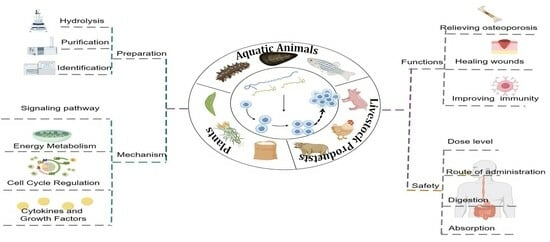
The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms
Abstract
1. Introduction
2. Source and Preparation of Cell Proliferation-Promoting Peptides
2.1. Cell Proliferation-Promoting Peptides Derived from Aquatic Animals
| Sequence | Source | Cell/Animal Model | Amount Added | Action Pathway | Cell Performance | Animal Performance | References |
|---|---|---|---|---|---|---|---|
| YRGDVVPK | Oyster | Mouse embryo osteoblast precursor cell (MC3T3-E1) | 100 nM | MAPK signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [9] |
| TPERYY | Tilapia scale | MC3T3-E1 cell | 109 μg/mL | Wnt/β-catenin signaling pathway | Cell proliferation, differentiation, and mineralization ↑ | Bone health ↑ Osteoporosis ↓ | [7] |
| KSA | Johnius belengerii | MC3T1-E1 cell | — | MAPK signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ bone formation ↑ | [23] |
| YPRKDETGAERT | Mytilus edulis | MC3T3-E1 cell | Bone morphogenetic protein type 2 (BMP-2) signaling pathway | Cell proliferation and differentiation ↑ | Femoral ↑, osteoporosis ↓ | [31] | |
| SCIH (28 peptides) | Sea Cucumber Intestine | MC3T3-E1 cell | 25 μg/mL | Wnt/β-catenin signaling pathway | Cell proliferation and differentiation ↑ | Bone growth ↑ | [29] |
| RPQYPQYPS, LSFSPY | Sea cucumber | MC3T3-E1 cell | 100 μg/mL | — | Proliferation and mineralization ↑ | Osteoporosis ↓ | [32] |
| FDNEGKGKLPEEY, FWDGRDGEVDGFK VLQTDNDALGKAK IVLDSGDGVTH, MVAPEEHP | Pinctada martensii | MC3T3-E1 cell | 2 μg/mL | — | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [33] |
| WSMP | Oyster shells | MC3T3-E1 cell | 100 μg/mL | BMP-2 signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [34] |
| MNKKREAEFQ | Gadus morhua | MC3T3-E1 cell | 100 μg/mL | BMP/WNT signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [35] |
| — | Chanos chanos | Human osteosarcoma cell (MG-63) | 100 μg/mL | — | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [36] |
| — | Mytilus coruscus | Mouse Mononuclear Macrophage cell (RAW264.7) | 100 μg/mL | MAPK signaling pathway | Cell proliferation ↑, the phagocytosis of cells ↑ | Immunomodulation ↑ | |
| — | Nibea japonica | Mouse Embryonic Fibroblast cell (NIH-3T3) | 25 μg/mL | NF-κB signaling pathway | Cell proliferation and migration ↑ | Wound healing ↑ | [14] |
| — | Sipunculus nudus | HUVEC, Human immortalized epidermal cells (HaCaT), HSF cell | — | — | Cell proliferation ↑ | Wound healing ↑, scar formation ↓ | [28] |
| VTPY, VLLY | Sea cucumber | HSF cell and HUVEC cell | 1000 nmol/mL | ERK/AKT signaling pathway | Cell proliferation ↑ | Wound healing ↑ | [12] |
| NINECFSSPCEN OGICODEIDGYN CVCOPGFTGTHCE | Sea cucumber | Human melanoma cell | 10 nM | MAPK and AKT signaling pathway | Cell proliferation ↑ | Wound healing ↑ | [3] |
| QIGFIW, IGIGPSGAS | Bigbelly seahorse | Mouse myoblast cell (C2C12) | 100 μg/mL | P38MAPK/AKT signaling pathway | Cell proliferation and differentiation ↑ | Skeletal muscle differentiation and endurance ↑ | [37] |
| VGRTNSH | Oyster | Human normal breast cell (MCF-10A) | 50 μg/mL | PRL/AKT/STAT5 and Mammalian target of rapamycin (mTOR)/Ribosomal protein S6 kinase B1 (S6KB1) signaling pathway | Cell proliferation ↑ | Lactation ↓ | [38] |
| — | Coryphaena hippurus | bone marrow-derived macrophage cell (BMMS) | 50 ng/mL | MAPK signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [27] |
| — | Mozambique tilapia | Human dermal papilla cells (hDPC) | 62.5 ppm | Wnt/β-catenin signaling pathway | Cell proliferation ↑ | Hair growth in the back skin tissue ↑ | [22] |
| MGLAGPR, MGDVLNF, EAPLMHV, TEAPLMHV, TEAPLMHV | Octopus | Mouse mammary epithelial cell (HC11) | 25 μg/mL | — | Cell proliferation ↑ | The synthesis of β-casein ↑ | [39] |
2.2. Cell Proliferation-Promoting Peptides Derived from Plants
| Sequence | Source | Cell/Animal Model | Amount Added | Action Pathway | Cell Performance | Animal Performance | References |
|---|---|---|---|---|---|---|---|
| IQDKEGIPPDQQR | Extruded Lupin | RAW 264.7 cell | 1 μg/mL | MARK signaling pathway | Cell proliferation ↑ | Inflammatory response ↓ | [20] |
| — | Mung bean | RAW264.7 cell | 200 mg/mL | — | Cell proliferation ↑, phagocytosis ↑ | Immunomodulation ↑ and anti-inflammation | [47] |
