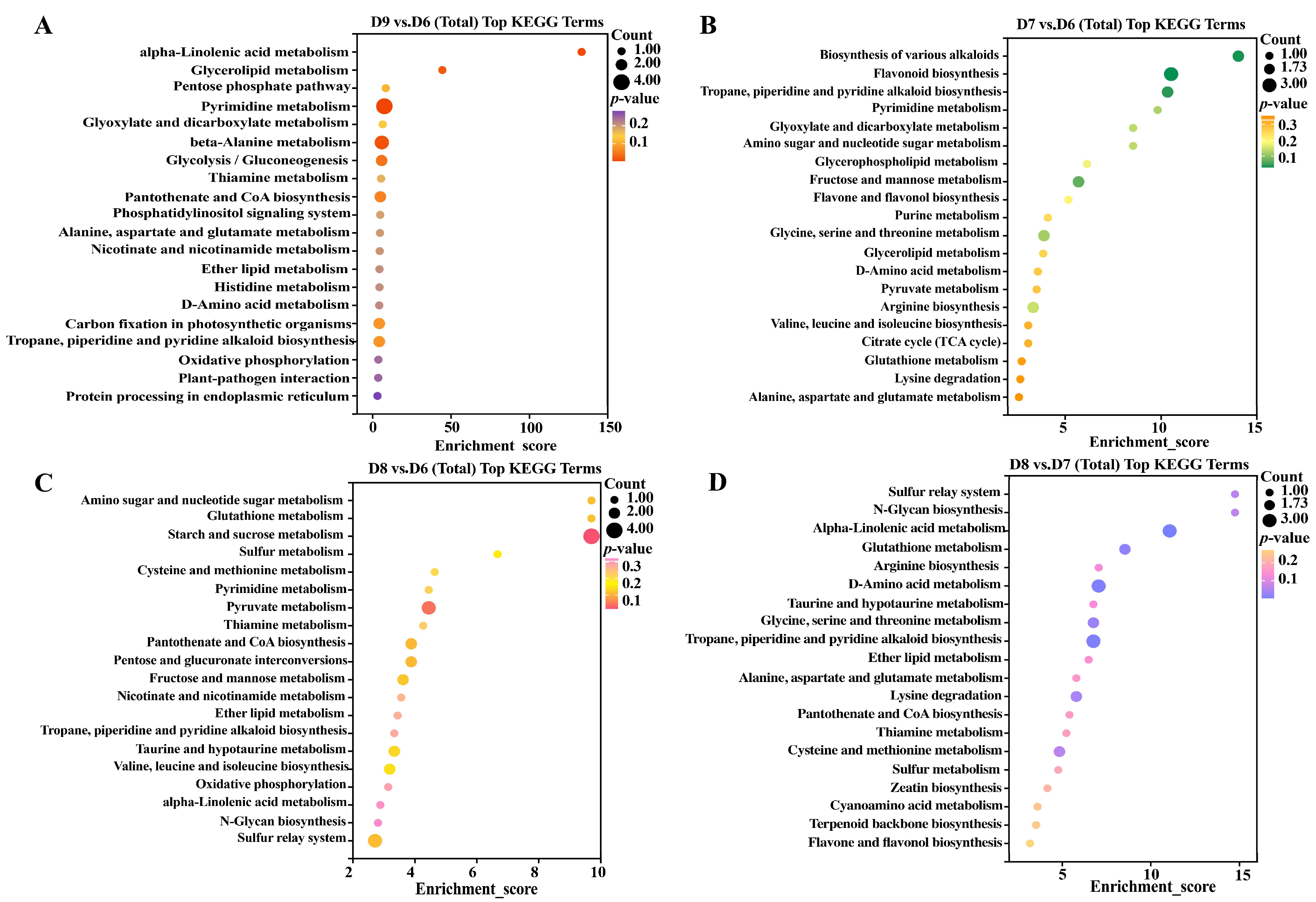
3.4. KEGG Pathway Enrichment Analysis in Tomato Roots
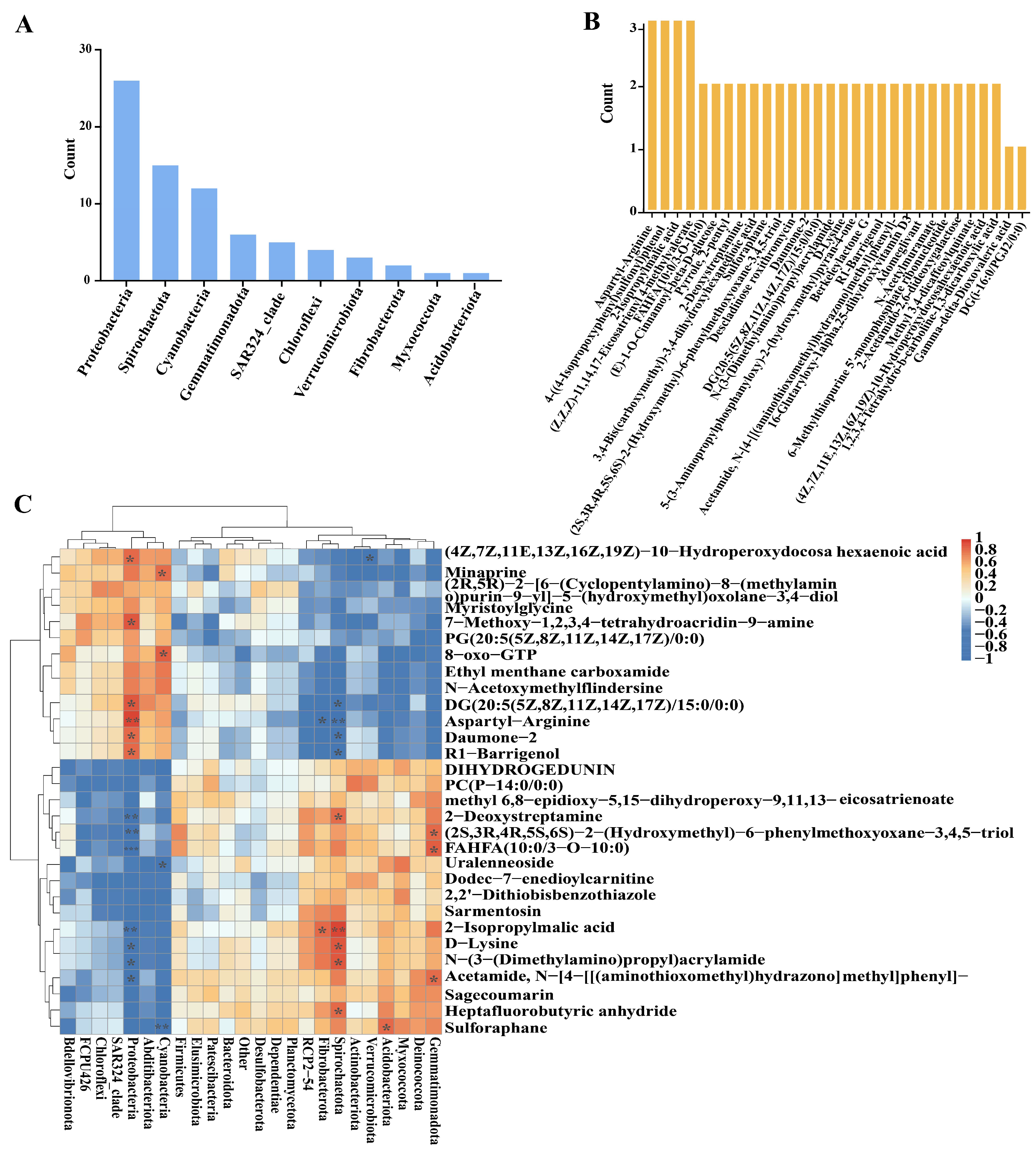
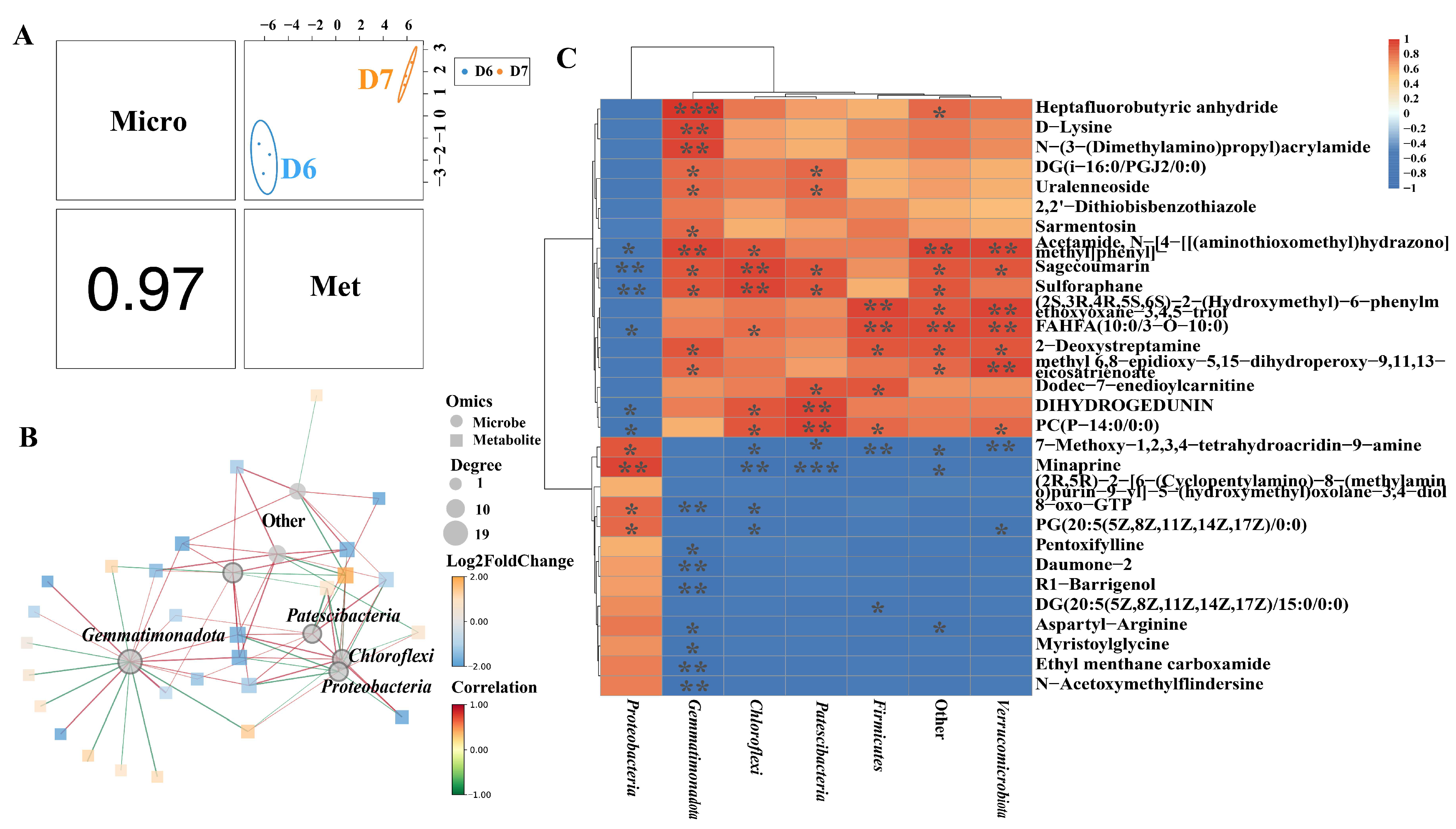
3.5. Correlation Analysis Between Metabolites and Microorganisms in Tomato
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol activity of aromatic and medicinal plants and their bioactive components against soil-borne pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef]
- Marsell, A.; Fröschel, C. Inoculation strategies to infect plant roots with soil-borne microorganisms. J. Vis. Exp. 2022, 181, e63446. [Google Scholar] [CrossRef]
- Windisch, S.; Walter, A.; Moradtalab, N.; Walker, F.; Höglinger, B.; El-Hasan, A.; Ludewig, U.; Neumann, G.; Grosch, R. Role of benzoic acid and lettucenin a in the defense response of lettuce against soil-borne pathogens. Plants 2021, 10, 2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, W.; Cheng, S.; Zhang, H.; Zong, J.; Zhang, Z. Ralstonia solanacearum-a soil borne hidden enemy of plants: Research development in management strategies, their action mechanism and challenges. Front. Plant Sci. 2023, 14, 1141902. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Dobhal, S.; Alvarez, A.M.; Arif, M. Taxonomy and phylogenetic research on Ralstonia solanacearum species complex: A complex pathogen with extraordinary economic consequences. Pathogens 2020, 9, 886. [Google Scholar] [CrossRef]
- Hikichi, Y.; Mori, Y.; Ishikawa, S.; Hayashi, K.; Ohnishi, K.; Kiba, A.; Kai, K. Regulation involved in colonization of intercellular spaces of host plants in Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 967. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, L.; Yu, G.; Macho, A.P. Tomato stem injection for the precise assessment of Ralstonia solanacearum fitness in planta. Bio Protoc. 2021, 11, e4134. [Google Scholar] [CrossRef]
- Vailleau, F.; Genin, S. Ralstonia solanacearum: An arsenal of virulence strategies and prospects for resistance. Annu. Rev. Phytopathol. 2023, 61, 25–47. [Google Scholar] [CrossRef]
- Liu, S.; Xue, Q.; Zhu, S.; Liu, Y.; Zou, H. Ralstonia solanacearum suppresses tomato root growth by downregulation of a wall-associated receptor kinase. Plants 2023, 12, 3600. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Jiang, N.; Yan, J.; Lin, W.; Cai, K. Silicon controls bacterial wilt disease in tomato plants and inhibits the virulence-related gene expression of Ralstonia solanacearum. Int. J. Mol. Sci. 2022, 23, 6965. [Google Scholar] [CrossRef]
