Comparative Study Between the Short-Term Effects of Replacement Therapy with Liquid and Tablet Formulations of Levothyroxine on Insulin Resistance Markers in Recently Thyroidectomized Female Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Post Hoc Aoutcome
2.2. Laboratory Analysis
2.3. Statistical Analysis
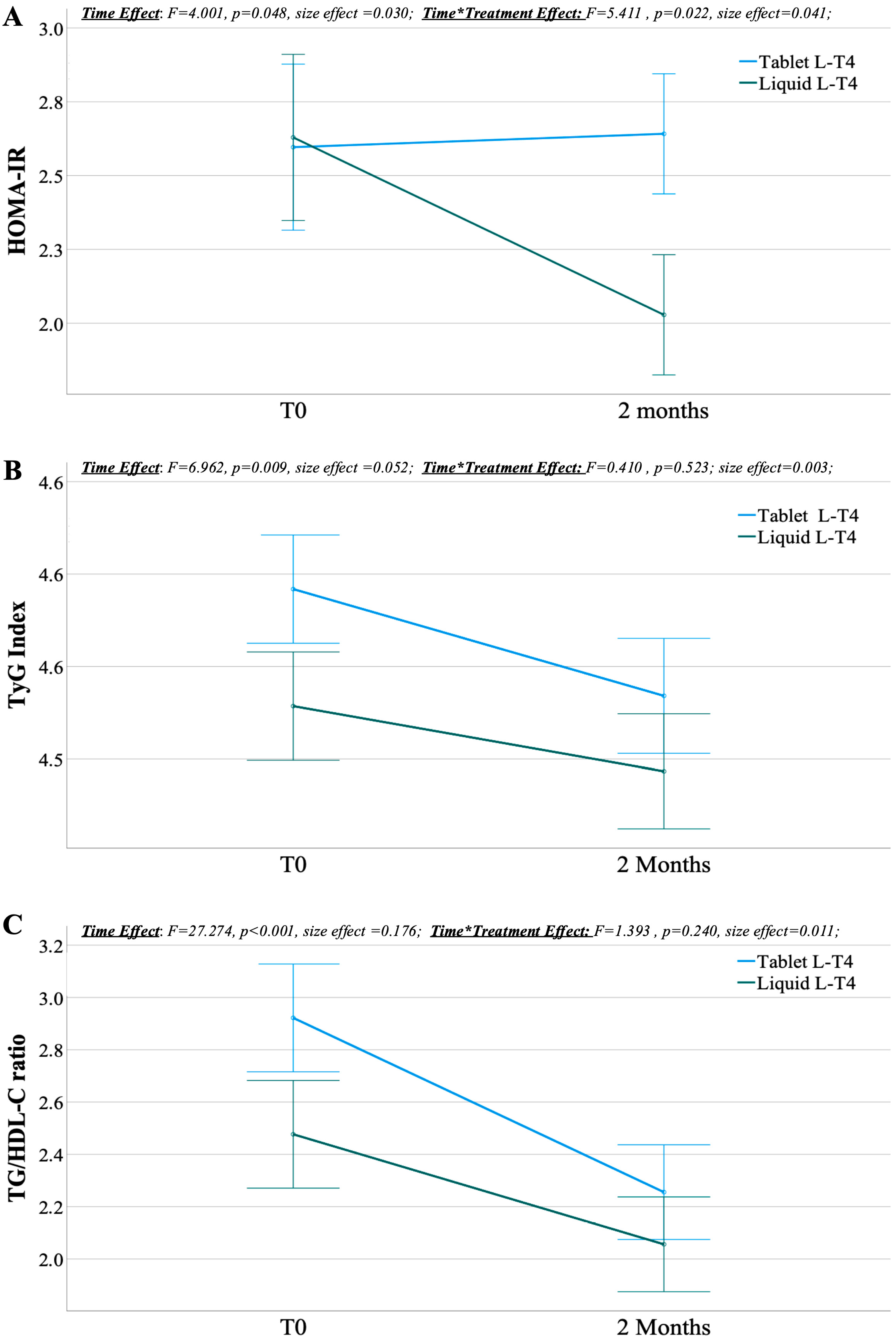
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L-T4 | Levothyroxine |
| IR | Insulin Resistance |
| HOMA | Homeostatic Model Assessment |
| TYG | Triglycerides-Glucose Index |
| TG | Triglycerides |
| HDL-C | High-Density Lipoprotein Cholesterol |
| TG/HDL-C | Triglyceride-To-High-Density Lipoprotein Cholesterol Ratio |
| PROBE | Prospective Randomized Open Blinded Endpoints |
| TSH | Thyroid-Stimulating Hormone |
| FT3 | Free Triiodothyronine |
| FT4 | Free Thyroxine |
| mg | Milligram |
| dl | Deciliter |
| SD | Standard Deviation |
| ANOVA | Analysis Of Variance |
| BMI | Body Mass Index |
| SBD | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| TC | Total Cholesterol |
| LDL-C | Low-Density Lipoprotein Cholesterol |
References
- Verhaert, N.; Vander Poorten, V.; Delaere, P.; Bex, M.; Debruyne, F. Levothyroxine replacement therapy after thyroid surgery. B-ENT 2006, 2, 129–133. [Google Scholar] [PubMed]
- Wiersinga, W.M. Thyroid hormone replacement therapy. Horm. Res. 2001, 56 (Suppl. S1), 74–81. [Google Scholar] [CrossRef] [PubMed]
- Nordenström, E.; Ranstam, J.; Bergenfelz, A. Effect of thyroid hormone replacement therapy on mortality rate in patients undergoing total or hemithyroidectomy for benign multinodular goitre. BJS Open 2024, 8, zrae012. [Google Scholar] [CrossRef]
- Eadala, P.; Waud, J.P.; Matthews, S.B.; Green, J.T.; Campbell, A.K. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions. Aliment. Pharmacol. Ther. 2009, 29, 677–687. [Google Scholar] [CrossRef]
- Rossi, L.; Paternoster, M.; Cammarata, M.; Bakkar, S.; Miccoli, P. Levothyroxine therapy in thyroidectomized patients: Ongoing challenges and controversies. Front. Endocrinol. 2025, 16, 1582734. [Google Scholar] [CrossRef]
- Vita, R.; Saraceno, G.; Trimarchi, F.; Benvenga, S. A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 2013, 43, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Antonelli, A. Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received L-T4 in tablet form after switching to an oral liquid formulation: A case series. BMC Gastroenterol. 2016, 16, 22. [Google Scholar] [CrossRef]
