Understanding the Metabolic Effects of Surgically Induced Renal Ischemia in Humans: A Temporal Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Procurement
2.2. Sample Grinding
2.3. Three-Phase Extraction
2.4. Protein Quantification Assay
2.5. NMR Analysis of Polar Extracts
2.6. Statistical Analysis
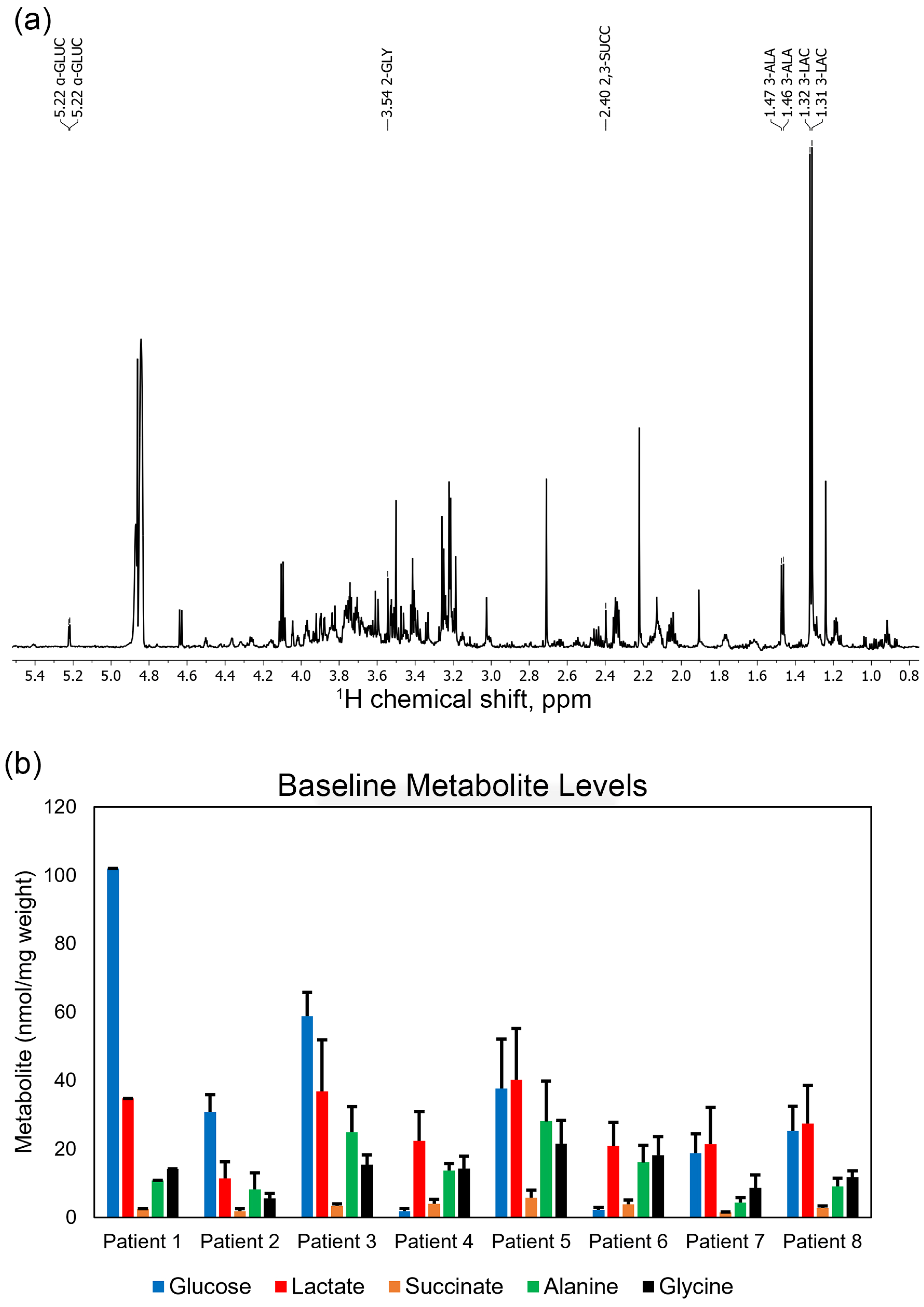
3. Results
3.1. Patient 1
3.2. Patient 2
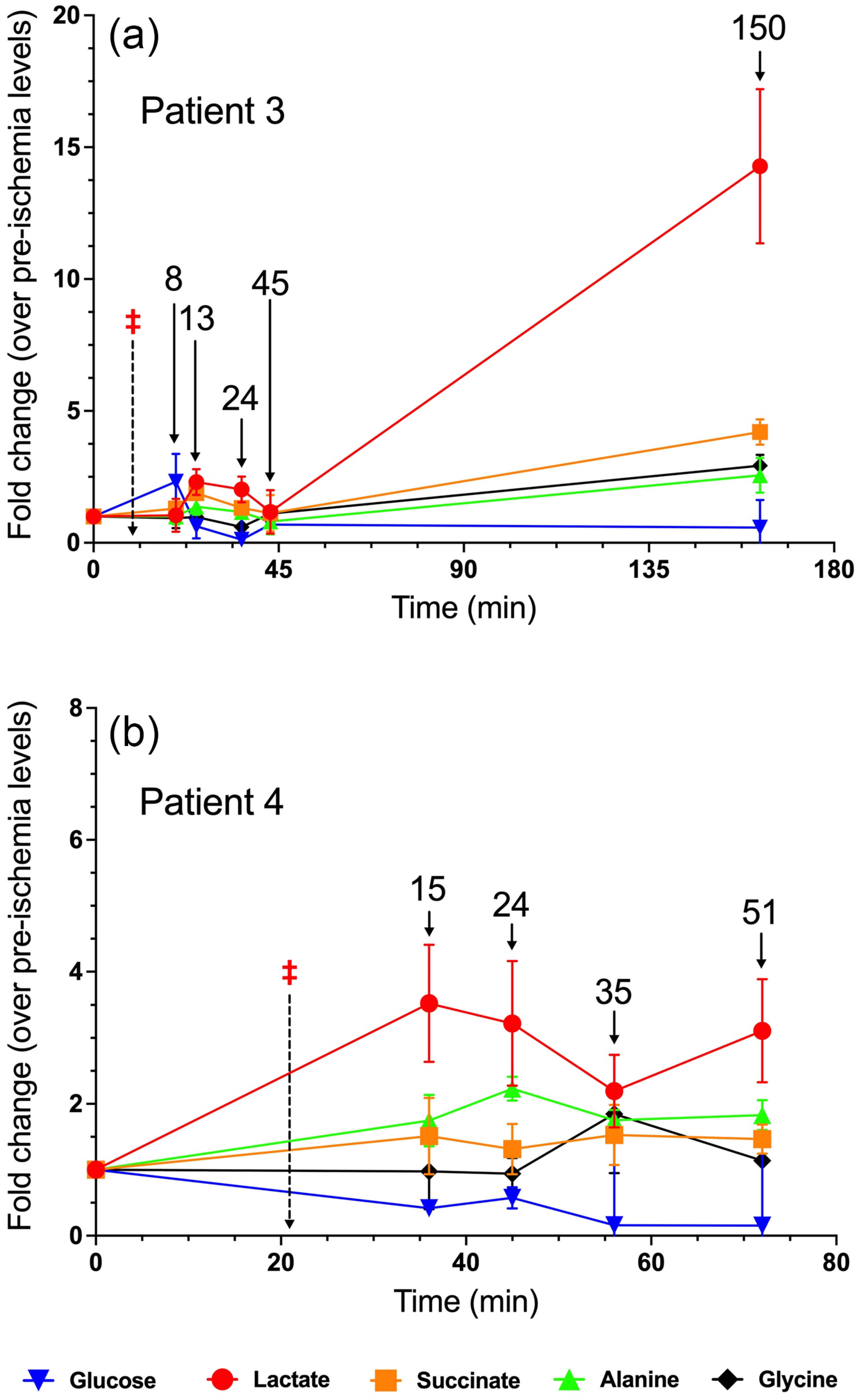
3.3. Patient 3
3.4. Patient 4
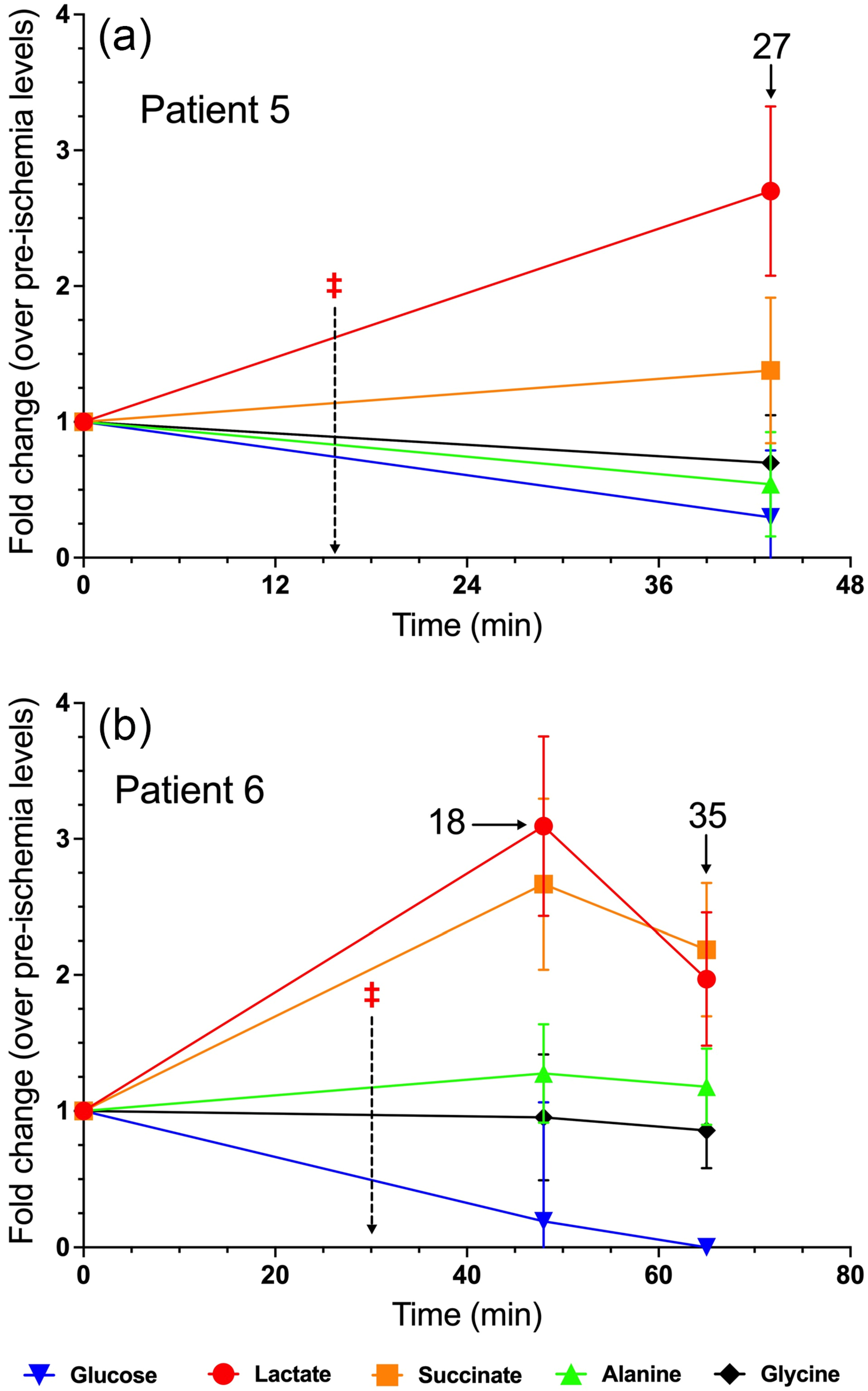
3.5. Patient 5
3.6. Patient 6
3.7. Patient 7
3.8. Patient 8
3.9. The Role of Fumarate in Ischemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Patient # | Genotype | Surgical Procedure | Tumor Size | Age at Time of Surgery | eGFR (Pre-Surgery) | Serum Creatinine (Pre-Surgery) | Intraoperative Medications | Prior Surgeries (Age at Procedure) | Co-Morbidities | Demographics |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sporadic | Robotic right radical nephrectomy | 4.2 cm with renal vein involvement | 69 | 53 | 1.43 | LR—2.6 L 0.9% NaCl—900 mL Albumin 25%—100 mL Ephedrine—20 mg | Cholecystectomy (49Y) Appendectomy (55Y) Left inguinal hernia repair (61Y) Prostate biospy (69Y) | CKD (Stage 3) HTN T2DM GG2 prostate → benign bony lesions Afib CHF with EF 38% | Male White |
| 2 | Sporadic | Robotic left radical nephrectomy | 7 cm | 88 | 65 | 1.09 | LR—2 L Albumin 25%—100 mL Ephedrine—20 mg Vasopressin—5 units | Cataract surgery (82Y) Renal biopsy (87Y) Percutaneous biopsy of liver lesion (88Y) Hemorrhoidectomy (88Y) | HTN HLD T2DM CAD/angina Macular degeneration | Male African American |
