Investigating Lipid and Energy Dyshomeostasis Induced by Per- and Polyfluoroalkyl Substances (PFAS) Congeners in Mouse Model Using Systems Biology Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Liver Transcriptomics Datasets
2.2. Transcriptomics and Pathway-Based Enrichment Analysis of Metabolic Genes
2.3. Transcriptomics Data Integration with iMM1865 Mouse GEM
2.4. Statistical Analysis
2.5. Data and Code Availability
3. Results
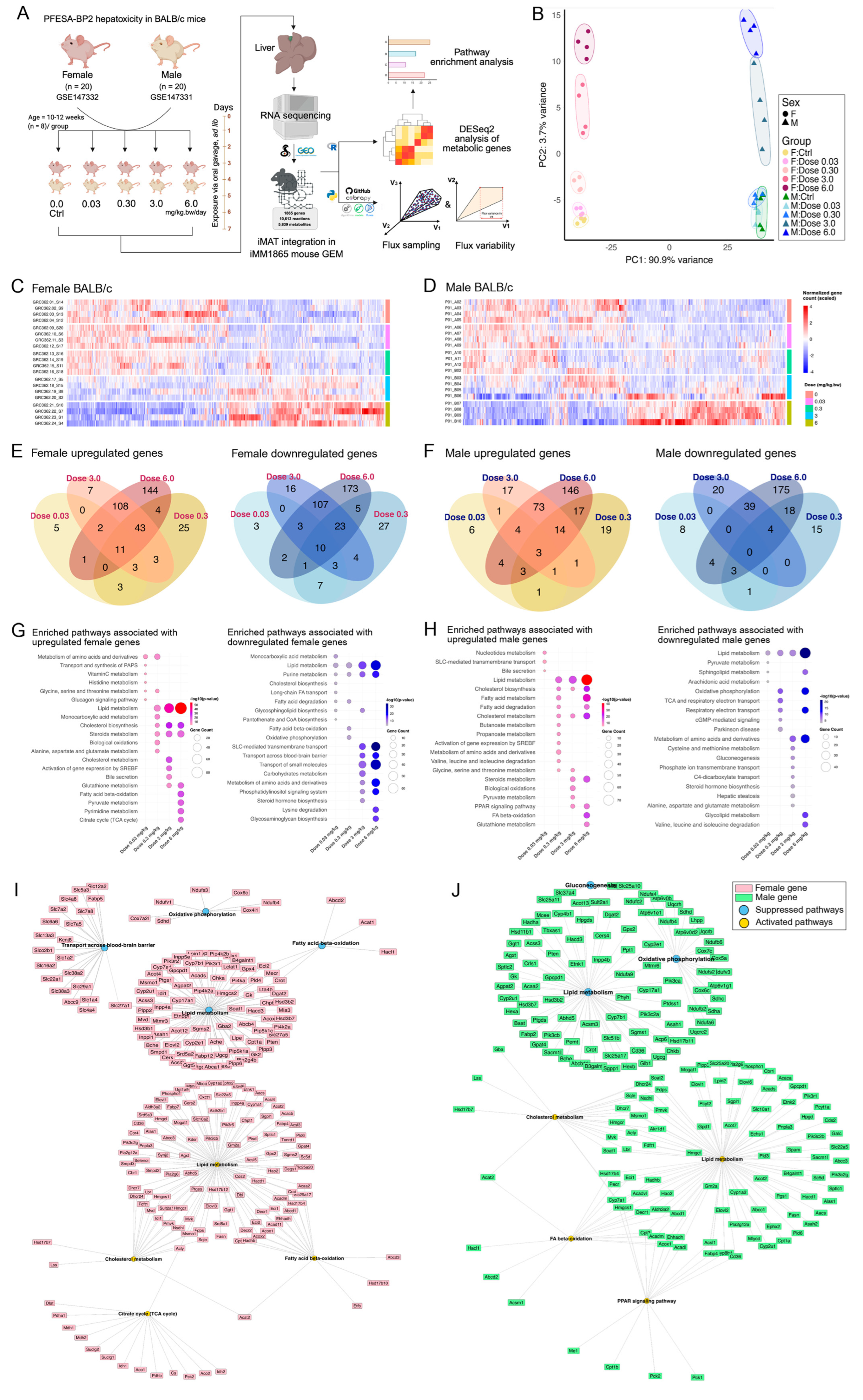
3.1. PFESA-BP2 Hepatotoxicity in BALB/c Mice Targets Lipid Metabolism in a Sex- and Dose-Dependent Pattern
3.2. PFESA-BP2 Hepatotoxicity in BALB/c Mice Is Associated with Energy Dyshomeostasis
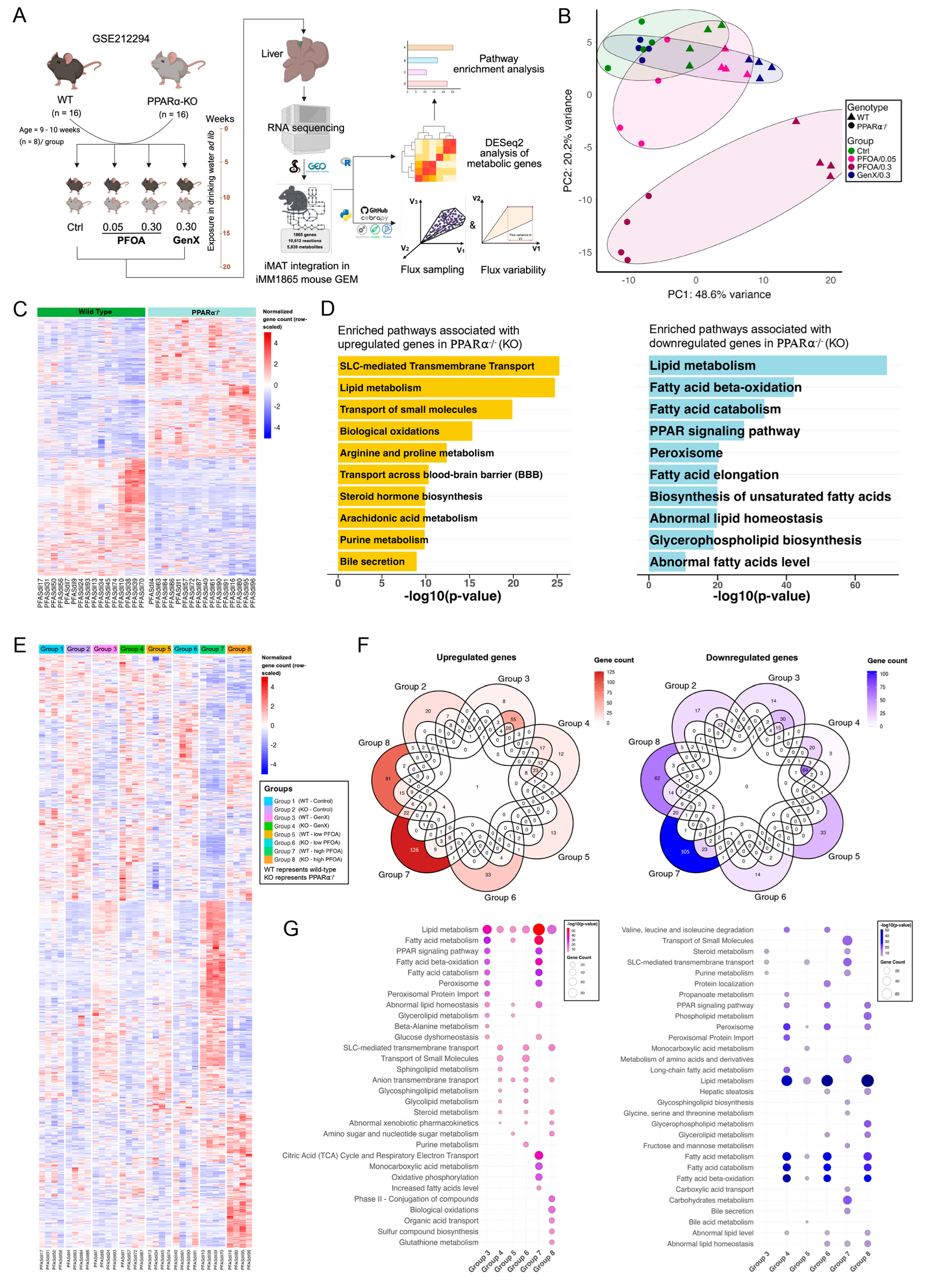
3.3. PFOA and GenX Exposure Targets Fatty Acid and Lipid Metabolism
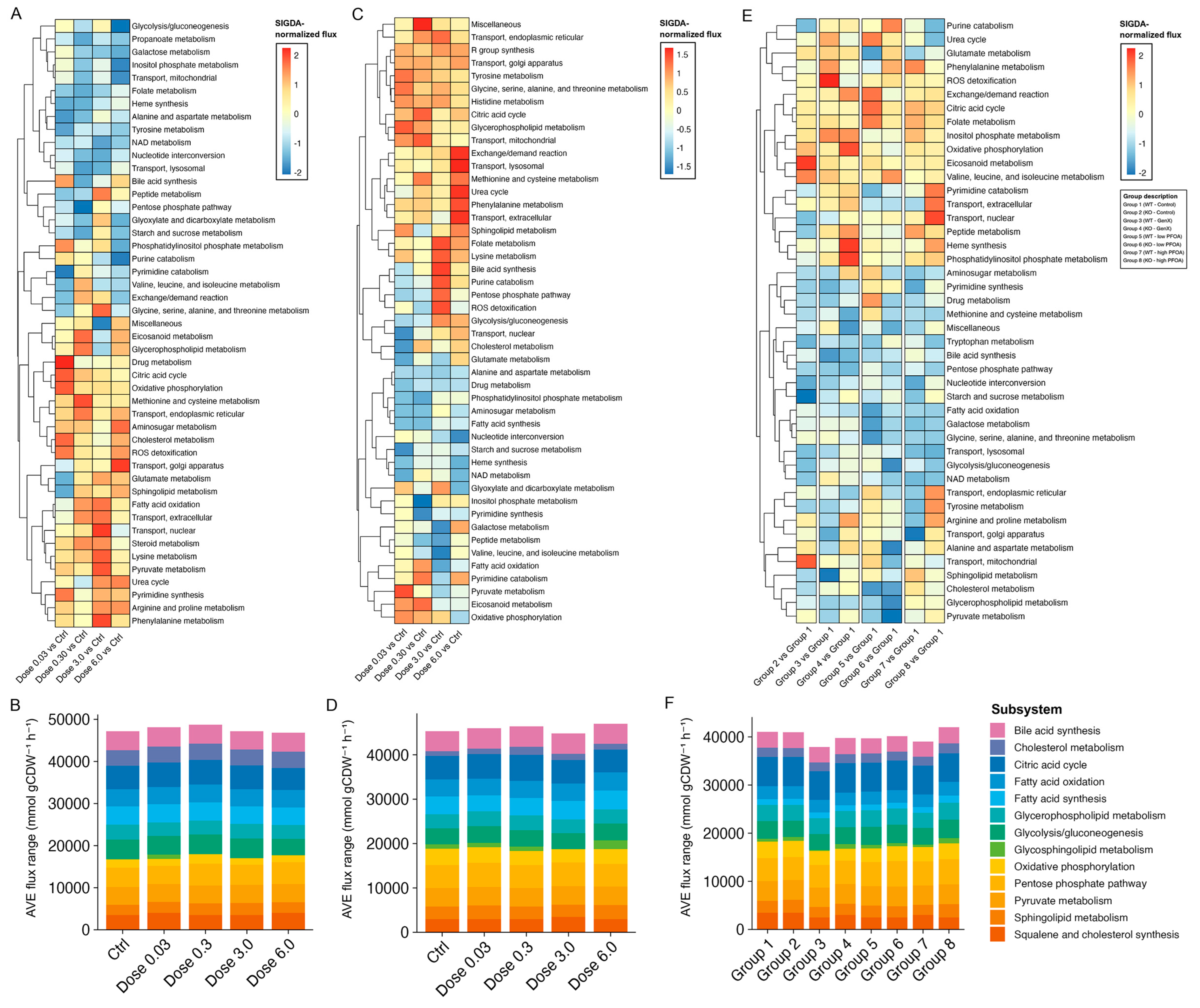
3.4. Identifying Perturbations in Energy and Lipid Metabolism Due to PFAS Exposure Using Integrated Genome-Scale Metabolic Models
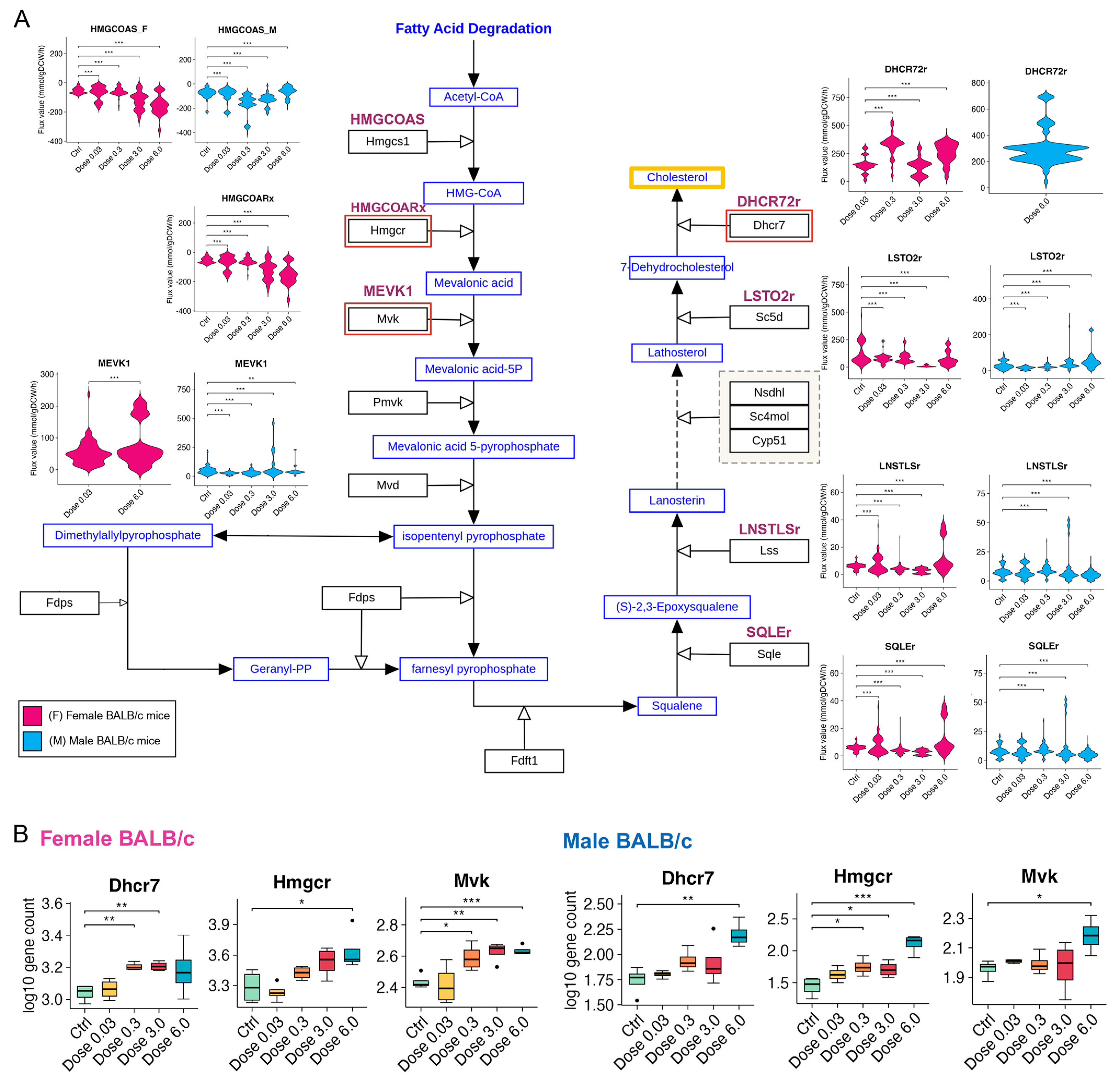
3.5. PFESA-BP2 Exposure Causes Activation of Cholesterol Biosynthesis in a Dose-Dependent Manner
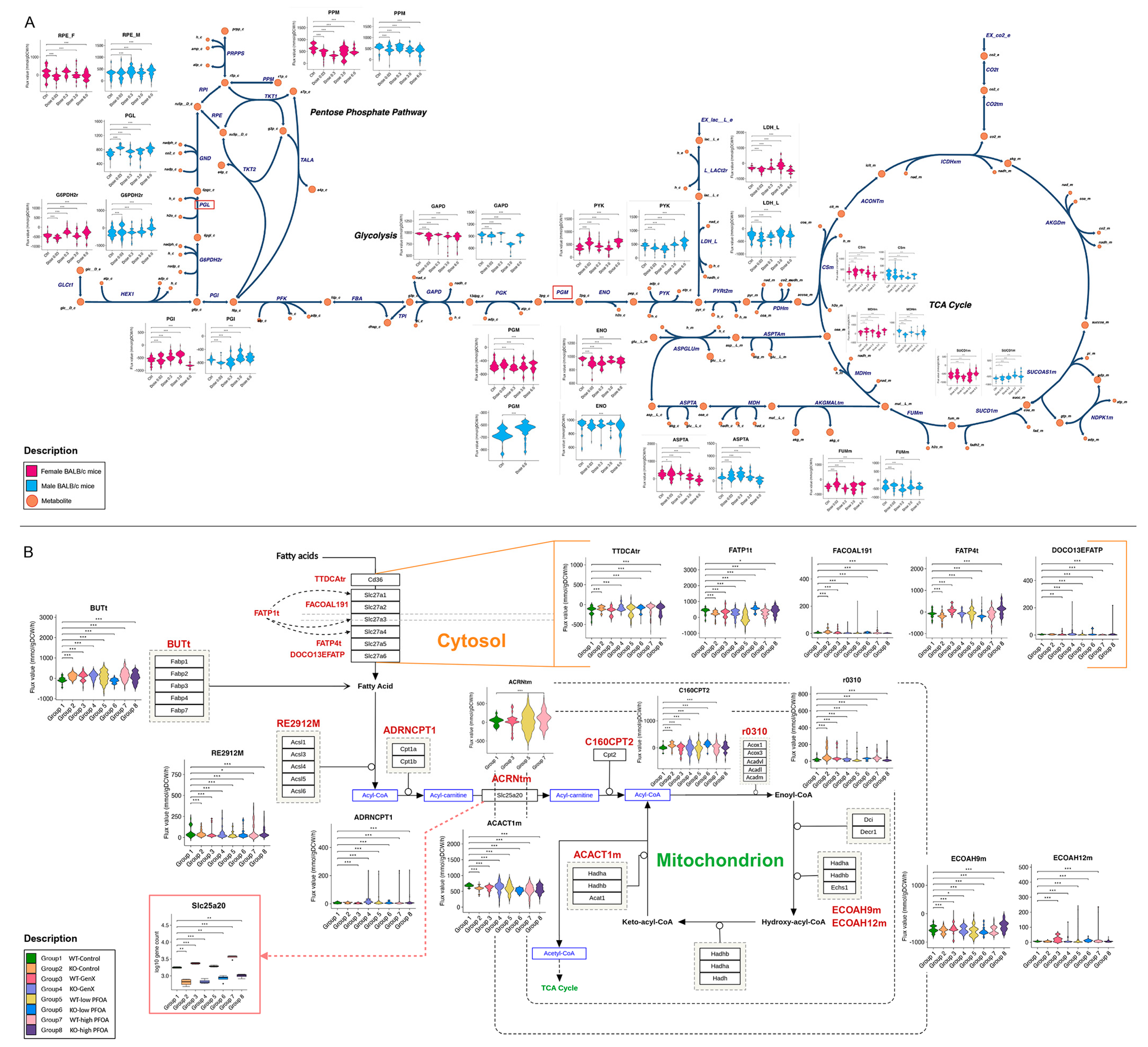
3.6. PFAS Exposure Causes Energy Dyshomeostasis via Targeting Carbon Metabolism and β-Oxidation
4. Discussion
4.1. Limitations of the Study
4.2. Applications of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase pathway |
| ACs | Acylcarnitines |
| BBB | Blood–brain barrier |
| BiGG | Biochemical, Genetic, and Genomic Knowledgebase |
| COBRA | Constraint-based reconstruction and analysis |
| DEGs | Differentially expressed genes |
| FA | Fatty acid |
| FBA | Flux balance analysis |
| FVA | Flux variability analysis |
| GEM | Genome-scale metabolic model |
| GenX | Hexafluoropropylene oxide dimer acid |
| GEO | Gene expression omnibus |
| GSEA | Gene set enrichment analysis |
| iMAT | Integrative metabolic analysis tool |
| mTOR | Mammalian target of rapamycin pathway |
| PPP | Pentose phosphate pathway |
| PPAR | Peroxisome proliferator-activated receptor |
| PFAS | Per-(poly) fluoroalkyl substances |
| PFOA | Perfluorooctanoic acid |
| PFESA-BP2 | 7H-Perfluoro-4-methyl-3,6-dioxaoctanesulfonic acid |
| SREPFs | Sterol regulatory element-binding transcription factors |
| TCA | Tricarboxylic acid |
References
- Panel, E.C.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Annexes to the Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food; Zenodo: Geneva, Switzerland, 2020. [Google Scholar]
- Cao, L.; Guo, Y.; Chen, Y.; Hong, J.; Wu, J.; Hangbiao, J. Per-/polyfluoroalkyl substance concentrations in human serum and their associations with liver cancer. Chemosphere 2022, 296, 134083. [Google Scholar] [CrossRef]