| YGPSSYGYG | Pseudostellaria heterophylla | RAW264.7 cell | 200 μg/mL | Toll-like receptors (TLR)/NF-κB/TNF-αsignaling pathway | Cell proliferation ↑, the endocytosis of macrophages ↑ | Immunomodulation ↑ | [21] |
| SSFSKGVQRAAF | Rice bran | HUVEC cell | 1 μM | — | Cell proliferation and migration ↑ | Wound healing ↑ | [46] |
| DIGGL | Ulva prolifera | HUVECs cell | 100 μM | — | Cell proliferation ↑ | Immunomodulation ↑ blood pressure ↓ | [43] |
| LRW | Pea | MC3T3-E1 cell | 50 μM | PI3K/AKT, AKT/Runx2 signal pathway | Cell proliferation, migration, differentiation, and mineralization ↑ | Osteoclast formation and the prevention of osteoporosis ↓ | [8] |
| DEDEQIPSHPPR | Soybean | MC3T3-E1 cell | 70 μM | MAPK signaling pathway | Cell proliferation, differentiation, and mineralization ↑ | Osteoporosis ↓ | [48] |
| — | Zein peptides | C2C12 cells | 200 μg/mL | Mechanistic Target of Rapamycin Complex 1/2 (mTORC1/mTORC2) signaling pathway | Cell proliferation and cell cycle progression ↑ | Sarcopenia ↓ | [49] |
| NQLDQMPR, PVNKPGRFE and the other 52 peptides | Soybean | Rat small intestine crypt epithelial cell (IEC-6) | 1 mg/mL | — | Cell proliferation ↑ | Intestinal inflammation ↓ | [45] |
| — | Porphyra haitanensis | IEC-6 cell | 100 μg/mL | — | Cell proliferation and migration ↑ | Intestinal epithelial wound healing ↑ | [44] |
| — | Cornus officinalis | Chicken Embryonic Fibroblasts (CEF) | 0.4 mg/mL | — | Cell proliferation ↑ | Free radicals ↓, anti-oxidation | [50] |
| SKWQHQQDSCRKQGVNLTPCEKHIMEKIQGRGDDDDDDDDD | Seed peptide | Male C57BL/6Jnarl mice, EL-4 T cell | — | — | Cell proliferation ↑, cytokines ↑ | Anti-inflammatory, antioxidant ↑ | [40] |
2.3. Cell Proliferation-Promoting Peptides Derived from Livestock Products
3. Cell Proliferation-Promoting Mechanism of Peptides
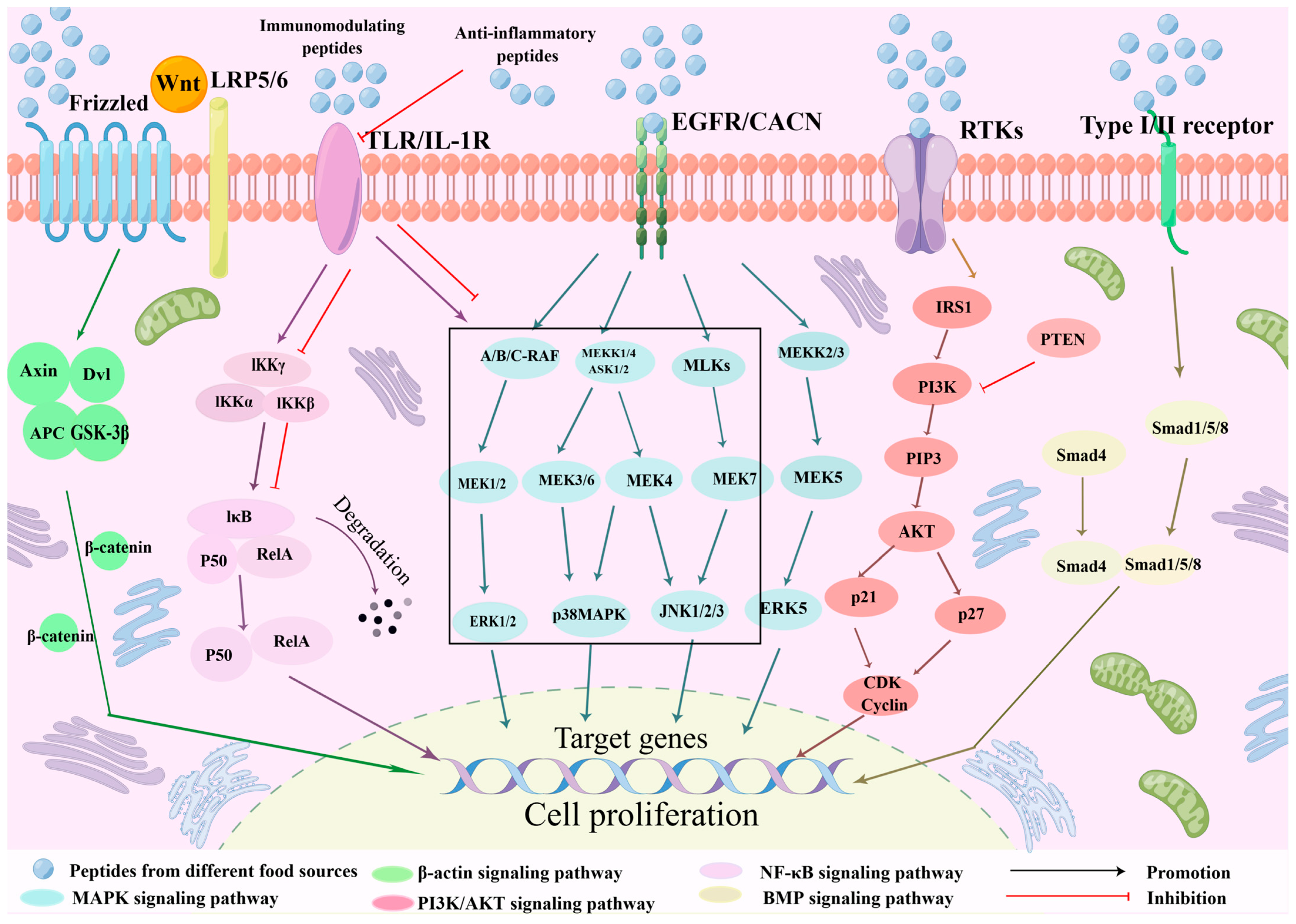
3.1. Signal Pathway
3.1.1. MAPK Signaling Pathway
3.1.2. Wnt/β-Catenin Signaling Pathway
3.1.3. NF-κB Signaling Pathway
3.1.4. PI3K/AKT Signaling Pathway
3.1.5. BMP Signaling Pathway
3.2. Regulation of Energy Metabolism
3.3. Cell Cycle Regulation
3.4. Regulation of Cytokines and Growth Factors
4. Safety and Regulatory Framework for Promoting Cell Proliferation Peptides
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| HUVEC | Human umbilical vein endothelial cells |
| TOF | Time-of-flight |
| HPLC-MS/MS | High-performance liquid chromatography-tandem mass spectrometry |
| MSCs | Mesenchymal stem cells |
| BMSCs | Bone marrow-derived mesenchymal stem cells |
| OCR | Oxygen consumption rate |
| COX2 | Cyclooxygenase-2 |
| NRF-1 | Nuclear respiratory factor 1 |
| TFAM | Mitochondrial transcription factor A |
| AKT | Protein kinases (ERK) and protein kinase B |
| TGF-α | Transforming growth factor-α |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor κ-B |
| IKKα | IκB kinase α |
| FGF | Fibroblast growth factor |
| EGF | Epidermal growth factor |
| VEGF | Vascular endothelial growth factor A |
| MCPs | Marine collagen peptides |
| CFDA | China Food and Drug Administration |
| IGF-1R | Insulin-like growth factor 1 receptor |
| ERK1/2 | Extracellular regulated protein kinases 1/2 |
| SMAD | Mothers against decapentaplegic |
| PI3K | Phosphoinositide 3-kinase |
| LRP1 | LDL receptor-related protein 1 |
| PGE2 | Prostaglandin E2 |
| IL-6 | Interleukin-6 |
| BMP-2 | Bone morphogenetic protein type 2 |
| S6KB1 | Ribosomal protein S6 kinase B1 |
| mTOR | Mammalian target of rapamycin |
| TLR | Toll-like receptors |
| RAW264.7 | Mouse mononuclear macrophage cells |
| MC3T3-E1 | Mouse embryo osteoblast precursor cells |
| mTORC1/mTORC2 | Mechanistic target of rapamycin complex 1/2 |
| HaCaT | Human immortalized epidermal cells |
| JNK | c-Jun N-terminal kinase |
| ERK | Extracellular-regulated protein kinases |
| AP-1 | Activator protein 1 |
| MyoD | Myogenic differentiation antigen |
| MyoG | Myogenin |
| MyHC | Myosin heavy chain |
| GSK3β | Glycogen synthase kinase 3 beta |
| LRP5/6 | Low-density lipoprotein receptor-related proteins 5 and 6 |
| UPLC | Ultra-Performance Liquid Chromatography |
| TCF/LEF | T cell factor/lymphoid enhancer factor family |
| LRP3 | Low-density lipoprotein receptor-related protein 3 |
| IκB | Inhibitor of NF-κB |
| ROS | Reactive oxygen species |
| MEK | Mitogen-activated extracellular signal-regulated kinase |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| PTEN | Phosphatase and tensin homolog |
