Annual Dynamic Changes in Lignin Synthesis Metabolites in Catalpa bungei ‘Jinsi’
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Sample Collection
2.2. Methods
2.2.1. Sample Extraction
2.2.2. UHPLC–MS/MS Conditions
2.2.3. Data Processing
3. Results and Analysis
3.1. Sample Quality Control Analysis
3.2. PCA
3.3. Qualitative and Quantitative Metabolite Analyses
3.3.1. Analysis of Metabolite Composition in Different Months
3.3.2. Change Patterns of Metabolites in Different Months and ANOVA
3.3.3. Changes in Metabolite Accumulation Rates
3.4. Correlation Analysis
3.5. Hierarchical Cluster Analysis
3.6. Differential Metabolite Screening
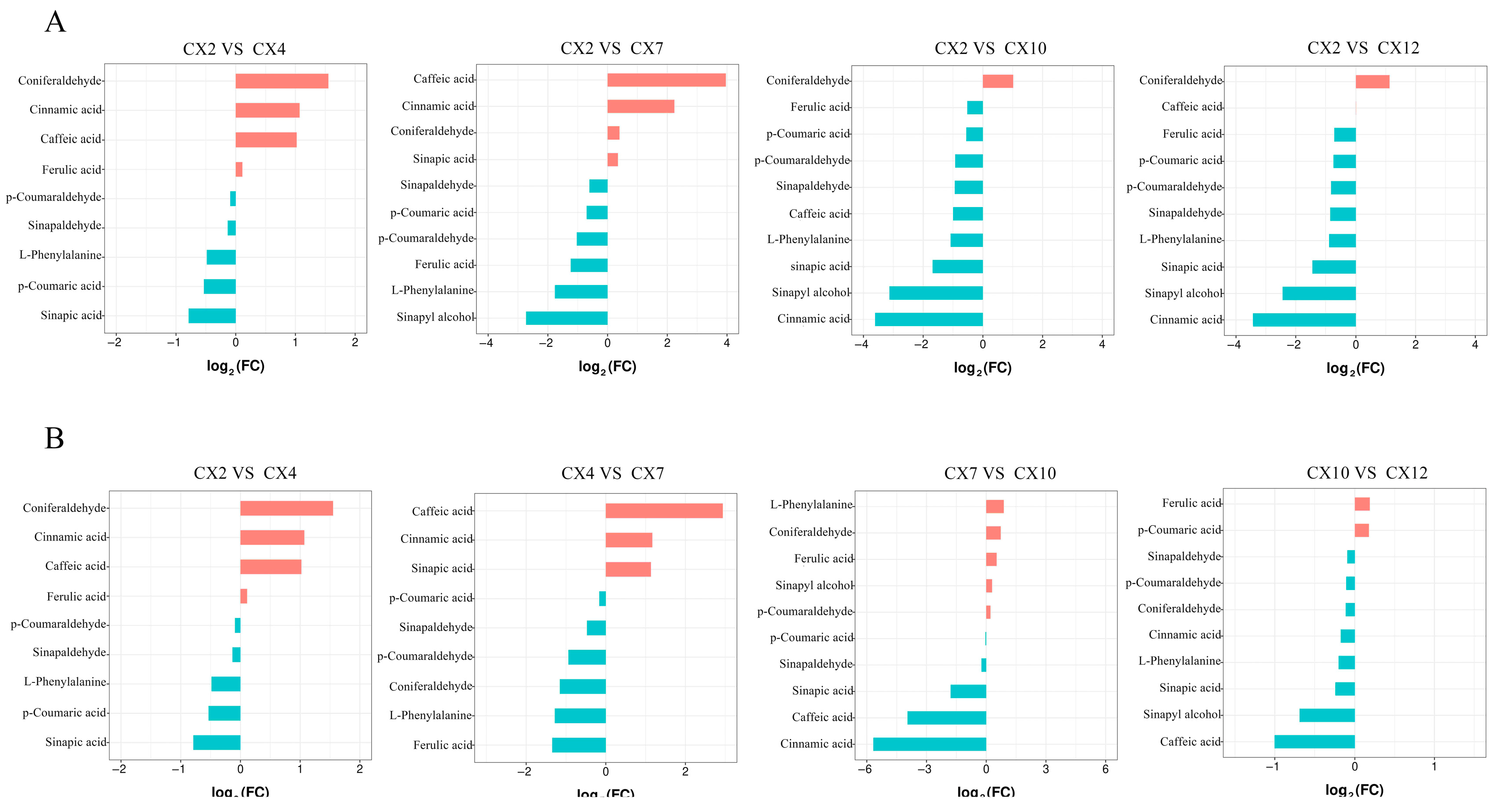
3.6.1. Statistical Analysis of the FC in Metabolites in Different Combinations
3.6.2. Screening of Differential Metabolites in Different Months
3.7. Analysis of Differential Metabolites
3.7.1. Analysis of Differential Metabolites for Different Differential Combinations
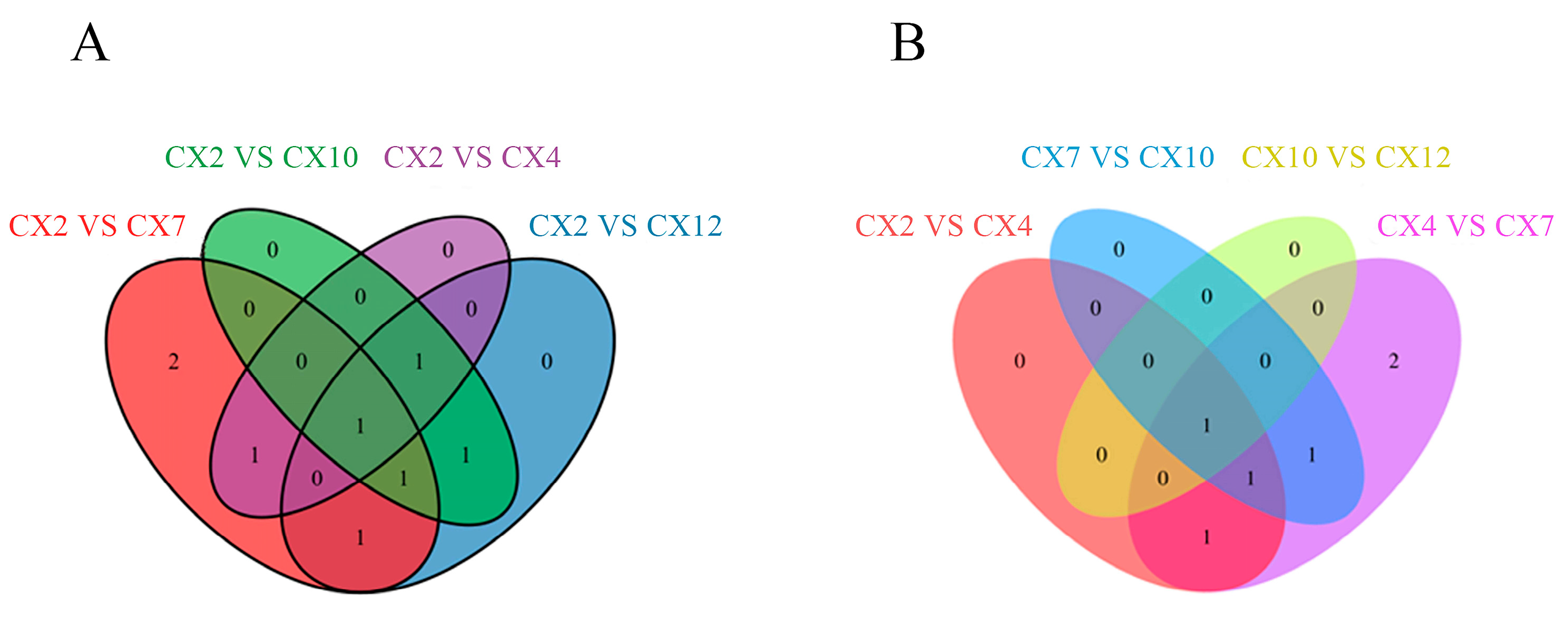
3.7.2. Analysis of Common Differential Metabolites in Different Differential Combinations
3.8. KEGG Enrichment Analysis
3.8.1. KEGG Enrichment Analysis of Metabolites
3.8.2. Changes in Annual Dynamics of Lignin Anabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.Q.; Wang, X.Y.; Jiao, W.; Li, N.; Wang, J.; Zheng, L.Y.; Wang, D.N.; Wang, X.Y.; Hou, L.J.; Shang, Z.Z.; et al. Physiological and transcriptomic analyses of Catalpa bungei ‘Jinsi’ seedlings in response to saline and alkaline stress. For. Res. 2023, 36, 166–178. [Google Scholar] [CrossRef]
- Guo, P.P.; Zhao, X.P.; Yang, Z.F.; Wang, Y.X.; Li, H.Y.; Zhang, L.P. Water, starch, and nuclear behavior in ray parenchyma during heartwood formation of Catalpa bungei ‘Jinsi’. Heliyon 2024, 10, e27231. [Google Scholar] [CrossRef]