- Corton, J.C.; Gift, J.S.; Auerbach, S.S.; Liu, J.; Das, K.P.; Ren, H.; Lang, J.R.; Chernoff, N.; Lau, C.; Hill, D. Dose–response modeling of effects in mice after exposure to a polyfluoroalkyl substance (Nafion byproduct 2). Toxicol. Sci. 2025, 205, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Medlock-Kakaley, E.; Hill, D.; McCord, J.; Strynar, M.J.; Wehmas, L.C.; Hester, S.; MacMillan, D.K.; et al. Developmental toxicity of Nafion byproduct 2 (NBP2) in the Sprague-Dawley rat with comparisons to hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) and perfluorooctane sulfonate (PFOS). Environ. Int. 2022, 160, 107056. [Google Scholar] [CrossRef]
- Quist, E.M.; Filgo, A.J.; Cummings, C.A.; Kissling, G.E.; Hoenerhoff, M.J.; Fenton, S.E. Hepatic Mitochondrial Alteration in CD-1 Mice Associated with Prenatal Exposures to Low Doses of Perfluorooctanoic Acid (PFOA). Toxicol. Pathol. 2015, 43, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tu, P.C.; Chi, L.; Shen, W.; Gao, N. Perfluorooctanoic Acid-Disturbed Serum and Liver Lipidome in C57BL/6 Mice. Chem. Res. Toxicol. 2022, 35, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Kostecki, G.; Chuang, K.; Buxton, A.; Dakshanamurthy, S. Dose-Dependent PFESA-BP2 Exposure Increases Risk of Liver Toxicity and Hepatocellular Carcinoma. Curr. Issues Mol. Biol. 2025, 47, 98. [Google Scholar] [CrossRef]
- Kirkwood-Donelson, K.I.; Chappel, J.; Tobin, E.; Dodds, J.N.; Reif, D.M.; DeWitt, J.C.; Baker, E.S. Investigating mouse hepatic lipidome dysregulation following exposure to emerging per- and polyfluoroalkyl substances (PFAS). Chemosphere 2024, 354, 141654. [Google Scholar] [CrossRef]
- Salihovic, S.; Fall, T.; Ganna, A.; Broeckling, C.D.; Prenni, J.E.; Hyötyläinen, T.; Kärrman, A.; Lind, P.M.; Ingelsson, E.; Lind, L. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 196–205. [Google Scholar] [CrossRef]
- Rashid, F.; Dubinkina, V.; Ahmad, S.; Maslov, S.; Irudayaraj, J.M.K. Gut Microbiome-Host Metabolome Homeostasis upon Exposure to PFOS and GenX in Male Mice. Toxics 2023, 11, 281. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; McCord, J.; Strynar, M.J.; Hill, D.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.E. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ. Int. 2021, 146, 106204. [Google Scholar] [CrossRef]
- Blake, B.E.; Cope, H.A.; Hall, S.M.; Keys, R.D.; Mahler, B.W.; McCord, J.; Scott, B.; Stapleton, H.M.; Strynar, M.J.; Elmore, S.A.; et al. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ. Health Perspect. 2020, 128, 27006. [Google Scholar] [CrossRef]
- Guillette, T.; McCord, J.; Guillette, M.; Polera, M.; Rachels, K.T.; Morgeson, C.; Kotlarz, N.; Knappe, D.R.; Reading, B.J.; Strynar, M.; et al. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ. Int. 2020, 136, 105358. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Zhang, X.; Zhu, J.; Wang, L.; Ji, M.; Zhang, Z.; Ji, X.M.; Wang, S.L. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ. Pollut. 2020, 256, 113429. [Google Scholar] [CrossRef]
- Brown-Leung, J.M.; Cannon, J.R. Neurotransmission Targets of Per- and Polyfluoroalkyl Substance Neurotoxicity: Mechanisms and Potential Implications for Adverse Neurological Outcomes. Chem. Res. Toxicol. 2022, 35, 1312–1333. [Google Scholar] [CrossRef] [PubMed]