| GAB1 | GRB2-associated binder 1 |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| GSK-3 | Glycogen synthase kinase-3 |
| BMP | Bone morphogenetic protein |
| ATP | Adenosine triphosphate |
| MCF-10A | Human normal breast cells |
| HO-1 | Heme oxygenase-1 |
| EGFR | Epidermal growth factor receptor |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| NIH-3T3 | Mouse embryonic fibroblast cells |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor-β |
| MG-63 | Human osteosarcoma cell |
| HSF | human skin fibroblast |
| BMMS | Bone marrow-derived macrophage cell |
| hDPC | Human dermal papilla cells |
| IEC-6 | Intestine crypt epithelial cell |
| CEF | Chicken embryonic fibroblast cell |
| GSK-3β | Glycogen synthase kinase 3 beta |
| IKKβ | IκB kinase β |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| BMP-2/4 | Bone morphogenetic proteins-2/4 |
| SMAD1/5 | Mothers against decapentaplegic homolog 1/5 |
| BMP-2/4 | Bone morphogenetic protein 2/4 |
| ADP | Adenosine diphosphate |
| IGF-1 | Insulin-like growth factor-1 |
References
- Li, M.; Dong, L.; Du, H.; Bao, Z.; Lin, S. Potential mechanisms underlying the protective effects of Tricholoma matsutake singer peptides against LPS-induced inflammation in RAW264.7 macrophages. Food Chem. 2021, 353, 129452. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Cardioprotective Peptides from Milk Processing and Dairy Products: From Bioactivity to Final Products including Commercialization and Legislation. Foods 2022, 11, 1270. [Google Scholar] [CrossRef]
- Pilus, N.S.M.; Muhamad, A.; Shahidan, M.A.; Yusof, N.Y.M. Potential of Epidermal Growth Factor-like Peptide from the Sea Cucumber Stichopus horrens to Increase the Growth of Human Cells: In Silico Molecular Docking Approach. Mar. Drugs 2022, 20, 596. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.G.; Chen, N.; Fu, Z.D.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Jiang, Y.; Lee, Y.-K.; Liang, D.; Yang, B.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Identification and validation of fermented milk-derived osteogenic peptides via molecular docking and osteoblastic cell model. Food Biosci. 2024, 58, 103698. [Google Scholar] [CrossRef]
- Guo, D.; Liu, W.; Zhang, X.; Zhao, M.; Zhu, B.; Hou, T.; He, H. Duck Egg White-Derived Peptide VSEE (Val-Ser-Glu-Glu) Regulates Bone and Lipid Metabolisms by Wnt/beta-Catenin Signaling Pathway and Intestinal Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900525. [Google Scholar] [CrossRef]
- Huang, W.; Yu, K.; Kang, M.; Wang, Q.; Liao, W.; Liang, P.; Liu, G.; Cao, Y.; Miao, J. Identification and functional analysis of three novel osteogenic peptides isolated from tilapia scale collagen hydrolysate. Food Res. Int. 2022, 162, 111993. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Shang, N.; Bhullar, K.S.; Wu, J. Pea protein-derived tripeptide LRW shows osteoblastic activity on MC3T3-E1 cells via the activation of the AKT/Runx2 pathway. Food Funct. 2020, 11, 7197–7207. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Fan, F.; Shi, P.; Tu, M.; Wang, Z.; Du, M. Identification and mechanism evaluation of a novel osteogenesis promoting peptide from Tubulin Alpha-1C chain in Crassostrea gigas. Food Chem. 2019, 272, 751–757. [Google Scholar] [CrossRef]
- Kaplani, K.; Koutsi, S.; Armenis, V.; Skondra, F.G.; Karantzelis, N.; Tsaniras, S.C.; Taraviras, S. Wound healing related agents: Ongoing research and perspectives. Adv. Drug Deliv. Rev. 2018, 129, 242–253. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wang, Y.H.; Bradbury, N.; Bravo, C.G.; Schnabl, B.; Di Nardo, A. Skin wound closure delay in metabolic syndrome correlates with SCF deficiency in keratinocytes. Sci. Rep. 2020, 10, 21732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, M.; Jiang, P.; Sun, N.; Lin, S. Peptides derived from sea cucumber accelerate cells proliferation and migration for wound healing by promoting energy metabolism and upregulating the ERK/AKT pathway. Eur. J. Pharmacol. 2022, 921, 174885. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Yang, F.; Jin, S.; Tang, Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-kappaB Signaling Pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and Characterization of a Novel Pentadecapeptide from Protein Hydrolysates of Cyclina sinensis and Its Immunomodulatory Effects on RAW264. 7 Cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Liu, C.; Liang, Y.; Lin, Q. Bioactive peptides from foods: Production, function, and application. Food Funct. 2021, 12, 7108–7125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, M.L.; Zhu, D.Y.; Wu, D.; Cheng, S.Z.; Wu, C.; El-Seedi, H.R.; Du, M. Immunomodulatory effect of ethanol-soluble polypeptides from Atlantic cod (Gadus morhua). Food Sci. Hum. Wellness 2023, 12, 1192–1203. [Google Scholar] [CrossRef]