- Dilbar, S.; Sher, H.; Ali, H.; Ullah, R.; Ali, A.; Ullah, Z. Antibacterial efficacy of green synthesized silver nanoparticles using salvia nubicola extract against Ralstonia solanacearum, the causal agent of vascular wilt of tomato. ACS Omega 2023, 8, 31155–31167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.J.; Wang, H.; Zhang, J.Z. Phytofabrication of silver nanoparticles using three flower extracts and their antibacterial activities against pathogen Ralstonia solanacearum strain YY06 of bacterial wilt. Front. Microbiol. 2020, 11, 2110. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in tomato using ZnO nanoparticles synthesized through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef]
- Huang, X.; Xing, Y.; Jiang, H.; Pu, Y.; Yang, S.; Kang, Z.; Cai, L. Nonphytotoxic and pH-responsive ZnO-ZIF-8 loaded with honokiol as a "nanoweapon" effectively controls the soil-borne bacterial pathogen Ralstonia solanacearum. J. Hazard. Mater. 2024, 472, 134502. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhou, X.; Wang, Y.; Liang, Y.; Luo, B.; Dai, Y.; Wei, Z.; Li, S.; He, R.; et al. Molecular mechanism of plant elicitor daphnetin-carboxymethyl chitosan nanoparticles against Ralstonia solanacearum by activating plant system resistance. Int. J. Biol. Macromol. 2023, 241, 124580. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, A.A.; Parveen, A.; Ansari, S.; Alam, M. Effect of phyto-assisted synthesis of magnesium oxide nanoparticles (MgO-NPs) on bacteria and the root-knot nematode. Bioinorg. Chem. Appl. 2022, 2022, 3973841. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, S.; Liu, H.; Lu, Q.; Du, P.; Li, H.; Li, S.; Liu, H.; Wang, R.; Huang, L.; et al. Silica nanoparticles conferring resistance to bacterial wilt in peanut (Arachis hypogaea L.). Sci. Total Environ. 2024, 915, 170112. [Google Scholar] [CrossRef]