- Vita, R.; Saraceno, G.; Trimarchi, F.; Benvenga, S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 2014, 99, 4481–4486. [Google Scholar] [CrossRef]
- Lombardi, C.P.; Bocale, R.; Barini, A.; D’Amore, A.; Boscherini, M.; Bellantone, R. Comparative study between the effects of replacement therapy with liquid and tablet formulations of levothyroxine on mood states, self-perceived psychological well-being and thyroid hormone profile in recently thyroidectomized patients. Endocrine 2017, 55, 51–59. [Google Scholar] [CrossRef]
- Hao, D.; Tian, L.; He, H.; Zhu, C.; Guo, L.; Zhang, K.; Zhang, J. Efficacy and safety of postoperative levothyroxine sodium tablets for improving serum thyroid hormone levels and tumor marker levels in patients with thyroid tumors. Eur. J. Transl. Myol. 2023, 33, 11582. [Google Scholar] [CrossRef]
- Pingitore, A.; Gaggini, M.; Mastorci, F.; Sabatino, L.; Cordiviola, L.; Vassalle, C. Metabolic Syndrome, Thyroid Dysfunction, and Cardiovascular Risk: The Triptych of Evil. Int. J. Mol. Sci. 2024, 25, 628. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Bocale, R.; D’Amore, A.M.; Carnassale, G.; Necozione, S.; Barini, A.; Lombardi, C.P. Thyroid hormones modulate uric acid metabolism in patients with recent onset subclinical hypothyroidism by improving insulin sensitivity. Intern. Emerg. Med. 2020, 15, 67–71. [Google Scholar] [CrossRef]
- Bocale, R.; Barini, A.; D’Amore, A.; Boscherin, M.; Necozione, S.; Desideri, G.; Lombardi, C.P. Thyroid hormones modulate irisin concentrations in patients with recently onset hypothyroidism following total thyroidectomy. J. Endocrinol. Investig. 2021, 44, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Nabavizadeh, A.; Vardanjani, H.M. The association between thyroid function and insulin resistance as measured by the metabolic score for insulin resistance (METS-IR): Insights from NHANES 2007–2012. BMC Endocr. Disord. 2024, 24, 267. [Google Scholar] [CrossRef]
- Brenta, G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J. Thyroid Res. 2011, 2011, 152850. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ziamanesh, F.; Mohammadi, M.; Ebrahimpour, S.; Tabatabaei-Malazy, O.; Mosallanejad, A.; Larijani, B. Unraveling the link between insulin resistance and Non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A Narrative Review. J. Diabetes Metab. Disord. 2023, 22, 1083–1094. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Singhal, A.; Goyal, P. TG/HDL Ratio: A marker for insulin resistance and atherosclerosis in prediabetics or not? J. Fam. Med. Prim. Care 2021, 10, 3700–3705. [Google Scholar] [CrossRef]