- Pouwer, M.G.; Pieterman, E.J.; Chang, S.C.; Olsen, G.W.; Caspers, M.P.M.; Verschuren, L.; Wouter Jukema, J. Princen HMG Dose Effects of Ammonium Perfluorooctanoate on Lipoprotein Metabolism in APOE*3-Leiden.CETP Mice. Toxicol. Sci. 2019, 168, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Schlezinger, J.J.; Puckett, H.; Oliver, J.; Nielsen, G.; Heiger-Bernays, W.; Webster, T.F. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol. Appl. Pharmacol. 2020, 405, 115204. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.C.; Shnyra, A.; Badr, M.Z.; Loveless, S.E.; Hoban, D.; Frame, S.R.; Cunard, R.; Anderson, S.E.; Meade, B.J.; Peden-Adams, M.M.; et al. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol. 2009, 39, 76–94. [Google Scholar] [CrossRef]
- Takacs, M.L.; Abbott, B.D. Activation of mouse and human peroxisome proliferator-activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007, 95, 108–117. [Google Scholar] [CrossRef]
- Attema, B.; Janssen, A.W.F.; Rijkers, D.; van Schothorst, E.M.; Hooiveld, G.J.E.J.; Kersten, S. Exposure to low-dose perfluorooctanoic acid promotes hepatic steatosis and disrupts the hepatic transcriptome in mice. Mol. Metab. 2022, 66, 101602. [Google Scholar] [CrossRef]
- Yang, S.L.; Ma, B.J.; Lu, Y.S.; Chen, J.; Yu, J.; Qiu, J.; Qian, Y.Z.; Xu, Y.Y. Multi-omics reveals the molecular mechanism of the combined toxic effects of PFOA and 4-HBP exposure in MCF-7 cells and the key player: mTORC1. Environ. Int. 2024, 188, 108778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Xu, L.L.; Zhong, M.T.; Chen, Y.K.; Lai, M.Q.; Wang, Q.; Xie, X.L. Gestational GenX and PFOA exposures induce hepatotoxicity, metabolic pathway, and microbiome shifts in weanling mice. Sci. Total Environ. 2024, 907, 168059. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, C.-F.; Han, F.; Liu, C.; Zhang, A.; Hsu, C.-C.; Peng, D.; Zhang, X.; Jin, G.; Rezaeian, A.-H.; et al. Phosphorylation of PDHA by AMPK Drives TCA Cycle to Promote Cancer Metastasis. Mol. Cell 2020, 80, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Peng, D.; Cai, Z.; Lin, H.K. AMPK signaling and its targeting in cancer progression and treatment. Semin. Cancer Biol. 2022, 85, 52–68. [Google Scholar] [CrossRef]
- Wallis, D.J.; Kotlarz, N.; Knappe, D.R.U.; Collier, D.N.; Lea, C.S.; Reif, D.; McCord, J.; Strynar, M.; DeWitt, J.C.; Hoppin, J.A. Estimation of the Half-Lives of Recently Detected Per- and Polyfluorinated Alkyl Ethers in an Exposed Community. Environ. Sci. Technol. 2023, 57, 15248–15355. [Google Scholar] [CrossRef]
- Wen, Y.; Mirji, N.; Irudayaraj, J. Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol. Vitr. 2020, 65, 104797. [Google Scholar] [CrossRef]
- Sheng, N.; Cui, R.; Wang, J.; Guo, Y.; Wang, J.; Dai, J. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch. Toxicol. 2018, 92, 359–369. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Strynar, M.J.; McCord, J.; McIntyre, B.S.; Travlos, G.S.; Cardon, M.C.; Medlock-Kakaley, E.; Hartig, P.C.; et al. Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ. Health Perspect. 2019, 127, 37008. [Google Scholar] [CrossRef]