- Pham, N.B.T.; Le, V.K.T.; Bui, T.T.T.; Phan, N.G.L.; Tran, Q.V.; Nguyen, M.L.; Dang, V.Q.; Nguyen, T.T.; Vo, T.N.H.; Tran, C.K. Improved synthesis of Ag/SiO2 colloidal nanocomposites and their antibacterial activity against Ralstonia solanacearum 15. J. Nanosci. Nanotechnol. 2021, 21, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Li, Z.; Cui, Z.; Ma, G.; Nassor, A.K.; Guan, Y.; Pan, X. Multi-stimuli-responsive pectin-coated dendritic mesoporous silica nanoparticles with Eugenol as a sustained release nanocarrier for the control of tomato bacterial wilt. J. Nanobiotechnol. 2025, 23, 191. [Google Scholar] [CrossRef]
- Luong, H.T.; Nguyen, C.X.; Lam, T.T.; Nguyen, T.H.; Dang, Q.L.; Lee, J.H.; Hur, H.G.; Nguyen, H.T.; Ho, C.T. Antibacterial effect of copper nanoparticles produced in a Shewanella-supported non-external circuit bioelectrical system on bacterial plant pathogens. RSC Adv. 2022, 12, 4428–4436. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ran, M.; Zhou, L.; Liu, Z.; Cai, L. g-C3N4@CuO electrostatic self-assembly toward Ralstonia solanacearum: Insights from cytomembrane and motility disruption. Pest Manag. Sci. 2024, 80, 3107–3115. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Khan, M.R.; Aziz, S.; Aqib. Use of manganese oxide nanoparticle (MnO2NPs) and Pseudomonas putida for the management of wilt disease complex of carrot. Exp. Parasitol. 2024, 257, 108698. [Google Scholar] [CrossRef]
- Matich, E.K.; Soria, N.G.C.; Aga, D.S.; Atilla-Gokcumen, G.E. Applications of metabolomics in assessing ecological effects of emerging contaminants and pollutants on plants. J. Hazard. Mater. 2019, 373, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Subramani, M.; Urrea, C.A.; Kalavacharla, V. Comparative analysis of untargeted metabolomics in tolerant and sensitive genotypes of common bean (Phaseolus vulgaris L.) seeds exposed to terminal drought stress. Metabolites 2022, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar application of SiO2 nanoparticles alters soil metabolite profiles and microbial community composition in the pakchoi (Brassica chinensis L.) rhizosphere grown in contaminated mine soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Stałanowska, K.; Szablińska-Piernik, J.; Okorski, A.; Lahuta, L.B. Zinc oxide nanoparticles affect early seedlings’ growth and polar metabolite profiles of pea (Pisum sativum L.) and wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2023, 24, 14992. [Google Scholar] [CrossRef]