| 3 | Sporadic | Robotic left radical nephrectomy | 6.5 cm | 76 | 54 | 1.35 | LR—4 L | Cholecystectomy (53Y) Umbilical hernia repair (73Y) Atrial appendage device placement (74Y) Afib ablation (75Y) Renal biopsy (76Y) Tonsillectomy Prostate vaporization | CKD RBBB BPH Afib | Male White |
| 4 | Sporadic | Robotic right radical nephrectomy | 4.4 cm | 65 | 103 | 0.69 | LR—5 L Albumin 25%—200 mL Calcium chloride 10%—1000 mg Ephedrine—10 mg | Ureteroscopy/TUBRT (65Y) Renal biopsy (65Y) | HTN HLD Bladder cancer | Male White |
| 5 | Sporadic | Robotic left radical nephrectomy | 2.5 cm | 81 | 79 | 0.96 | LR—3 L Ephedrine—20 mg Phenylephrine—250 mcg | 2001, 2006: Prostate biopsy (59,65Y) Anal fistula repair (71Y) Left hand fracture repair with pins Tonsillectomy | HTN HLD Onychomycosis Seborrheic Dermatitis | Male White |
| 6 | Sporadic | Robotic left radical nephrectomy with retroperitoneal lymph node dissection | 8 cm | 59 | 80 | 0.84 | LR—2 L Albumin 25%—100 mL Phenylephrine—80 mcg Hydrocortisone—25 mg | Left thoracentesis (58Y) Liver biopsy | HTN (off BP meds for 6 months) HLD Hypothyroidism | Female African American |
| 7 | Tuberous Sclerosis | Open left radical nephrectomy | Multiple solid and cystic renal masses—3.0 cm and 2.2 cm | 37 | 75 | 1.23 | LR—2 L | Right radical nephrectomy (20Y) Left kidney RFA ×3 (23Y) Left renal biopsy (29Y) Left robotic partial nephrectomy (31Y) Left open partial nephrectomy (33Y) Left ablation for presumed ccRCC (35Y) Wisdom tooth extraction | TSC-associated seizures Depression Anxiety | Male White |