- Lang, J.R.; Strynar, M.J.; Lindstrom, A.B.; Farthing, A.; Huang, H.; Schmid, J.; Hill, D.; Chernoff, N. Toxicity of Balb-c mice exposed to recently identified 1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3-(1,1,2,2-tetrafluoroethoxy)propan-2-yl]oxyethane-1-sulfonic acid (PFESA-BP2). Toxicology 2020, 441, 152529. [Google Scholar] [CrossRef]
- Baloni, P.; Sangar, V.; Yurkovich, J.T.; Robinson, M.; Taylor, S.; Karbowski, C.M.; Hamadeh, H.K.; He, Y.D.; Price, N.D. Genome-scale metabolic model of the rat liver predicts effects of diet restriction. Sci. Rep. 2019, 9, 9807. [Google Scholar] [CrossRef]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Louie, G.; Saykin, A.J.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef]
- Turanli, B.; Zhang, C.; Kim, W.; Benfeitas, R.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Discovery of therapeutic agents for prostate cancer using genome-scale metabolic modeling and drug repositioning. eBioMedicine 2019, 42, 386–396. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.L.; Kocabas, P.; Gustafsson, J.; Anton, M.; Cholley, P.-E.; Huang, S.; Gobom, J.; Svensson, T.; Uhlen, M.; et al. Genome-scale metabolic network reconstruction of model animals as a platform for translational research. Proc. Natl. Acad. Sci. USA 2021, 118, 2102344118. [Google Scholar] [CrossRef]
- Baloni, P.; Arnold, M.; Buitrago, L.; Nho, K.; Moreno, H.; Huynh, K.; Brauner, B.; Louie, G.; Kueider-Paisley, A.; Suhre, K.; et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. Commun. Biol. 2022, 5, 1074. [Google Scholar] [CrossRef]
- Focil, C.; Canto-Encalada, G.; Campos, D.T.; Zuñiga, C.; Zepeda, A. Applying genome-scale metabolic modeling tools to understand microbial communities in wastewater treatment. In Development in Waste Water Treatment Research and Processes: Role of Environmental Microbiology in Industrial Wastewater Research; Elsevier: Amsterdam, The Netherlands, 2024; pp. 297–332. [Google Scholar]
- Ofaim, S.; Zarecki, R.; Porob, S.; Gat, D.; Lahav, T.; Kashi, Y.; Aly, R.; Eizenberg, H.; Ronen, Z.; Freilich, S. Genome-scale reconstruction of Paenarthrobacter aurescens TC1 metabolic model towards the study of atrazine bioremediation. Sci. Rep. 2020, 10, 13019. [Google Scholar] [CrossRef]
- Sun, J.; Sayyar, B.; Butler, J.E.; Pharkya, P.; Fahland, T.R.; Famili, I.; Schilling, C.H.; Lovley, D.R.; Mahadevan, R. Genome-scale constraint-based modeling of Geobacter metallireducens. BMC Syst. Biol. 2009, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Nogales, J.; Palsson, B.; Thiele, I. A genome-scale metabolic reconstruction of Pseudomonas putida KT2440: iJN746 as a cell factory. BMC Syst. Biol. 2008, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Canto-Encalada, G.; Tec-Campos, D.; Tibocha-Bonilla, J.D.; Zengler, K.; Zepeda, A.; Zuñiga, C. Flux balance analysis of the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC19718 unravels specific metabolic activities while degrading toxic compounds. PLoS Comput. Biol. 2022, 18, e1009828. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, R.; Bond, D.R.; Butler, J.E.; Esteve-Nuñez, A.; Coppi, M.V.; Palsson, B.O.; Schilling, C.H.; Lovley, D.R. Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl. Environ. Microbiol. 2006, 72, 1558–1568. [Google Scholar] [CrossRef]
- Navid, A.; Jiao, Y.; Wong, S.E.; Pett-Ridge, J. System-level analysis of metabolic trade-offs during anaerobic photoheterotrophic growth in Rhodopseudomonas palustris. BMC Bioinform. 2019, 20, 233. [Google Scholar] [CrossRef]
- Pannala, V.R.; Hari, A.; Abdulhameed, M.D.M.; Balik-Meisner, M.R.; Mav, D.; Phadke, D.P.; Scholl, E.H.; Shah, R.R.; Auerbach, S.S.; Wallqvist, A. Quantifying liver-toxic responses from dose-dependent chemical exposures using a rat genome-scale metabolic model. Toxicol. Sci. 2025, 204, 154–168. [Google Scholar] [CrossRef]
- Khodaee, S.; Asgari, Y.; Totonchi, M. Karimi-Jafari MH iMM1865: A New Reconstruction of Mouse Genome-Scale Metabolic Model. Sci. Rep. 2020, 10, 6177. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets–Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.M.; Mohamed, W.M.A.; Lau, A.C.C.; Chatanga, E.; Qiu, Y.; Hayashi, N.; Naguib, D.; Sato, K.; Takano, A.; Matsuno, K.; et al. R A language and environment for statistical computing, R Foundation for Statistical. Computing 2020, 20, 1979–1992. [Google Scholar]
- Evangelista, J.E.; Xie, Z.; Marino, G.B.; Nguyen, N.; Clarke, D.J.B.; Ma’Ayan, A. Enrichr-KG: Bridging enrichment analysis across multiple libraries. Nucleic Acids Res. 2023, 51, W168–W179. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Smith, C.L.; Eppig, J.T. The mammalian phenotype ontology: Enabling robust annotation and comparative analysis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 390–399. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Wilkinson, L. ggplot2: Elegant Graphics for Data Analysis by WICKHAM, H. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Gao, C.H.; Chen, C.; Akyol, T.; Dusa, A.; Yu, G.; Cao, B.; Cai, P. ggVennDiagram: Intuitive Venn diagram software extended. iMeta 2024, 3, e177. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.B. Tidyplots empowers life scientists with easy code-based data visualization. iMeta 2025, 4, e70018. [Google Scholar] [CrossRef] [PubMed]
- Zur, H.; Ruppin, E.; Shlomi, T. iMAT: An integrative metabolic analysis tool. Bioinformatics 2010, 26, 3140–3142. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef]
- Robinson, M.; Zimmer, A.; Farrah, T.; Mauldin, D.; Price, N.; Hood, L.; Glusman, G. Scale-invariant geometric data analysis (SIGDA) provides robust, detailed visualizations of human ancestry specific to individuals and populations. bioRxiv 2018. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Thiele, I. Computationally efficient flux variability analysis. BMC Bioinform. 2010, 11, 489. [Google Scholar] [CrossRef]