- Pan, C.; Bao, Y.; Guo, A.; Ma, J. Environmentally relevant-level CeO2 NP with ferrous amendment alters soil bacterial community compositions and metabolite profiles in rice-planted soils. J. Agric. Food Chem. 2020, 68, 8172–8184. [Google Scholar] [CrossRef]
- Khan, M.; Alkhathlan, H.Z.; Adil, S.F.; Shaik, M.R.; Siddiqui, M.R.H.; Khan, M.; Khan, S.T. Secondary metabolite profile of Streptomyces spp. changes when grown with the sub-lethal concentration of silver nanoparticles: Possible implication in novel compound discovery. Antonie Leeuwenhoek 2024, 117, 95. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A.; Kulpińska, A.; Dębska, B.; Michalska, A.; Nowakowska, J.; Wichrowska, D.; Wojnarowicz, J.; Szałaj, U. Nanoparticles in plant cryopreservation: Effects on genetic stability, metabolic profiles, and structural integrity in bleeding heart (Papaveraceae) cultivars. Nanotechnol. Sci. Appl. 2025, 18, 35–56. [Google Scholar] [CrossRef]
- Shang, H.; Ma, C.; Li, C.; Cai, Z.; Shen, Y.; Han, L.; Wang, C.; Tran, J.; Elmer, W.H.; White, J.C.; et al. Aloe vera extract gel-biosynthesized selenium nanoparticles enhance disease resistance in lettuce by modulating the metabolite profile and bacterial endophytes composition. ACS Nano 2023, 17, 13672–13684. [Google Scholar] [CrossRef]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef]
- Mao, H.; Zhao, W.; Yang, X.; Sheng, L.; Zhu, S. Recruitment and metabolomics between Canna indica and rhizosphere bacteria under Cr stress. Front. Microbiol. 2023, 14, 1187982. [Google Scholar] [CrossRef]
- Sheng, L.; Zhao, W.; Yang, X.; Mao, H.; Zhu, S. Response characteristics of rhizosphere microbial community and metabolites of Iris tectorum to Cr stress. Ecotoxicol. Environ. Saf. 2023, 263, 115218. [Google Scholar] [CrossRef]
- Shao, L.; Li, X.; Xiao, T.; Lu, T.; Li, J.; Deng, J.; Xiao, E. Variations in microbial assemblage between rhizosphere and root endosphere microbiomes contribute to host plant growth under cadmium stress. Appl. Environ. Microbiol. 2023, 89, e0096023. [Google Scholar] [CrossRef]
- Mesa, V. Rhizosphere and endosphere bacterial communities survey by metagenomics approach. Methods Mol. Biol. 2022, 2512, 181–197. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Rahman, M.K.U.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fu, R.; Feng, H.; Jiang, G.; Finkel, O.; Sun, T.; Liu, M.; Huang, B.; Li, S.; Wang, X.; et al. RIN enhances plant disease resistance via root exudate-mediated assembly of disease-suppressive rhizosphere microbiota. Mol. Plant 2023, 16, 1379–1395. [Google Scholar] [CrossRef]