- Soriano-Romani, L.; Nieto, J.A.; Garcia-Benlloch, S. Immunomodulatory role of edible bone collagen peptides on macrophage and lymphocyte cell cultures. Food Agric. Immunol. 2022, 33, 546–562. [Google Scholar] [CrossRef]
- He, K.; Zeng, Y.; Tian, H.; Zhang, Z.; Zhang, H.; Huang, F.; Yu, F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. J. Funct. Foods 2021, 83, 104562. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.; Yao, Y. Peptides from Extruded Lupin (Lupinus albus L.) Regulate Inflammatory Activity via the p38 MAPK Signal Transduction Pathway in RAW 264.7 Cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Huang, M.; Chen, X.; Tian, Y.; Chen, G.; Wang, M.; Wang, S.; Xiao, J. Isolation, Identification, and Immunomodulatory Effect of a Peptide from Pseudostellaria heterophylla Protein Hydrolysate. J. Agric. Food Chem. 2020, 68, 12259–12270. [Google Scholar] [CrossRef]
- Hwang, S.B.; Park, H.J.; Lee, B.H. Hair-Growth-Promoting Effects of the Fish Collagen Peptide in Human Dermal Papilla Cells and C57BL/6 Mice Modulating Wnt/beta-Catenin and BMP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11904. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.Y.; Ko, S.C.; Nam, S.Y.; Oh, J.; Kim, Y.M.; Kim, J.I.; Kim, N.; Yi, M.; Jung, W.K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef]
- Yue, H.; Tian, Y.; Feng, X.; Bo, Y.; Leng, Z.; Dong, P.; Xue, C.; Wang, J. Novel peptides from sea cucumber intestinal hydrolysates promote longitudinal bone growth in adolescent mice through accelerating cell cycle progress by regulating glutamine metabolism. Food Funct. 2022, 13, 7730–7739. [Google Scholar] [CrossRef]
- Tian, M.; Han, Y.-b.; Yang, G.-y.; Li, J.-l.; Shi, C.-s.; Tian, D. The role of lactoferrin in bone remodeling: Evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front. Endocrinol. 2023, 14, 1218148. [Google Scholar] [CrossRef]
- Han, L.; Mao, X.; Wang, K.; Li, Y.; Zhao, M.; Wang, J.; Xue, C. Phosphorylated peptides from Antarctic krill (Euphausia superba) ameliorated osteoporosis by activation of osteogenesis-related MAPKs and PI3K/AKT/GSK-3β pathways in dexamethasone-treated mice. J. Funct. Foods 2018, 47, 447–456. [Google Scholar] [CrossRef]
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; de Val, J.; Wu, W. Collagen Peptide Upregulates Osteoblastogenesis from Bone Marrow Mesenchymal Stem Cells through MAPK- Runx2. Cells 2019, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zheng, Z.; Yuan, J.; Zhang, C.; Cao, W.; Qin, X. Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules 2021, 26, 1385. [Google Scholar] [CrossRef]
- Yue, H.; Tian, Y.; Feng, X.; Bo, Y.; Xue, C.; Dong, P.; Wang, J. Novel Peptides Derived from Sea Cucumber Intestine Promotes Osteogenesis by Upregulating Integrin-Mediated Transdifferentiation of Growth Plate Chondrocytes to Osteoblasts. J. Agric. Food Chem. 2022, 70, 13212–13222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Lu, W.; Liu, J.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; Guo, Y. Cell Proliferation Stimulation Ability and Osteogenic Activity of Low Molecular Weight Peptides Derived from Bovine Gelatin Hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, F.; Chen, H.; Shi, P.; Zhu, D.; Yang, M.; Wang, Z.; Ei-Seedi, H.R.; Du, M. Absorption and transport of a Mytilus edulis-derived peptide with the function of preventing osteoporosis. Food Funct. 2021, 12, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yue, H.; Bo, Y.Y.; Yin, H.W.; Tian, Y.Y.; Zhao, Z.F.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Purification and identification of novel osteogenic peptides from sea cucumber intestine hydrolysates and their pro-osteogenesis effects on MC3T3-E1 cells. FOOD Biosci. 