- Ma, Q.G.; Wang, Z.J.; Xu, H.M.; Li, M.K.; Li, H.M.; Zhang, J.P.; Zhang, J.G. AFLP analysis of genetic diversity and kinship among superior clones of Catalpa bungei ‘Jinsi’. For. Res. 2020, 33, 145–153. [Google Scholar] [CrossRef]
- Lu, N.; Mei, F.; Wang, Z.; Wang, N.; Xiao, Y.; Kong, L.S.; Qu, G.Z.; Ma, W.J.; Wang, J.H. Single-nucleotide polymorphisms (SNPs) in a sucrose synthase gene are associated with wood properties in Catalpa fargesii bur. BMC Genet. 2018, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zhang, Q.W.; Niu, Z.T.; Lu, M.Z.; Douglas, C.J. Advances in lignin biosynthesis and its gene regulation. World For. Res. 2007, 20, 29–37. [Google Scholar] [CrossRef]
- Bano, N.; Mohammad, N.; Ansari, M.I.; Ansari, S.A. Genotyping SNPs in lignin biosynthesis gene (CAD1) and transcription factors (MYB1 and MYB2) exhibits association with wood density in teak (Tectona grandis L.f.). Mol. Biol. Rep. 2024, 51, 169. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.Y. Inhomogeneity of chemical composition in wood of Catalpa bungei ‘Jinsi’. Sci. Silvae Sin. 2021, 57, 169–177. [Google Scholar] [CrossRef]
- Yi, F. Research on the Mechanism of Heartwood Color Formation in Catalpa bungei ‘Jinsi’. Ph.D. Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2023; pp. 103–104. [Google Scholar] [CrossRef]
- Xu, Y.H.; Yu, X.C.; Yi, F.; Wang, J.H.; Liang, H.W.; Ma, W.J. Cloning and expression analysis of CbuCYP71D15 gene from Catalpa bungei ‘jinsi’. J. Cent. South. Univ. Technol. 2024, 44, 150–158. [Google Scholar]
- Chao, N.; Chen, W.Q.; Cao, X.; Jiang, X.N.; Gai, Y.; Liu, L. Plant hormones coordinate monolignol biosynthesis with seasonal changes in Populus tomentosa. Environ. Exp. Bot. 2022, 195, 104784. [Google Scholar] [CrossRef]