- Benaissa, A. Rhizosphere: Role of bacteria to manage plant diseases and sustainable agriculture-a review. J. Basic Microbiol. 2024, 64, e2300361. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Chen, J.; Zhang, L.; Feng, F.; Cheng, J.; Ma, L.; Li, M.; Wang, Y.; Jiang, W.; et al. Rhizosphere bacteria help to compensate for pesticide-induced stress in plants. Environ. Sci. Technol. 2024, 58, 12542–12553. [Google Scholar] [CrossRef]
- Łuniewski, S.; Rogowska, W.; Łozowicka, B.; Iwaniuk, P. Plants, microorganisms and their metabolites in supporting asbestos detoxification-a biological perspective in asbestos treatment. Materials 2024, 17, 1644. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yuan, M.; Tang, L.; Shen, Y.; Yu, Q.; Li, S. Integrated microbiology and metabolomics analysis reveal responses of soil microorganisms and metabolic functions to phosphorus fertilizer on semiarid farm. Sci. Total Environ. 2022, 817, 152878. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, A.U.; Rafatullah, M.; Alam, M.; Bogdanchikova, N.; Garibo, D. Search for effective approaches to fight microorganisms causing high losses in agriculture: Application of P. lilacinum metabolites and mycosynthesised silver nanoparticles. Biomolecules 2022, 12, 174. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using plant growth-promoting microorganisms (PGPMs) to improve plant development under in vitro culture conditions. Planta 2022, 255, 117. [Google Scholar] [CrossRef] [PubMed]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in plant growth and development: Roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary metabolites produced by plant growth-promoting bacterial endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef]
- Ning, W.; Luo, X.; Zhang, Y.; Tian, P.; Xiao, Y.; Li, S.; Yang, X.; Li, F.; Zhang, D.; Zhang, S.; et al. Broad-spectrum nano-bactericide utilizing antimicrobial peptides and bimetallic Cu-Ag nanoparticles anchored onto multiwalled carbon nanotubes for sustained protection against persistent bacterial pathogens in crops. Int. J. Biol. Macromol. 2024, 265, 131042. [Google Scholar] [CrossRef]