2024, 61, 104390. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, P.; Liu, X.; Wei, L.; Bai, Y.; Liu, X.; Li, S. Production and identification of peptides with activity promoting osteoblast proliferation from meat dregs of Pinctada martensii. J. Food Biochem. 2021, 45, e13890. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, S.; Zhang, F.; Wang, R.; Zhao, Y.; Zeng, M. Shell water-soluble matrix protein from oyster shells promoted proliferation, differentiation and mineralization of osteoblasts in vitro and vivo. Int. J. Biol. Macromol. 2022, 201, 288–297. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, S.; Ma, W.; Wu, D.; El-Seedi, H.R.; Wang, Z.; Du, M. Myosin heavy chain-derived peptide of Gadus morhua promotes proliferation and differentiation in osteoblasts and bone formation and maintains bone homeostasis in ovariectomized mice. Food Funct. 2023, 14, 5151–5166. [Google Scholar] [CrossRef]
- Chuu, J.J.; Lu, J.W.; Chang, H.J.; Chu, Y.H.; Peng, Y.J.; Ho, Y.J.; Shen, P.H.; Chen, Y.S.; Chen, C.H.; Liu, Y.C.; et al. Attenuative effects of collagen peptide from milkfish (Chanos chanos) scales on ovariectomy-induced osteoporosis. FOOD Sci. Nutr. 2024, 12, 116–130. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Kim, S.-Y.; Kim, Y.; Kim, H.-S.; Jeon, Y.-J.; Cho, M. Bigbelly seahorse (Hippocampus abdominalis)-derived peptides enhance skeletal muscle differentiation and endurance performance via activated P38MAPK/AKT signalling pathway: An in vitro and in vivo analysis. J. Funct. Foods 2019, 52, 147–155. [Google Scholar] [CrossRef]
- Chen, S.; Qin, X.; Zhang, C.; Cao, W.; Zheng, H.; Lin, H. Lactation Activity and Mechanism of Milk-Protein Synthesis by Peptides from Oyster Hydrolysates. Nutrients 2022, 14, 1786. [Google Scholar] [CrossRef]
- Cai, B.; Wan, P.; Chen, H.; Chen, X.; Sun, H.; Pan, J. Identification of octopus peptide and its promotion of beta-casein synthesis in a mouse mammary epithelial cell line. J. Food Biochem. 2020, 44, e13467. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Wang, Y.F.; Lin, P.Y.; Peng, S.H.; Chou, M.J. Seed peptide lunasin ameliorates obesity-induced inflammation and regulates immune responses in C57BL/6J mice fed high-fat diet. Food Chem. Toxicol. 2021, 147, 111908. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Rivera-Jimenez, J.; Berraquero-Garcia, C.; Perez-Galvez, R.; Garcia-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibiting anti-inflammatory activity: Sources, structural features and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; He, H.; Zhou, W.; Li, M.; Lu, A.; Che, T.; Shen, S. Purification identification and function analysis of ACE inhibitory peptide from Ulva prolifera protein. Food Chem. 2023, 401, 134127. [Google Scholar] [CrossRef]
- Qiu, H.M.; Veeraperumal, S.; Lv, J.H.; Wu, T.C.; Zhang, Z.P.; Zeng, Q.K.; Liu, Y.; Chen, X.Q.; Aweya, J.J.; Cheong, K.L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure characterization of soybean peptides and their protective activity against intestinal inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Saito, K.; Aida, R.; Ochiai, A.; Saitoh, E.; Tanaka, T. Wound healing activity and mechanism of action of antimicrobial and lipopolysaccharide-neutralizing peptides from enzymatic hydrolysates of rice bran proteins. J. Biosci. Bioeng. 2019, 128, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Miao, X.; Chen, H. Anti-inflammatory effects of mung bean protein hydrolysate on the lipopolysaccharide- induced RAW264.7 macrophages. Food Sci. Biotechnol. 2022, 31, 849–856. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X.; Du, M.; Yu, W.; Wang, Z.; Xu, B.; Yang, J.; Xu, J.; Liu, Z.; Cheng, Y.; et al. Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TbetaRI-p38-MAPK-Depending RUNX2 Activation. Nutrients 2022, 14, 1940. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.S.; Yu, B.B.; Wu, D.J.; Lu, Y.J.; Wu, W.; Wang, J.; Zhang, Y.H.; Fu, Y. Zein-Derived Peptides from Corn Promote the Proliferation of C2C12 Myoblasts via Crosstalk of mTORC1 and mTORC2 Signaling Pathways. Foods 2024, 13, 919. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Jiao, P.; Xia, M.; Tang, B. Preparation and Evaluation of Antioxidant Activities of Bioactive Peptides Obtained from Cornus officinalis. Molecules 2022, 27, 1232. [Google Scholar] [CrossRef]