| 8 | Sporadic | Robotic left radical nephrectomy with retroperitoneal lymph node dissection | 12.2 cm | 67 | 74 | 1.1 | LR—3.5 L | none | HLD, HCM, CAD | Male, White |
| Patient 2 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 6 | 0.051 | 0.075 | 0.100 | 0.126 | 0.120 |
| t = 12 | 0.010 * | 0.022 * | 0.551 | 0.034 * | 0.074 |
| t = 27 | 0.002 * | 0.054 | 0.639 | 0.067 | 0.016 * |
| t = 90 | 0.006 * | 0.001 * | 0.113 | 0.049 * | 0.008 * |
| Patient 3 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 8 | 0.894 | 0.094 | 0.310 | 0.999 | 0.740 |
| t = 13 | 0.005 * | 0.001 * | 0.058 | 0.147 | 0.848 |
| t = 24 | 0.015 * | 0.058 | 0.001 * | 0.398 | 0.018 * |
| t = 31 | 0.709 | 0.764 | 0.010 * | 0.463 | 0.554 |
| t = 150 | 0.018 * | 0.009 * | 0.197 | 0.041 * | 0.009 * |
| Patient 4 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 15 | 0.007 * | 0.190 | 0.038 * | 0.033 * | 0.933 |
| t = 24 | 0.018 * | 0.285 | 0.098 | 0.001 * | 0.780 |
| t = 35 | 0.018 * | 0.128 | 0.013 * | 0.003 * | 0.200 |
| t = 51 | 0.007 * | 0.103 | 0.013 * | 0.005 * | 0.481 |
| Patient 5 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 27 | 0.011 * | 0.226 | 0.020 * | 0.116 | 0.187 |
| Patient 6 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 18 | 0.0002 * | 0.001 * | 0.00035 * | 0.174 | 0.816 |
| t = 35 | 0.003 * | 0.001 * | 0.00004 * | 0.323 | 0.399 |
| Patient 7 | Lactate | Succinate | Glucose | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 37 | 0.200 | 0.313 | 0.897 | 0.292 | 0.504 |