- Ning, W.; Luo, X.; Zhang, Y.; Li, S.; Yang, X.; Wang, X.; Chen, Y.; Xu, Y.; Zhang, D.; Zhang, S.; et al. Comparative Analysis of Nano-Bactericides and Thiodiazole-Copper on Tomato Rhizosphere Microbiome. Microorganisms 2025, 13, 1327. [Google Scholar] [CrossRef]
- Ning, W.; Bao, X.; Jiang, L.; Yang, M.; Lei, T.; Liu, M.; Liu, Y. Cu-Ag nanoparticles positively modulating the endophytic bacterial community in tomato roots affected by bacterial wilt. Front. Microbiol. 2025, 16, 1579517. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Wachsmuth, C.; Buchholz, C.; Bentley, M. A complex matrix characterization approach, applied to cigarette smoke, that integrates multiple analytical methods and compound identification strategies for non-targeted liquid chromatography with high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8571. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, A.; Mardal, M.; Linnet, K.; Dalsgaard, P.W. How to perform spectrum-based LC-HR-MS screening for more than 1000 NPS with HighResNPS consensus fragment ions. PLoS ONE 2020, 15, e0242224. [Google Scholar] [CrossRef]
- Li, H.; Shi, H.; Xu, P.; Yu, D. Metabolomics and microbiome reveal potential root microbiota affecting the alkaloidal metabolome in Aconitum vilmorinianum Kom. BMC Microbiol. 2022, 22, 70. [Google Scholar] [CrossRef]
- He, M.; Hou, G.; Liu, M.; Peng, Z.; Guo, H.; Wang, Y.; Sui, J.; Liu, H.; Yin, X.; Zhang, M.; et al. Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes. J. Transl. Med. 2024, 22, 448. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; El-Shiekh, R.A.; Mohamed, O.G.; Al-Karmalawy, A.A.; Tripathi, A.; Abdel-Baki, P.M. LC/MS-based metabolomics reveals chemical variations of two broccoli varieties in relation to their Anticholinesterase activity: In vitro and in silico studies. Plant Foods Hum. Nutr. 2024, 79, 359–366. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Tsentalovich, Y.P.; Sharshov, K.A.; Yanshole, V.V. The application of quantitative metabolomics for the taxonomic differentiation of birds. Biology 2022, 11, 1089. [Google Scholar] [CrossRef]
- Sui, H.; Sheng, M.; Luo, H.; Liu, G.; Meng, F.; Cao, Z.; Zhang, Y. Characterization of freezability-associated metabolites in boar semen. Theriogenology 2023, 196, 88–96. [Google Scholar] [CrossRef]