- Schellenberger, J.; Que, R.; Fleming, R.M.T.; Thiele, I.; Orth, J.D.; Feist, A.M.; Zielinski, D.C.; Bordbar, A.; Lewis, N.E.; Rahmanian, S.; et al. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox v2.0. Nat. Protoc. 2011, 6, 1290–1307. [Google Scholar] [CrossRef]
- Ebrahim, A.; Lerman, J.A.; Palsson, B.O.; Hyduke, D.R. COBRApy: COnstraints-Based Reconstruction and Analysis for Python. BMC Syst. Biol. 2013, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kotlarz, N.; McCord, J.; Collier, D.; Lea, C.S.; Strynar, M.; Lindstrom, A.B.; Wilkie, A.A.; Islam, J.Y.; Matney, K.; Tarte, P.; et al. Measurement of novel, drinking water-associated pfas in blood from adults and children in Wilmington, North Carolina. Environ. Health Perspect. 2020, 128, 77005. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, Z.R.; Sun, M.; DeWitt, J.C.; Knappe, D.R.U. Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. Am. Water Work. Assoc. 2018, 110, 13–28. [Google Scholar] [CrossRef]
- Wang, G.; Li, M.; Wang, Y.; Wang, B.; Pu, H.; Mao, J.; Zhang, S.; Zhou, S.; Luo, P. Characterization of differentially expressed and lipid metabolism-related lncRNA-mRNA interaction networks during the growth of liver tissue through rabbit models. Front. Vet. Sci. 2022, 9, 998796. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Gao, R.; Liu, M.; Xie, W. A comprehensive review of the family of very-long-chain fatty acid elongases: Structure, function, and implications in physiology and pathology. Eur. J. Med. Res. 2023, 28, 532. [Google Scholar] [CrossRef]
- Bishop-Bailey, D.; Thomson, S.; Askari, A.; Faulkner, A.; Wheeler-Jones, C. Lipid-metabolizing CYPs in the regulation and dysregulation of metabolism. Annu. Rev. Nutr. 2014, 34, 261–279. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Zhang, H.; Yao, J.; Sheng, N.; Li, Q.; Guo, Y.; Wu, C.; Xie, W.; Dai, J. Exposure to GenX and Its Novel Analogs Disrupts Hepatic Bile Acid Metabolism in Male Mice. Environ. Sci. Technol. 2022, 56, 6133–6143. [Google Scholar] [CrossRef]
- Kersten, S.; Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 2017, 136, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, X.; Sun, W.; Griffin, N.; Wang, L.; Liu, H. PFOA exposure induces aberrant glucose and lipid metabolism in the rat liver through the AMPK/mTOR pathway. Toxicology 2023, 493, 153551. [Google Scholar] [CrossRef] [PubMed]
- Kuriya, Y.; Murata, M.; Yamamoto, M.; Watanabe, N.; Araki, M. Prediction of Metabolic Flux Distribution by Flux Sampling: As a Case Study, Acetate Production from Glucose in Escherichia coli. Bioengineering 2023, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Agrawal, A.; Balcı, H.; Hanspers, K.; Coort, S.L.; Martens, M.; Slenter, D.N.; Ehrhart, F.; Digles, D.; Waagmeester, A.; Wassink, I.; et al. WikiPathways 2024: Next generation pathway database. Nucleic Acids Res. 2024, 52, D679–D689. [Google Scholar] [CrossRef]