- Shi, P.; Fan, F.; Chen, H.; Xu, Z.; Cheng, S.; Lu, W.; Du, M. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J. Dairy Sci. 2020, 103, 3950–3960. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Wei, Y.; Feng, Z.; Fang, L.; Li, M.; Ren, J.; Liu, W.; Gan, J. In vitro immunomodulatory and antioxidant effects of oligopeptides and four characteristic peptides in black-bone silky fowl (Gallus gallus domesticus Brisson). J. Food Biochem. 2022, 46, e14469. [Google Scholar] [CrossRef]
- Jung, H.; Jung, D.; Lee, J.; Ki, W.; Lee, J.M.; Kim, E.M.; Nam, M.S.; Kim, K.K. Bioactive peptides in the pancreatin-hydrolysates of whey protein support cell proliferation and scavenge reactive oxygen species. Anim. Cells Syst. 2022, 26, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Guo, Y. Cell Growth Stimulation, Cell Cycle Alternation, and Anti-Apoptosis Effects of Bovine Bone Collagen Hydrolysates Derived Peptides on MC3T3-E1 Cells Ex Vivo. Molecules 2020, 25, 2305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xie, Y.; Wen, B.; Ye, M.; Liu, Y.; Imam, K.M.S.U.; Cai, H.; Zhang, C.; Wang, F.; Xin, F. Porcine bone collagen peptides promote osteoblast proliferation and differentiation by activating the PI3K/AKT signaling pathway. J. Funct. Foods 2020, 64, 103697. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, C.; Zhu, L.; Jia, W.; Shen, Q. Yak (Bos grunniens) bones collagen-derived peptides stimulate osteoblastic proliferation and differentiation via the activation of Wnt/beta-catenin signaling pathway. J. Sci. Food Agric. 2020, 100, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, S.; Lee, H.J.; Suh, H.J.; Jo, K. Stimulating effect of whey protein hydrolysate on bone growth in MC3T3-E1 cells and a rat model. Food Funct. 2021, 12, 5109–5117. [Google Scholar] [CrossRef]
- Qiu, Y.; Ying, J.; Yan, F.; Yu, H.; Zhao, Y.; Li, H.; Xia, S.; Chen, J.; Zhu, J. Novel antiosteoporotic peptides purified from protein hydrolysates of taihe black-boned silky fowl: By larval zebrafish model and molecular docking. Food Res. Int. 2023, 169, 112850. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Li, Y.H.; Li, A.; Liu, R.H.; Gao, X.; Li, D.; Kou, X.H.; Xue, Z.H. Nutritional constituent and health benefits of chickpea (Cicer arietinum L.): A review. Food Res. Int. 2021, 150, 110790. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.; Xu, H.; Yang, S.; Chen, Z.; Wang, H.; Zheng, B.; Mao, B.; Wu, X. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2021, 150, 112073. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.H.; Glimcher, L.H. Mitogen-activated protein kinase pathways in osteoblasts. Annu. Rev. Cell Dev. Biol. 2013, 29, 63–79. [Google Scholar] [CrossRef]
- Vlashi, R.; Zhang, X.; Wu, M.; Chen, G. Wnt signaling: Essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes Dis. 2023, 10, 1291–1317. [Google Scholar] [CrossRef]
- Guo, D.; He, H.; Zhao, M.; Zhang, G.; Hou, T. Desalted duck egg white peptides promoted osteogenesis via wnt/beta-catenin signal pathway. J. Food Sci. 2020, 85, 834–842. [Google Scholar] [CrossRef]
- Jang, E.; Jin, S.; Cho, K.J.; Kim, D.; Rho, C.R.; Lyu, J. Wnt/β-catenin signaling stimulates the self-renewal of conjunctival stem cells and promotes corneal conjunctivalization. Exp. Mol. Med. 2022, 54, 1156–1164. [Google Scholar] [CrossRef]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Jin, W.; Li, D.; Li, Y.; Yuan, L. Peptide fraction from sturgeon muscle by pepsin hydrolysis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via MAPK and NF-κB pathways. Food Sci. Hum. Wellness 2021, 10, 103–111. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Huang, P.; Cai, Z.; Zhang, N.; Wang, Y.; Li, Y. The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway. Foods 2023, 12, 2398. [Google Scholar] [CrossRef]