- Paiva, J.A.; Garnier-Géré, P.H.; Rodrigues, J.C.; Alves, A.; Santos, S.; Graça, J.; Le Provost, G.; Chaumeil, P.; Da Silva-Perez, D.; Bosc, A.; et al. Plasticity of maritime pine (Pinus pinaster) wood-forming tissues during a growing season. New Phytol. 2008, 179, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Bugos, R.C.; Chiang, V.L.; Campbell, W.H. cDNA cloning, sequence analysis and seasonal expression of lignin-bispecific caffeic acid/5-hydroxyferulic acid O-methyltransferase of aspen. Plant Mol. Biol. 1991, 17, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, P.P.; Liu, X.W.; Xu, T.Y.; Zhang, Y.Q.; Meng, H.F.; Xia, T. High temperature increased lignin contents of poplar (Populus spp) stem via inducing the synthesis caffeate and coniferaldehyde. Front. Genet. 2022, 13, 1007513. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Xu, H.M.; Zhao, Y.Y.; Wu, H.Y.; Lin, J.X. Advances in plant lignification and its regulation. Sci. Sin. Vitae. 2020, 50, 111–122. [Google Scholar]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; De, M.B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Barros, J. Modeling lignin biosynthesis: A pathway to renewable chemicals. Trends Plant Sci. 2024, 29, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuo, C.L.; Xiao, X.R.; Wang, X.Q.; Docampo-Palacios, M.; Chen, F.; Dixon, R.A. Substrate-Specificity of LACCASE 8 Facilitates Polymerization of Caffeyl Alcohol for C-Lignin Biosynthesis in the Seed Coat of Cleome hassleriana. Plant Cell. 2020, 32, 3825–3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, Y.; Chen, Y.; Xu, J.; Jiang, C.; Wang, X.; Zhuo, R.; Lu, M.Z.; Zhang, J. Lignin biosynthesis and accumulation in response to abiotic stresses in woody plants. For. Res. 2022, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Heidarvand, L.; Maali-Amiri, R. Physio-biochemical and proteome analysis of chickpea in early phases of cold stress. J. Plant Physiol. 