- Colantoni, A.; Bucci, T.; Cocomello, N.; Angelico, F.; Ettorre, E.; Pastori, D.; Lip, G.Y.H.; Del Ben, M.; Baratta, F. Lipid-based insulin-resistance markers predict cardiovascular events in metabolic dysfunction associated steatotic liver disease. Cardiovasc. Diabetol. 2024, 23, 175. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Manzari, T.; Gohari-Lasaki, S.; Tutunchi, H.; Mobasseri, M.; Sadra, V.; Najafipour, F. Effects of levothyroxine replacement therapy on insulin resistance in patients with untreated primary hypothyroidism. BMC Res. Notes 2023, 16, 237. [Google Scholar] [CrossRef]
- Benabdelkamel, H.; Jaber, M.A.; Dahabiyeh, L.A.; Masood, A.; Almalki, R.H.; Musambil, M.; Abdel Rahman, A.M.; Alfadda, A.A. Metabolomic profile of patients on levothyroxine treatment for hypothyroidism. Eur. Thyroid J. 2023, 12, e230062. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Bartolone, L.; Squadrito, S.; Lo Giudice, F.; Trimarchi, F. Delayed intestinal absorption of levothyroxine. Thyroid 1995, 5, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bach-Huynh, T.G.; Nayak, B.; Loh, J.; Soldin, S.; Jonklaas, J. Timing of levothyroxine administration affects serum thyrotropin concentration. J. Clin. Endocrinol. Metab. 2009, 94, 3905–3912. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Teixeira, P.F.D.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef]
- Roos, A.; Bakker, S.J.; Links, T.P.; Gans, R.O.; Wolffenbuttel, B.H. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J. Clin. Endocrinol. Metab. 2007, 92, 491–496. [Google Scholar] [CrossRef]
- Wang, R.; Qin, S.; Qiao, T.; Jiang, W.; Tong, J.; Lu, G.; Gao, D.; Zhang, M.; Lv, Z.; Li, D.; et al. Body composition changes in patients with differentiated thyroid cancer after iodine-131 treatment and short-term levothyroxine replacement and suppression therapy. Hormones 2024, 23, 257–265. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Thyroid hormone promotes insulin-induced glucose uptake by enhancing Akt phosphorylation and VAMP2 translocation in 3T3-L1 adipocytes. J. Cell. Physiol. 2011, 226, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Kang, G.H.; Kim, H.H.; Han, S.K.; Koo, Y.D.; Cho, S.W.; Kim, Y.A.; Oh, B.C.; Park, d.J.; Chung, S.S.; et al. Thyroid-stimulating hormone improves insulin sensitivity in skeletal muscle cells via cAMP/PKA/CREB pathway-dependent upregulation of insulin receptor substrate-1 expression. Mol. Cell. Endocrinol. 2016, 436, 50–58. [Google Scholar] [CrossRef] [PubMed]