| t = 39 | 0.264 | 0.210 | 0.197 | 0.253 | 0.288 |
| t = 45 | 0.120 | 0.204 | 0.025 * | 0.128 | 0.571 |
| t = 50 | 0.228 | 0.002 * | 0.008 * | 0.044 * | 0.036 * |
| t = 60 | 0.643 | 0.007 * | 0.009 * | 0.094 | 0.048 * |
| t = 70 | 0.935 | 0.234 | 0.009 * | 0.174 | 0.080 |
| t = 120 | 0.008 * | 0.003 * | 0.049 * | 0.011 * | 0.006 * |
| Patient 8 | Glucose | Lactate | Succinate | Alanine | Glycine |
|---|---|---|---|---|---|
| t = 0 | |||||
| t = 12 | 0.008 * | 0.002 * | 0.004 * | 0.220 | 0.300 |
| t = 26 | 0.008 * | 0.052 | 0.024 * | 0.197 | 0.699 |
| t = 43 | 0.017 * | 0.007 * | 0.006 * | 0.009 * | 0.040 * |
| t = 140 | 0.000 * | 0.000 * | 0.000 * | 0.002 * | 0.005 * |
| Glucose | Lactate | Succinate | Fumarate | Alanine | Glycine | |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| Patient 2 | 0.16 | 0.42 | 0.38 | 0.60 | 0.26 | |
| Patient 3 | 0.12 | 0.41 | 0.17 | 0.30 | 0.20 | |
| Patient 4 | 0.45 | 0.38 | 0.31 | 0.15 | 0.25 | |
| Patient 5 | 0.25 | 0.22 | 0.23 | 0.45 | 0.17 | |
| Patient 6 | 0.32 | 0.33 | 0.30 | 0.31 | 0.24 | |
| Patient 7 | 0.30 | 0.50 | 0.08 | 0.33 | 0.43 | |
| Patient 8 | 0.29 | 0.41 | 0.23 | 0.27 | 0.15 |
References
- Davison, D.L.; Patel, K.; Chawla, L.S. Hemodynamic monitoring in the critically ill: Spanning the range of kidney function. Am. J. Kidney Dis. 2012, 59, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.N.; Schreiber, M.J.; Gill, I.S. Surgical renal ischemia: A contemporary overview. J. Urol. 2008, 180, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Brezis, M.; Rosen, S. Hypoxia of the renal medulla—Its implications for disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Brezis, M.; Heyman, S.N.; Epstein, F.H. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am. J. Physiol. 1994, 267, F1063–F1068. [Google Scholar] [CrossRef]
- Gronow, G.H.; Cohen, J.J. Substrate support for renal functions during hypoxia in the perfused rat kidney. Am. J. Physiol. 1984, 247, F618–F631. [Google Scholar] [CrossRef]
- Mazzeo, A.; Sincos, A.; Leite, K.R.M.; Goes, M.A., Jr.; Dos Pavao, O.F.S.; Kaufmann, O.G. Study of kidney morphologic and structural changes related to different ischemia times and types of clamping of the renal vascular pedicle. Int. Braz. J. Urol. 2019, 45, 754–762. [Google Scholar] [CrossRef]
- Chihanga, T.; Ma, Q.; Nicholson, J.D.; Ruby, H.N.; Edelmann, R.E.; Devarajan, P.; Kennedy, M.A. NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2018, 314, F154–F166. [Google Scholar] [CrossRef]
- Weinberg, J.M.; Venkatachalam, M.A.; Roeser, N.F.; Nissim, I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl. Acad. Sci. USA 2000, 97, 2826–2831. [Google Scholar] [CrossRef]
- Mitchell, P. Keilin’s respiratory chain concept and its chemiosmotic consequences. Science 1979, 206, 1148–1159. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 3rd ed.; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Key Statistics About Kidney Cancer. Available online: https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html (accessed on 4 August 2024).