- Attiq, A.; Yao, L.J.; Afzal, S.; Khan, M.A. The triumvirate of NF-κB, inflammation and cytokine storm in COVID-19. Int. Immunopharmacol. 2021, 101, 108255. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mo, S.R.; Shen, M.Y.; Chen, Y.; Yu, Q.; Li, Z.D.; Xie, J.H. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-Kappa B and PI3K-AKT signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Crawford, R.; Xiao, Y. Vertical inhibition of the PI3K/AKT/mTOR pathway for the treatment of osteoarthritis. J. Cell. Biochem. 2012, 114, 245–249. [Google Scholar] [CrossRef]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/AKT signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. 2023, 21, 201. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Qu, M.; Yang, X.; Fang, Y.; Ying, X.; Zhang, M.; Wei, J.; Pan, Y. Exploring the potential of treating chronic liver disease targeting the PI3K/AKT pathway and polarization mechanism of macrophages. Heliyon 2023, 9, e17116. [Google Scholar] [CrossRef]
- Huang, X.Q.; You, L.; Nepovimova, E.; Psotka, M.; Malinak, D.; Valko, M.; Sivak, L.; Korabecny, J.; Heger, Z.; Adam, V.; et al. Inhibitors of phosphoinositide 3-kinase (PI3K) and phosphoinositide 3-kinase-related protein kinase family (PIKK). J. Enzym. Inhib. Med. Chem. 2023, 38, 2237209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Long, Y.F.; Jin, L.Y.; Li, C.H.; Long, J. Non-coding RNAs regulate the BMP/Smad pathway during osteogenic differentiation of stem cells. Acta Histochem. 2023, 125, 151998. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gong, W.; Li, F.; Xie, M. Pilose antler aqueous extract promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells by stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway. Chin. J. Nat. Med. 2019, 17, 756–767. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mazloomnejad, R.; Kasravi, M.; Gholamine, B.; Bahrami, S.; Sarzaeem, M.M.; Niknejad, H. Recent advances on small molecules in osteogenic differentiation of stem cells and the underlying signaling pathways. Stem Cell Res. Ther. 2022, 13, 518. [Google Scholar] [CrossRef]
- Wang, X.-H.; Yang, F.; Pan, J.-B.; Kang, B.; Xu, J.-J.; Chen, H.-Y. Quantitative Imaging of pN Intercellular Force and Energetic Costs during Collective Cell Migration in Epithelial Wound Healing. Anal. Chem. 2020, 92, 16180–16187. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.; Cao, Y.; Zhang, J.; Zhao, J. Sea buckthorn (Hippophae rhamnoides L.) oil enhances proliferation, adipocytes differentiation and insulin sensitivity in 3T3-L1 cells. Food Sci. Biotechnol. 2020, 29, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Veeraperumal, S.; Qiu, H.-M.; Zeng, S.-S.; Yao, W.-Z.; Wang, B.-P.; Liu, Y.; Cheong, K.-L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef]
- Zhong, W.; He, J.; Huang, W.; Yin, G.; Liu, G.; Cao, Y.; Miao, J. Effect of the phosphorylation structure in casein phosphopeptides on the proliferation, differentiation, and mineralization of osteoblasts and its mechanism. Food Funct. 2023, 14, 10107–10118. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Yamamoto, A.; Shimizu, N.; Ishihara, E.; Ohno, H.; Takeda, A.; Kuroyanagi, Y. Development of cultured dermal substitute composed of hyaluronic acid and collagen spongy sheet containing fibroblasts and epidermal growth factor. J. Biomater. Sci.-Polym. Ed. 2014, 25, 1133–1143. [Google Scholar] [CrossRef]
- Yoo, Y.; Hyun, H.; Yoon, S.-J.; Kim, S.Y.; Lee, D.-W.; Um, S.; Hong, S.O.; Yang, D.H. Visible light-cured glycol chitosan hydrogel dressing containing endothelial growth factor and basic fibroblast growth factor accelerates wound healing in vivo. J. Ind. Eng. Chem. 2018, 67, 365–372. [Google Scholar] [CrossRef]
- Ning, J.L.; Zhao, H.L.; Chen, B.; Mi, E.Z.L.; Yang, Z.; Qing, W.H.; Lam, K.W.J.; Yi, B.; Chen, Q.; Gu, J.T.; et al. Argon Mitigates Impaired Wound Healing Process and Enhances Wound Healing In Vitro and In Vivo. Theranostics 2019, 9, 477–490. [Google Scholar] [CrossRef]
- Chunhui, Y.; Wenjun, C.; Hui, W.; Liquan, S.; Changwei, Z.; Tianzhu, Z.; Wenhai, Z. Pilose antler peptide protects osteoblasts from inflammatory and oxidative injury through EGF/EGFR signaling. Int. J. Biol. Macromol. 2017, 99, 15–20. [Google Scholar] [CrossRef]
- Moldagaliyeva, D.; Uzakov, Y.; Sarsembayeva, N.; Ibazhanova, A.; Jussipbekova, B.; Nurakhova, A.; Artykbayeva, U.; Baimuratova, M. Functional semi-finished fish product evaluation: Organoleptic and evidence in vivo. Front. Sustain. Food Syst. 2023, 7, 1190340. [Google Scholar] [CrossRef]
- Madhu, M.; Kumar, D.; Sirohi, R.; Tarafdar, A.; Dhewa, T.; Aluko, R.E.; Badgujar, P.C.; Awasthi, M.K. Bioactive peptides from meat: Current status on production, biological activity, safety, and regulatory framework. Chemosphere 2022, 307, 135650. [Google Scholar] [CrossRef]