| Liquid L-T4 (n = 65) | Tablet L-T4 (n = 65) | p | |
|---|---|---|---|
| Age (years) | 51.9 ± 12.6 | 51.2 ± 13.0 | 0.774 |
| BMI (kg/m2) | 26.6 ± 4.9 | 26.2 ± 5.0 | 0.909 |
| SBP (mmHg) | 124.5 ± 10.0 | 125.5 ± 9.0 | 0.588 |
| DBP (mmHg) | 76.5 ± 6.3 | 77.7 ± 7.2 | 0.309 |
| TC (mmol/L) | 4.7 ± 0.8 | 4.8 ± 1.0 | 0.384 |
| LDL-C (mmol/L) | 2.8 ± 0.7 | 3.0 ± 0.9 | 0.339 |
| HDL-C (mmol/L) | 1.3 ± 0.3 | 1.2 ± 0.4 | 0.303 |
| TG (mmol/L) | 1.3 ± 0.5 | 1.4 ± 0.6 | 0.174 |
| Glucose (mmol/L) | 4.7 ± 1.0 | 4.9 ± 0.8 | 0.352 |
| Insulin (mU/L) | 11.8 ± 9.0 | 11.7 ± 7.8 | 0.932 |
| HOMA-IR | 2.6 ± 2.6 | 2.6 ± 1.9 | 0.934 |
| TyG Index | 4.5 ± 0.2 | 4.6 ± 0.2 | 0.129 |
| TG/HDL-C ratio | 2.5 ± 1.3 | 2.9 ± 2.0 | 0.129 |
| TSH (μU/mL) | 8.3 ± 5.6 | 7.7 ± 4.3 | 0.491 |
| FT3 (pg/mL) | 2.0 ± 0.6 | 2.1 ± 0.7 | 0.493 |
| FT4 (pg/mL) | 9.2 ± 3.3 | 9.4 ± 3.6 | 0.660 |
| Δ HOMA-IR | Δ TyG Index | Δ TG/HDL-C Ratio | |
|---|---|---|---|
| Δ BMI (kg/m2) | rs: 0.016; p = 0.855 | rs: −0.049; p = 0.580 | rs: −0.044; p = 0.623 |
| Δ TSH (μU/mL) | rs: −0.206; p = 0.019 | rs: 0.084; p = 0.344 | rs: 0.048; p = 0.585 |
| Δ FT3 (pg/mL) | rs: 0.123; p = 0.163 | rs: −0.065; p = 0.460 | rs: −0.240; p = 0.006 |
| Δ FT4 (pg/mL) | rs: 0.094; p = 0.287 | rs: −0.089; p = 0.315 | rs: −0.216; p = 0.013 |
| Δ HOMA-IR | |||||
| Model 1 | HOMA | Beta: −0.677 | B: −0.483 | S.E: 0.046 | p < 0.001 |
| Model 2 | HOMA | Beta: −1.301 | B: −0.928 | S.E: 0.138 | p < 0.001 |
| Insulin | Beta: 0.659 | B: 0.127 | S.E: 0.037 | p < 0.001 | |
| Model 3 | HOMA | Beta: −1.917 | B: −1.367 | S.E: 0.219 | p < 0.001 |
| Insulin | Beta: 1.170 | B: 0.226 | S.E: 0.053 | p < 0.001 | |
| Glucose | Beta: 0.263 | B: 0.463 | S.E: 0.182 | p = 0.012 | |
| Δ TyG Index | |||||
| Model 1 | TyG | Beta: −0.355 | B: −0.300 | S.E: 0.070 | p < 0.001 |
| Model 2 | TyG | Beta: −0.514 | B: −0.434 | S.E: 0.071 | p < 0.001 |
| HDL | Beta: −0.384 | B: −0.224 | S.E: 0.049 | p < 0.001 | |
| Model 3 | TyG | Beta: −0.541 | B: −0.456 | S.E: 0.071 | p < 0.001 |
| HDL-C | Beta: −0.369 | B: −0.215 | S.E: 0.049 | p < 0.001 | |
| BMI | Beta: 0.155 | B: 0.006 | S.E: 0.003 | p = 0.049 | |
| Δ TG/HDL-C Ratio | |||||
| Model 1 | TG/HDL-C ratio | Beta: −0.522 | B: −0.372 | S.E: 0.054 | p < 0.001 |
| Model 2 | TG/HDL-C ratio | Beta: −0.556 | B: −0.396 | S.E: 0.051 | p < 0.001 |
| Glucose | Beta: 0.296 | B: 0.384 | S.E: 0.093 | p < 0.001 | |