- Spanò, R.; Gena, P.; Linsalata, V.; Sini, V.; D’Antuono, I.; Cardinali, A.; Cotugno, P.; Calamita, G.; Mascia, Z. Spotlight on secondary metabolites produced by an early-flowering apulian artichoke ecotype sanitized from virus infection by meristem-tip-culture and thermotherapy. Antioxidants 2024, 13, 852. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K. mixOmics: An R package for ’omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- MacIntyre, A.M.; Meline, V.; Gorman, Z.; Augustine, S.P.; Dye, C.J.; Hamilton, C.D.; Iyer-Pascuzzi, A.S.; Kolomiets, M.V.; McCulloh, K.A.; Allen, C. Trehalose increases tomato drought tolerance, induces defenses, and increases resistance to bacterial wilt disease. PLoS ONE 2022, 17, e0266254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G.; et al. Emerging nanochitosan for sustainable agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Naz, S.; Raziq, F.; Qudratullah, Q.; Khan, N.A.; Laudadio, V.; Tufarelli, V.; Ragni, M. Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ. Sci. Pollut. Res. Int. 2022, 29, 32594–32604. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, Q.; Zhao, B. Exogenous organic acids promoted phytoremediation by Hydrangea macrophylla in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2025, 290, 117551. [Google Scholar] [CrossRef]
- Ackah, M.; Shi, Y.; Wu, M.; Wang, L.; Guo, P.; Guo, L.; Jin, X.; Li, S.; Zhang, Q.; Qiu, C.; et al. Metabolomics response to drought stress in Morus alba l. variety yu-711. Plants 2021, 10, 1636. [Google Scholar] [CrossRef]
- Vinchira-Villarraga, D.; Dhaouadi, S.; Milenkovic, V.; Wei, J.; Grace, E.R.; Hinton, K.G.; Webster, A.J.; Vadillo-Dieguez, A.; Powell, S.E.; Korotania, N.; et al. Metabolic profiling and antibacterial activity of tree wood extracts obtained under variable extraction conditions. Metabolomics 2024, 21, 13. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Zeng, L.; Lv, Y.; Ding, X.; Cheng, Y.; Zou, X. Plant lipid phosphate phosphatases: Current advances and future outlooks. Crit. Rev. Biotechnol. 2023, 43, 384–392. [Google Scholar] [CrossRef]