2013, 170, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, P.X.; Shen, X.X.; Zhang, Y.; Li, X.W.; Jiang, L.J.; Xie, Y.P.; Niu, C.D.; Zhang, J.; Huang, X.H.; et al. MDMYB88 and MDMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.W.; Choi, S.; Jin, X.; Jung, S.E.; Choi, J.W.; Seo, J.S.; Kim, J.K. Transcriptional activation of rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, contributes to drought tolerance by modulating lignin accumulation in roots. Plant Biotechnol. J. 2022, 20, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Augstein, F.; Mazumdar, S.; Nguyen, T.V.; Minina, E.A.; Melnyk, C.W.; Carlsbecker, A. Abscisic acid signaling activates distinct VND transcription factors to promote xylem differentiation in Arabidopsis. Curr. Biol. 2021, 31, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Choi, Y.D. Drought stress promotes xylem differentiation by modulating the interaction between cytokinin and jasmonic acid. Plant Signal Behav. 2018, 13, e1451707. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.B.; Dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; De Oliveira Petkowicz, C.L. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; De Oliveira, D.M.; Andreotti, G.C.; De Souza, L.A.; Dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased gibberellins and light levels promotes cell wall thickness and enhance lignin deposition in xylem fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Xu, H.M.; Li, H.Y.; Wei, D.M.; Lin, J.X.; Li, X.J. Seasonal development of cambial activity in relation to xylem formation in Chinese fir. J. Plant Physiol. 2016, 195, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Guo, R.X.; Yang, Y.; Luo, Y.; Wei, G.Q.; Bian, L.M.; Xu, J. Integrative analysis of the transcriptome, targeted metabolome, and anatomical observation provides insights into the brassinosteroids-mediated seasonal variation of cambial activity in Chinese fir. Ind. Crops Prod. 2024, 222, 119977. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Xu, H.M.; Wu, H.Y.; Shen, W.; Lin, J.; Zhao, Y. Seasonal changes in cambium activity from active to dormant stage affect the formation of secondary xylem in Pinus tabulaeformis Carr. Tree Physiol. 2022, 42, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Calfapietra, C.; Mugnozza, G.S.; Liberloo, M.; Polle, A. Carbon-based secondary metabolites and internal nitrogen pools in Populus nigra under Free Air CO2 Enrichment (FACE) and nitrogen fertilisation. Plant Soil 2008, 304, 45–57. [Google Scholar] [CrossRef]
- Larisch, C.; Dittrich, M.; Wildhagen, H.; Lautner, S.; Fromm, J.; Polle, A.; Hedrich, R.; Rennenberg, H.; Müller, T.; Ache, P. Poplar wood rays are involved in seasonal remodeling of tree physiology. Plant Physiol. 2012, 160, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Antonova, G.F.; Varaksina, T.N.; Zheleznichenko, T.V.; Bazhenov, A.V. Changes in lignin structure during earlywood and latewood formation in Scots pine stems. Wood Sci. Technol. 2019, 53, 927–952. [Google Scholar] [CrossRef]
- Liszka, A.; Wightman, R.; Latowski, D.; Bourdon, M.; Krogh, K.B.; Pietrzykowski, M.; Lyczakowski, J.J. Structural differences of cell walls in earlywood and latewood of Pinus sylvestris and their contribution to biomass recalcitrance. Front. Plant Sci. 2023, 14, 1283093. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Bae, E.K.; Tran, T.N.A.; Lee, H.; Ko, J.H. Exploring the Seasonal Dynamics and Molecular Mechanism of Wood Formation in Gymnosperm Trees. Int. J. Mol. Sci. 2023, 24, 8624. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Feng, K.; Xi, M.; Barros, J.; Tschaplinski, T.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Phylogenetic occurrence of the phenylpropanoid pathway and lignin biosynthesis in plants. Front. Plant Sci. 2021, 12, 704697. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ling, J.; Yi, F.; Ma, W.J.; Lu, N.; Zhu, T.Q.; Wang, J.H.; Zhao, K.; Yun, H.L. Transcriptomic, proteomic, and metabolic profiles of Catalpa bungei tension wood reveal new insight into lignin biosynthesis involving transcription factor regulation. Front. Plant Sci. 2021, 12, 704262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.C.; Lyu, K.; Zeng, S.M.; Wang, X.A.; Chen, X.M. A Combined Metabolome and Transcriptome Reveals the Lignin Metabolic Pathway during the Developmental Stages of Peel Coloration in the ‘Xinyu’Pear. Int. J. Mol. Sci. 2024, 25, 7481. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.M.; Liu, Z.B.; Feng, X.H.; Xiao, W.F. Research progress on the effects of climate change on xylem growth of trees. Sci. Silvae Sin. 2015, 51, 147–154. [Google Scholar]
- Yadav, S.; Chattopadhyay, D. Lignin: The building block of defense responses to stress in plants. J. Plant Growth Regul. 2023, 42, 6652–6666. [Google Scholar] [CrossRef]
- Voelker, S.L.; Lachenbruch, B.; Meinzer, F.C.; Jourdes, M.; Ki, C.Y.; Patten, A.M.; Davin, L.B.; Lewis, N.G.; Tuskan, G.A.; Gunter, L.; et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 2010, 154, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.R.; Dwivedi, U. Lignin genetic engineering for improvement of wood quality: Applications in paper and textile industries, fodder and bioenergy production. S. Afr. J. Bot. 2014, 91, 107–125. [Google Scholar] [CrossRef]
- Xing, X.Y.; Wang, B.B.; Guan, Y.; Zhou, L.; Liu, Y.M.; Liu, S.Q.; Yun, H.L.; Gao, H. Radial variation in selected wood properties among different clones of Catalpa bungei. J. For. Eng. 2022, 7, 72–77. [Google Scholar]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mahonen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Matthews, M.; Williams, C.M.; Rui, S.; Yang, C.M.; Tunlaya-Anukit, S.; Chen, H.-C.; Li, Q.; Liu, J.; Lin, C.-Y.; et al. Improving wood properties for wood utilization through multi-omics integration in lignin biosynthesis. Nat. Commun. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]