- King, Z.A.; Dräger, A.; Ebrahim, A.; Sonnenschein, N.; Lewis, N.E.; Palsson, B.O. Escher: A Web Application for Building, Sharing, and Embedding Data-Rich Visualizations of Biological Pathways. PLoS Comput. Biol. 2015, 11, e1004321. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFAS) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Schillemans, T.; Bergdahl, I.A.; Hanhineva, K.; Shi, L.; Donat-Vargas, C.; Koponen, J.; Kiviranta, H.; Landberg, R.; Åkesson, A.; Brunius, C. Associations of PFAS-related plasma metabolites with cholesterol and triglyceride concentrations. Environ. Res. 2023, 216, 114570. [Google Scholar] [CrossRef]
- India-Aldana, S.; Yao, M.; Midya, V.; Colicino, E.; Chatzi, L.; Chu, J.; Gennings, C.; Jones, D.P.; Loos, R.J.F.; Setiawan, V.W.; et al. PFAS Exposures and the Human Metabolome: A Systematic Review of Epidemiological Studies. Curr. Pollut. Rep. 2023, 9, 510–568. [Google Scholar] [CrossRef]
- Permuth-Wey, J.; Chen, Y.A.; Tsai, Y.-Y.; Chen, Z.; Qu, X.; Lancaster, J.M.; Stockwell, H.; Dagne, G.; Iversen, E.; Risch, H.; et al. Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1131–1145. [Google Scholar] [CrossRef]
- Mikkelsen, A.C.D.; Kjærgaard, K.; Schapira, A.H.V.; Mookerjee, R.P.; Thomsen, K.L. The liver–brain axis in metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 2025, 10, 248–258. [Google Scholar] [CrossRef]
- Pan, Y.; Short, J.L.; Choy, K.H.C.; Zeng, A.X.; Marriott, P.J.; Owada, Y.; Scanlon, M.J.; Porter, C.J.H.; Nicolazzo, J.A. Fatty Acid-Binding Protein 5 at the Blood–Brain Barrier Regulates Endogenous Brain Docosahexaenoic Acid Levels and Cognitive Function. J. Neurosci. 2016, 36, 11755. [Google Scholar] [CrossRef]
- Canova, C.; Barbieri, G.; Zare Jeddi, M.; Gion, M.; Fabricio, A.; Daprà, F.; Russo, F.; Fletcher, T.; Pitter, G. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ. Int. 2020, 145, 106117. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Zobel, L.R. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health 2007, 81, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Tinker, S.; Frisbee, S.; Ducatman, A.; Vaccarino, V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol. 2009, 170, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, K.; Li, X.; Zhao, H.; Fu, J.; Wang, L.; Zhu, N.; Cai, Z.; Liang, Y.; Wang, Y.; et al. Mass Spectrometry-Based Metabolomics Reveals Occupational Exposure to Per- and Polyfluoroalkyl Substances Relates to Oxidative Stress, Fatty Acid β-Oxidation Disorder, and Kidney Injury in a Manufactory in China. Environ. Sci. Technol. 2019, 53, 9800–9809. [Google Scholar] [CrossRef]
- Panagopoulos Abrahamsson, D.; Wang, A.; Jiang, T.; Wang, M.; Siddharth, A.; Morello-Frosch, R.; Park, J.S.; Sirota, M.; Woodruff, T.J. A Comprehensive Non-targeted Analysis Study of the Prenatal Exposome. Environ. Sci. Technol. 2021, 55, 10542–10557. [Google Scholar] [CrossRef]