- Kunath, F.; Schmidt, S.; Krabbe, L.M.; Miernik, A.; Dahm, P.; Cleves, A.; Walther, M.; Kroeger, N. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst. Rev. 2017, 5, CD012045. [Google Scholar] [CrossRef]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Linehan, W.M.; Walther, M.M.; Zbar, B. The genetic basis of cancer of the kidney. J. Urol. 2003, 170, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Metwalli, A.R.; Linehan, W.M. Nephron-sparing surgery for multifocal and hereditary renal tumors. Curr. Opin. Urol. 2014, 24, 466–473. [Google Scholar] [CrossRef]
- Baiocco, J.A.; Metwalli, A.R. Multiplex Partial Nephrectomy, Repeat Partial Nephrectomy, and Salvage Partial Nephrectomy Remain the Primary Treatment in Multifocal and Hereditary Kidney Cancer. Front. Oncol. 2017, 7, 244. [Google Scholar] [CrossRef]
- Antony, M.B.; Kozel, Z.; Gopal, N.; Loebach, L.; Metwalli, A.R.; Gurram, S.; Linehan, W.M.; Ball, M.W. Cumulative Impact of Serial Partial Nephrectomy for the Treatment of Recurrent Renal Masses. J. Urol. 2024, 212, 431–440. [Google Scholar] [CrossRef]
- Pavlovic, N.; Krizanac, M.; Kumric, M.; Vukojevic, K.; Bozic, J. Mitochondrial Dysfunction: The Silent Catalyst of Kidney Disease Progression. Cells 2025, 14, 749. [Google Scholar] [CrossRef]
- Kallingal, G.J.; Weinberg, J.M.; Reis, I.M.; Nehra, A.; Venkatachalam, M.A.; Parekh, D.J. Long-term response to renal ischaemia in the human kidney after partial nephrectomy: Results from a prospective clinical trial. BJU Int. 2016, 117, 766–774. [Google Scholar] [CrossRef]
- Bidani, A.K.; Griffin, K.A. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 2004, 44, 595–601. [Google Scholar] [CrossRef]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–60. [Google Scholar] [CrossRef]
- Twombley, K.; Baum, M.; Gattineni, J. Accidental and iatrogenic causes of acute kidney injury. Curr. Opin. Pediatr. 2011, 23, 208–214. [Google Scholar] [CrossRef]
- Keller, A.K.; Jorgensen, T.M.; Olsen, L.H.; Stolle, L.B. Early detection of renal ischemia by in situ microdialysis: An experimental study. J. Urol. 2008, 179, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Crooks, D.R.; Fan, T.W.; Linehan, W.M. Metabolic Labeling of Cultured Mammalian Cells for Stable Isotope-Resolved Metabolomics: Practical Aspects of Tissue Culture and Sample Extraction. In Cancer Metabolism; Humana Press: New York, NY, USA, 2019; Volume 1928, pp. 1–27. [Google Scholar] [CrossRef]
- Nyamundanda, G.; Gormley, I.C.; Fan, Y.; Gallagher, W.M.; Brennan, L. MetSizeR: Selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinform. 2013, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Cano, N. Bench-to-bedside review: Glucose production from the kidney. Crit. Care 2002, 6, 317–321. [Google Scholar] [CrossRef]
- Meyer, C.; Stumvoll, M.; Dostou, J.; Welle, S.; Haymond, M.; Gerich, J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E428–E434. [Google Scholar] [CrossRef]
- Didwania, A.; Miller, J.; Kassel, D.; Jackson, E.V., Jr.; Chernow, B. Effect of intravenous lactated Ringer’s solution infusion on the circulating lactate concentration: Part 3. Results of a prospective, randomized, double-blind, placebo-controlled trial. Crit. Care Med. 1997, 25, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Zitek, T.; Skaggs, Z.D.; Rahbar, A.; Patel, J.; Khan, M. Does Intravenous Lactated Ringer’s Solution Raise Serum Lactate? J. Emerg. Med. 2018, 55, 313–318. [Google Scholar] [CrossRef]
- Aber, G.M.; Morris, L.O.; Housley, E. Gluconeogenesis by the human kidney. Nature 1966, 212, 1589–1590. [Google Scholar] [CrossRef]
- Hanson, R.W.; Garber, A.J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am. J. Clin. Nutr. 1972, 25, 1010–1021. [Google Scholar] [CrossRef]