| Model 3 | TG/HDL-C ratio | Beta: −0.577 | B: −0.411 | S.E: 0.050 | p < 0.001 |
| Glucose | Beta: 0.264 | B: 0.343 | S.E: 0.092 | p < 0.001 | |
| BMI | Beta: 0.183 | B: 0.044 | S.E: 0.017 | p = 0.011 | |
| Model 4 | TG/HDL-C ratio | Beta: −0.700 | B: −0.499 | S.E: 0.065 | p < 0.001 |
| Glucose | Beta: 0.273 | B: 0.355 | S.E: 0.091 | p < 0.001 | |
| BMI | Beta: 0.167 | B: 0.040 | S.E: 0.017 | p = 0.020 | |
| HDL-C | Beta: −0.192 | B: −0.662 | S.E: 0.314 | p = 0.037 | |
| Model 5 | TG/HDL-C ratio | Beta: −0.668 | B: −0.476 | S.E: 0.065 | p < 0.001 |
| Glucose | Beta: 0.232 | B: 0.301 | S.E: 0.094 | p = 0.002 | |
| BMI | Beta: 0.152 | B: 0.037 | S.E: 0.017 | p = 0.033 | |
| HDL-C | Beta: −0.191 | B: −0.660 | S.E: 0.310 | p = 0.035 | |
| FT3 | Beta: 0.147 | B: 0.251 | S.E: 0.124 | p = 0.045 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, F.; Moscucci, F.; Bocale, R.; Savoia, C.; Cocomello, N.; Lospinuso, I.; Ettorre, E.; Desideri, G.; Pontecorvi, A. Comparative Study Between the Short-Term Effects of Replacement Therapy with Liquid and Tablet Formulations of Levothyroxine on Insulin Resistance Markers in Recently Thyroidectomized Female Patients. Metabolites 2025, 15, 547. https://doi.org/10.3390/metabo15080547
Baratta F, Moscucci F, Bocale R, Savoia C, Cocomello N, Lospinuso I, Ettorre E, Desideri G, Pontecorvi A. Comparative Study Between the Short-Term Effects of Replacement Therapy with Liquid and Tablet Formulations of Levothyroxine on Insulin Resistance Markers in Recently Thyroidectomized Female Patients. Metabolites. 2025; 15(8):547. https://doi.org/10.3390/metabo15080547
Chicago/Turabian StyleBaratta, Francesco, Federica Moscucci, Raffaella Bocale, Carmine Savoia, Nicholas Cocomello, Ilaria Lospinuso, Evaristo Ettorre, Giovambattista Desideri, and Alfredo Pontecorvi. 2025. "Comparative Study Between the Short-Term Effects of Replacement Therapy with Liquid and Tablet Formulations of Levothyroxine on Insulin Resistance Markers in Recently Thyroidectomized Female Patients" Metabolites 15, no. 8: 547. https://doi.org/10.3390/metabo15080547
APA StyleBaratta, F., Moscucci, F., Bocale, R., Savoia, C., Cocomello, N., Lospinuso, I., Ettorre, E., Desideri, G., & Pontecorvi, A. (2025). Comparative Study Between the Short-Term Effects of Replacement Therapy with Liquid and Tablet Formulations of Levothyroxine on Insulin Resistance Markers in Recently Thyroidectomized Female Patients. Metabolites, 15(8), 547. https://doi.org/10.3390/metabo15080547