- You, L.; Zheng, F.; Su, C.; Wang, L.; Li, X.; Chen, Q.; Kou, J.; Wang, X.; Wang, Y.; Wang, Y.; et al. Metabolome-wide association study of serum exogenous chemical residues in a cohort with 5 major chronic diseases. Environ. Int. 2022, 158, 106919. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, T.; Walker, D.I.; Thomas, D.C.; Qiu, C.; Chatzi, L.; Alderete, T.L.; Kim, J.S.; Conti, D.V.; Breton, C.V.; et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ. Int. 2020, 145, 106091. [Google Scholar] [CrossRef]
- Sinisalu, L.; Yeung, L.W.Y.; Wang, J.; Pan, Y.; Dai, J.; Hyötyläinen, T. Prenatal exposure to poly-/per-fluoroalkyl substances is associated with alteration of lipid profiles in cord-blood. Metabolomics 2021, 17, 103. [Google Scholar] [CrossRef]
- Fragki, S.; Dirven, H.; Fletcher, T.; Grasl-Kraupp, B.; Bjerve Gützkow, K.; Hoogenboom, R.; Kersten, S.; Lindeman, B.; Louisse, J.; Peijnenburg, A.; et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? Crit. Rev. Toxicol. 2021, 51, 141–164. [Google Scholar] [CrossRef]
- Rosen, E.M.; Kotlarz, N.; Knappe, D.R.U.; Lea, C.S.; Collier, D.N.; Richardson, D.B.; Hoppin, J.A. Drinking Water–Associated PFAS and Fluoroethers and Lipid Outcomes in the GenX Exposure Study. Environ. Health Perspect. 2022, 130, 97002. [Google Scholar] [CrossRef]
- Peng, S.; Yan, L.; Zhang, J.; Wang, Z.; Tian, M.; Shen, H. An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid. J. Pharm. Biomed. Anal. 2013, 86, 56–64. [Google Scholar] [CrossRef]
- Yu, N.; Wei, S.; Li, M.; Yang, J.; Li, K.; Jin, L.; Xie, Y.; Giesy, J.P.; Zhang, X.; Yu, H. Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Sci. Rep. 2016, 6, 23963. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- Labine, L.M.; Simpson, M.J. The use of nuclear magnetic resonance (NMR) and mass spectrometry (MS)–based metabolomics in environmental exposure assessment. Curr. Opin. Environ. Sci. Health 2020, 15, 7–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabal, E.; Azaizeh, M.; Baloni, P. Investigating Lipid and Energy Dyshomeostasis Induced by Per- and Polyfluoroalkyl Substances (PFAS) Congeners in Mouse Model Using Systems Biology Approaches. Metabolites 2025, 15, 499. https://doi.org/10.3390/metabo15080499
Gabal E, Azaizeh M, Baloni P. Investigating Lipid and Energy Dyshomeostasis Induced by Per- and Polyfluoroalkyl Substances (PFAS) Congeners in Mouse Model Using Systems Biology Approaches. Metabolites. 2025; 15(8):499. https://doi.org/10.3390/metabo15080499
Chicago/Turabian StyleGabal, Esraa, Marwah Azaizeh, and Priyanka Baloni. 2025. "Investigating Lipid and Energy Dyshomeostasis Induced by Per- and Polyfluoroalkyl Substances (PFAS) Congeners in Mouse Model Using Systems Biology Approaches" Metabolites 15, no. 8: 499. https://doi.org/10.3390/metabo15080499
APA StyleGabal, E., Azaizeh, M., & Baloni, P. (2025). Investigating Lipid and Energy Dyshomeostasis Induced by Per- and Polyfluoroalkyl Substances (PFAS) Congeners in Mouse Model Using Systems Biology Approaches. Metabolites, 15(8), 499. https://doi.org/10.3390/metabo15080499