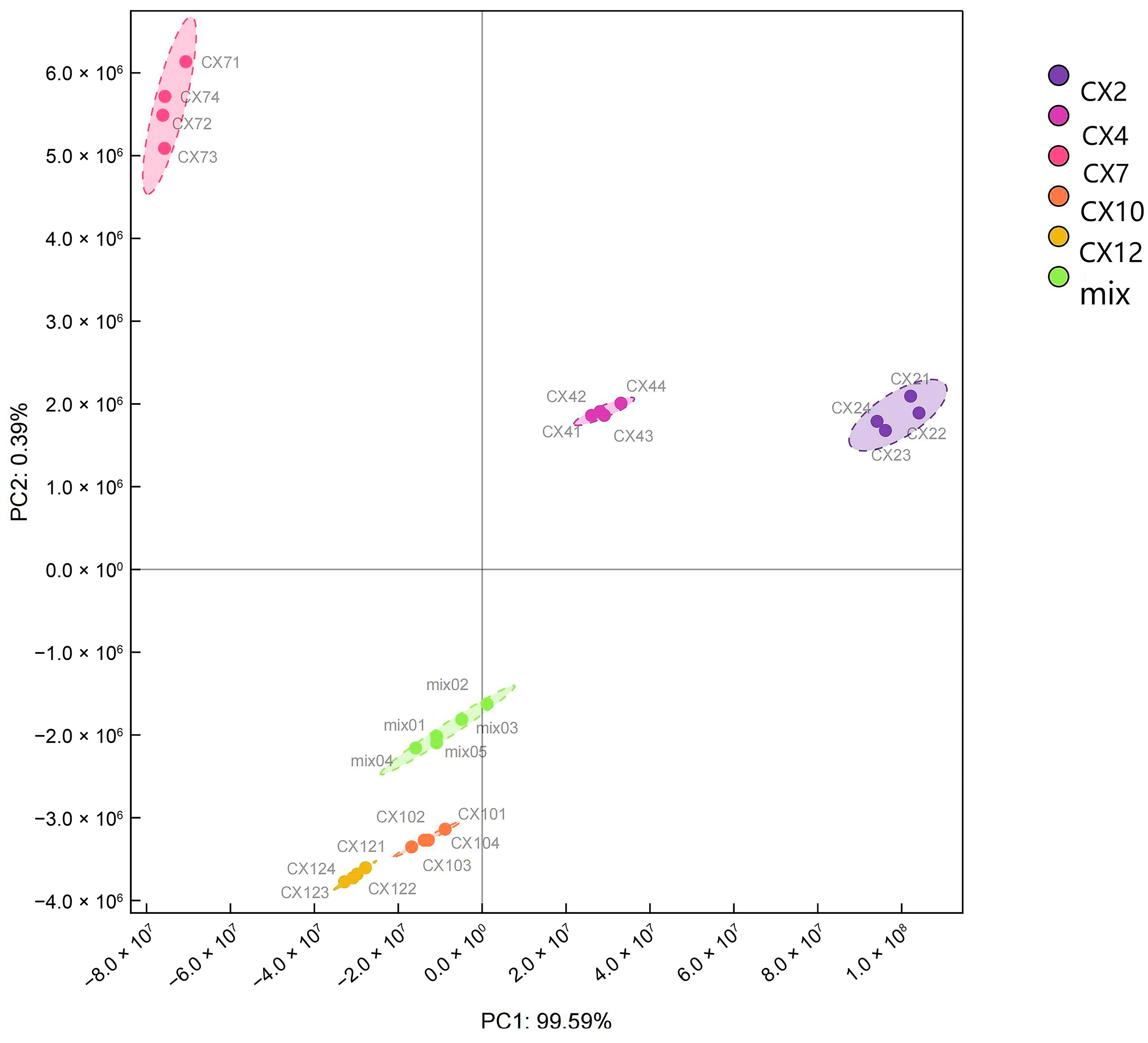
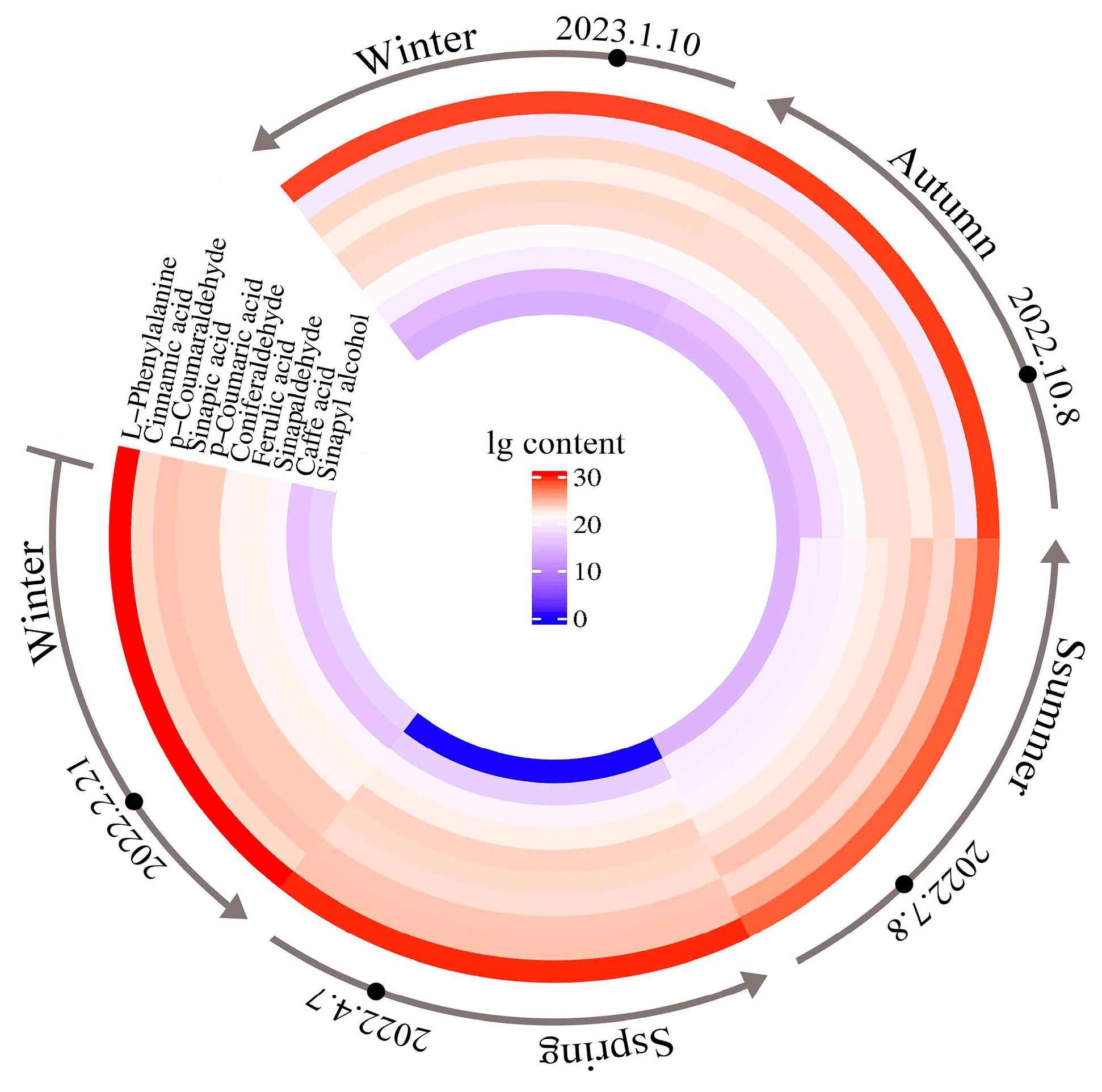


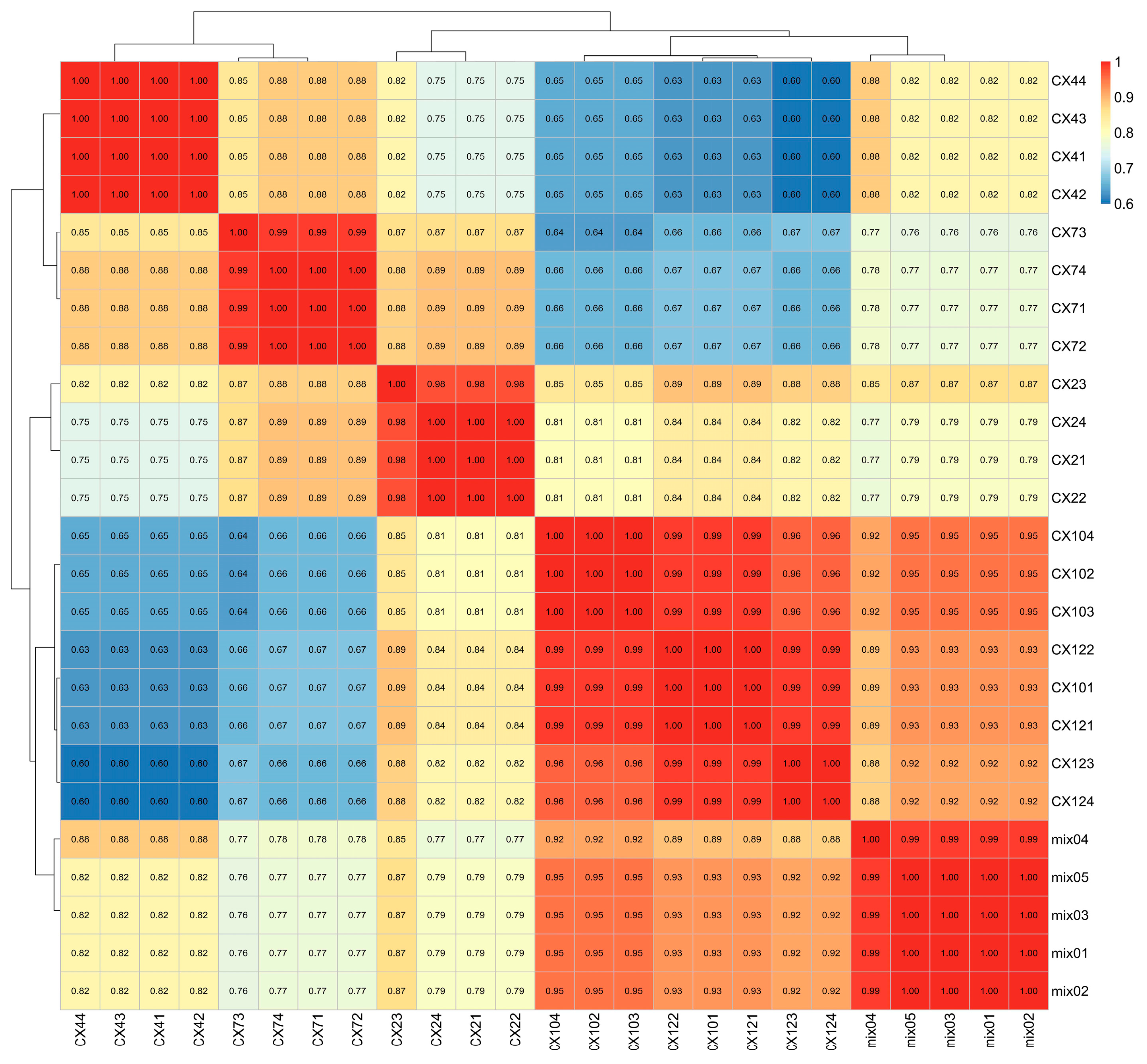



| Sample Location | Group | Sample Numbers | Acquisition Date |
|---|---|---|---|
| Xylem | CX2 | CX21, CX22, CX23, and CX24 | 21 February 2022 |
| Xylem | CX4 | CX41, CX42, CX43, and CX44 | 7 April 2022 |
| Xylem | CX7 | CX71, CX72, CX73, and CX74 | 8 July 2022 |
| Xylem | CX10 | CX101, CX102, CX103, and CX104 | 8 October 2022 |
| Xylem | CX12 | CX121, CX122, CX123, and CX124 | 10 January 2023 |
| Differential Comparison Combination | Differential Metabolites | Upregulated Expression of Differential Metabolites | Downregulated Expression of Differential Metabolites |
|---|---|---|---|
| CX2 vs. CX4 | 3 | caffeic acid, coniferaldehyde, cinnamic acid | / |
| CX2 vs. CX7 | 6 | caffeic acid, cinnamic acid | p-coumaraldehyde, L-phenylalanine, ferulic acid, sinapyl alcohol |
| CX2 vs. CX10 | 4 | coniferaldehyde | cinnamic acid, sinapic acid, sinapyl alcohol |
| CX2 vs. CX12 | 5 | coniferaldehyde | L-phenylalanine, cinnamic acid, sinapic acid, sinapyl alcohol |
| CX4 vs. CX7 | 6 | sinapic acid, caffeic acid, cinnamic acid | L-phenylalanine, ferulic acid, coniferaldehyde |
| CX4 vs. CX10 | 2 | / | caffeic acid, cinnamic acid |
| CX4 vs. CX12 | 2 | / | caffeic acid, cinnamic acid |
| CX7 vs. CX10 | 3 | / | sinapic acid, caffeic acid, cinnamic acid |
| CX7 vs. CX12 | 3 | / | sinapic acid, caffeic acid, cinnamic acid |
| CX10 vs. CX12 | 1 | / | caffeic acid |
| Metabolite | Classification | KEGG ID | Main KEGG Map |
|---|---|---|---|
| caffeic acid | Phenylpropanoids | C01197 | ko01110, ko01061, ko00940, ko01220, and ko01100 |
| coniferaldehyde | Phenylpropanoids | C02666 | ko01110, ko01061, ko00940, and ko01100 |