- Correa, S.M.; Fernie, A.R.; Nikoloski, Z.; Brotman, Y. Towards model-driven characterization and manipulation of plant lipid metabolism. Prog. Lipid Res. 2020, 80, 101051. [Google Scholar] [CrossRef]
- Le, Y.; He, L.; Li, S.; Xiong, C.; Lu, C.; Yang, X. Chlorogenic acid exerts antibacterial effects by affecting lipid metabolism and scavenging ROS in Streptococcus pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef] [PubMed]
- Moormann, J.; Heinemann, B.; Hildebrandt, T.M. News about amino acid metabolism in plant-microbe interactions. Trends Biochem. Sci. 2022, 47, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Ye, Z.; Hu, F.; Shan, C.; Wen, D.; Cao, J. Improvement of plant quality by amino acid transporters: A comprehensive review. Plant Physiol. Biochem. 2024, 215, 109084. [Google Scholar] [CrossRef] [PubMed]
- Du, G.F.; Yin, X.F.; Yang, D.H.; He, Q.Y.; Sun, X. Proteomic investigation of the antibacterial mechanism of trans-cinnamaldehyde against Escherichia coli. J. Proteome Res. 2021, 20, 2319–2328. [Google Scholar] [CrossRef]
- Arellano, H.; Nardello-Rataj, V.; Szunerits, S.; Boukherroub, R.; Fameau, A.L. Saturated long chain fatty acids as possible natural alternative antibacterial agents: Opportunities and challenges. Adv. Colloid Interface Sci. 2023, 318, 102952. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Valle, R.G.D.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, W.; Jiang, L.; Yang, M.; Lei, T.; Liu, C.; Zhao, F.; Shu, P.; Liu, Y. Metabolomic Profiling Reveals the Effects of Cu-Ag Nanoparticles on Tomato Bacterial Wilt. Metabolites 2025, 15, 548. https://doi.org/10.3390/metabo15080548
Ning W, Jiang L, Yang M, Lei T, Liu C, Zhao F, Shu P, Liu Y. Metabolomic Profiling Reveals the Effects of Cu-Ag Nanoparticles on Tomato Bacterial Wilt. Metabolites. 2025; 15(8):548. https://doi.org/10.3390/metabo15080548
Chicago/Turabian StyleNing, Weimin, Lei Jiang, Mei Yang, Tianhao Lei, Chan Liu, Fei Zhao, Pan Shu, and Yong Liu. 2025. "Metabolomic Profiling Reveals the Effects of Cu-Ag Nanoparticles on Tomato Bacterial Wilt" Metabolites 15, no. 8: 548. https://doi.org/10.3390/metabo15080548
APA StyleNing, W., Jiang, L., Yang, M., Lei, T., Liu, C., Zhao, F., Shu, P., & Liu, Y. (2025). Metabolomic Profiling Reveals the Effects of Cu-Ag Nanoparticles on Tomato Bacterial Wilt. Metabolites, 15(8), 548. https://doi.org/10.3390/metabo15080548