- Kruger, C.L.; Mann, S.W. Safety evaluation of functional ingredients. Food Chem. Toxicol. 2003, 41, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 3294. [Google Scholar] [CrossRef]
- Najafian, L. A review of bioactive peptides as functional food ingredients: Mechanisms of action and their applications in active packaging and food quality improvement. Food Funct. 2023, 14, 5835–5857. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.L.; Tan, C.M.; Shinali, T.S.; Zhang, B.; Zhang, L.L.; Han, Z.X.; Shang, N. Plant antimicrobial peptides: A comprehensive review of their classification, production, mode of action, functions, applications, and challenges. Food Funct. 2023, 14, 5492–5515. [Google Scholar] [CrossRef]
- Cui, P.B.; Li, M.Y.; Yu, M.X.; Liu, Y.F.; Ding, Y.T.; Liu, W.L.; Liu, J.H. Advances in sports food: Sports nutrition, food manufacture, opportunities and challenges. Food Res. Int. 2022, 151, 111258. [Google Scholar] [CrossRef]
- O’Brien, P. Regulation of functional foods in China: A framework in flux. Regul. Rapp. 2016, 12, 15–19. [Google Scholar]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides−Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control 2019, 98, 24–33. [Google Scholar] [CrossRef]
| Sequence | Source | Cell/Animal Model | Amount Added | Action Pathway | Cell Performance | Animal Performance | References |
|---|---|---|---|---|---|---|---|
| FKSETKNLL | Bovine lactoferrin | MC3T3-E1 cell | 200 μg/mL | MAPK signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [51] |
| VSEE | Duck Egg White | MC3T3-E1 cell | 1 mM | Wnt/β-catenin signaling pathway | Cell proliferation, differentiation, and mineralization ↑ | Osteoporosis ↓, dyslipidemia ↓ | [6] |
| — | Porcine bone | MC3T3-E1 cell | 0.5 mg/mL | PI3K/AKT signaling pathway | Cell proliferation and differentiation ↑, Cell cycle progression ↑ | Osteoporosis ↓ | [55] |
| GPAGPPGPIGNV | Yak bones | MC3T3-E1 cell | 60.6 mg/mL | Wnt/β-catenin signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [56] |
| GPAGPSGPAGK, GPPGSPGPR | Bovine Gelatin | MC3T3-E1 cell | 3 mg/mL | MAPK/ERK1/2 signaling pathway | Cell proliferation, differentiation, and mineralization ↑ | Osteoporosis ↓ Osteoarthritis ↓ | [30] |
| — | Whey protein | MC3T3-E1 cell | 500 μg/mL | — | Cell proliferation, differentiation, and mineralization ↑ | Osteoporosis ↓protects bones | [57] |
| ARHPHPHLSF, AAGGPGAPADPGRPTGY, NIPPLTQTPVVVPPFLQPE | Fermented milk | MC3T3-E1 cell | 2 μM | MAPK signaling pathway | Cell proliferation, differentiation, and mineralization ↑ | Osteoporosis ↓protects bones | [5] |
| HHGDQGAPGAVGPAGPRGPAGPSGPAGKDGR, GPAGANDRGEAGPAGPAGPR | Bovine Bone | MC3T3-E1 cell | 48.0 mg/mL | — | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [54] |
| PASTGAAK, PGPPGTPF | black-boned silky fowl | MC3T3-E1 cell | 400 μg/mL | BMP-2/Smad signaling pathway | Cell proliferation and differentiation ↑ | Osteoporosis ↓ | [58] |
| VLVLDTDYKK, VGINYWLAHK | Whey protein | RAW 264.7 cell | 1.25 mg/mL | — | Cell proliferation ↑ | Free radicals ↓, anti-inflammatory | [53] |
| COLPROPURD | Porcine fresh bones | Monocytic, lymphocyte, and Caco-2 | 0.15 mg/mL, 1.4 mg/mL,137.5 μg/mL | — | Cell proliferation ↑ and cytokine ↑ | Anti-intestinal inflammation, immunomodulation ↑ | [18] |
| EF, AGGF, EHPT | Black-bone silky fowl | Mice spleen | 1 mM | — | Lymphocyte proliferation ↑ | Immunomodulation ↑ | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Liu, Y.; Zhang, X.; Zan, L.; Fang, X. The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms. Metabolites 2025, 15, 505. https://doi.org/10.3390/metabo15080505
Yan Y, Liu Y, Zhang X, Zan L, Fang X. The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms. Metabolites. 2025; 15(8):505. https://doi.org/10.3390/metabo15080505
Chicago/Turabian StyleYan, Yuhao, Yinuo Liu, Xinwei Zhang, Liting Zan, and Xibi Fang. 2025. "The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms" Metabolites 15, no. 8: 505. https://doi.org/10.3390/metabo15080505
APA StyleYan, Y., Liu, Y., Zhang, X., Zan, L., & Fang, X. (2025). The Promotion of Cell Proliferation by Food-Derived Bioactive Peptides: Sources and Mechanisms. Metabolites, 15(8), 505. https://doi.org/10.3390/metabo15080505