| cinnamic acid | Phenylpropanoids | C10438 | – |
| L-phenylalanine | Amino acid and derivatives | C00079 | ko01110, ko01061, ko00940, ko01100, ko00360, ko00997, ko00400, and ko00999 |
| sinapic acid | Phenylpropanoids | C00482 | ko01110, ko01061, ko00940, and ko01100 |
| p-coumaric acid | Phenylpropanoids | C00811 | ko01110, ko01061, ko00940, ko01220, and ko01100 |
| ferulic acid | Phenylpropanoids | C01494 | ko01110, ko01061, ko00940, and ko01100 |
| sinapyl alcohol | Phenylpropanoids | C02325 | ko01110, ko01061, ko00940, and ko01100 |
| caffeic alcohol | Phenylpropanoids | C09066 | – |
| caffeic aldehyde | Phenylpropanoids | C10945 | ko00940 and ko01110 |
| sinapaldehyde | Phenylpropanoids | – | – |
| p-coumaraldehyde | Phenylpropanoids | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Wang, Y.; Sun, T.; Han, Y.; Mu, Y.; Ji, X.; Zhang, S.; Sun, Y.; Wu, F.; Liu, T.; et al. Annual Dynamic Changes in Lignin Synthesis Metabolites in Catalpa bungei ‘Jinsi’. Metabolites 2025, 15, 493. https://doi.org/10.3390/metabo15080493
Song C, Wang Y, Sun T, Han Y, Mu Y, Ji X, Zhang S, Sun Y, Wu F, Liu T, et al. Annual Dynamic Changes in Lignin Synthesis Metabolites in Catalpa bungei ‘Jinsi’. Metabolites. 2025; 15(8):493. https://doi.org/10.3390/metabo15080493
Chicago/Turabian StyleSong, Chenxia, Yan Wang, Tao Sun, Yi Han, Yanjuan Mu, Xinyue Ji, Shuxin Zhang, Yanguo Sun, Fusheng Wu, Tao Liu, and et al. 2025. "Annual Dynamic Changes in Lignin Synthesis Metabolites in Catalpa bungei ‘Jinsi’" Metabolites 15, no. 8: 493. https://doi.org/10.3390/metabo15080493
APA StyleSong, C., Wang, Y., Sun, T., Han, Y., Mu, Y., Ji, X., Zhang, S., Sun, Y., Wu, F., Liu, T., Li, N., Han, Q., Tong, B., Lu, X., & Lu, Y. (2025). Annual Dynamic Changes in Lignin Synthesis Metabolites in Catalpa bungei ‘Jinsi’. Metabolites, 15(8), 493. https://doi.org/10.3390/metabo15080493