- Legouis, D.; Faivre, A.; Cippa, P.E.; de Seigneux, S. Renal gluconeogenesis: An underestimated role of the kidney in systemic glucose metabolism. Nephrol. Dial. Transpl. 2022, 37, 1417–1425. [Google Scholar] [CrossRef]
- Cersosimo, E.; Garlick, P.; Ferretti, J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes 2000, 49, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Garlick, P.; Ferretti, J. Regulation of splanchnic and renal substrate supply by insulin in humans. Metabolism 2000, 49, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef]
- Biava, C.; Grossman, A.; West, M. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Lab. Investig. 1966, 15, 330–356. [Google Scholar]
- Khandelwal, R.L.; Zinman, S.M.; Knull, H.R. The effect of streptozotocin-induced diabetes on glycogen metabolism in rat kidney and its relationship to the liver system. Arch. Biochem. Biophys. 1979, 197, 310–316. [Google Scholar] [CrossRef]
- Needleman, P.; Passonneau, J.V.; Lowry, O.H. Distribution of glucose and related metabolites in rat kidney. Am. J. Physiol. 1968, 215, 655–659. [Google Scholar] [CrossRef]
- Moyle, D.M. A Quantitative Study of Succinic Acid in Muscle. I. Biochem. J. 1924, 18, 351–364. [Google Scholar] [CrossRef]
- Needham, D.M. A Quantitative Study of Succinic Acid in Muscle. II: The Metabolic Relationships of Succinic, Malic and Fumaric Acids. Biochem. J. 1927, 21, 739–750. [Google Scholar] [CrossRef]
- Freminet, A.; Leclerc, L. Simultaneous use of carbohydrates and amino acids during total ischemia in the isolated rat heart (author’s transl). J. Physiol. 1980, 76, 893–899. [Google Scholar]
- Peuhkurinen, K.J.; Takala, T.E.; Nuutinen, E.M.; Hassinen, I.E. Tricarboxylic acid cycle metabolites during ischemia in isolated perfused rat heart. Am. J. Physiol. 1983, 244, H281–H288. [Google Scholar] [CrossRef]
- Pisarenko, O.; Studneva, I.; Khlopkov, V. Metabolism of the tricarboxylic acid cycle intermediates and related amino acids in ischemic guinea pig heart. Biomed. Biochim. Acta 1987, 46, S568–S571. [Google Scholar] [PubMed]
- Wiesner, R.J.; Deussen, A.; Borst, M.; Schrader, J.; Grieshaber, M.K. Glutamate degradation in the ischemic dog heart: Contribution to anaerobic energy production. J. Mol. Cell Cardiol. 1989, 21, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Rosen, P.C.; Sprenger, H.G.; Puszynska, A.M.; Mann, J.L.; Roessler, J.M.; Cangelosi, A.L.; Henne, A.; Condon, K.J.; Zhang, T.; et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 2021, 374, 1227–1237. [Google Scholar] [CrossRef]
- Oh, C.J.; Kim, M.J.; Lee, J.M.; Kim, D.H.; Kim, I.Y.; Park, S.; Kim, Y.; Lee, K.B.; Lee, S.H.; Lim, C.W.; et al. Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion. Kidney Int. 2023, 104, 724–739. [Google Scholar] [CrossRef]
- Hunter, F.E., Jr. Anaerobic phosphorylation due to a coupled oxidation-reduction between alpha-ketoglutaric acid and oxalacetic acid. J. Biol. Chem. 1949, 177, 361–372. [Google Scholar] [CrossRef]
- Chinopoulos, C. Succinate in ischemia: Where does it come from? Int. J. Biochem. Cell Biol. 2019, 115, 105580. [Google Scholar] [CrossRef]
- Sanadi, D.R.; Fluharty, A.L. On the Mechanism of Oxidative Phosphorylation. Vii. The Energy-Requiring Reduction of Pyridine Nucleotide by Succinate and the Energy-Yielding Oxidation of Reduced Pyridine Nucleotide by Fumarate. Biochemistry 1963, 2, 523–528. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Dressendorfer, R.H. Succinate accumulation in man during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1976, 35, 235–242. [Google Scholar] [CrossRef]
- Taegtmeyer, H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ. Res. 1978, 43, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ravasz, D.; Bui, D.; Nazarian, S.; Pallag, G.; Karnok, N.; Roberts, J.; Marzullo, B.P.; Tennant, D.A.; Greenwood, B.; Kitayev, A.; et al. Residual Complex I activity and amphidirectional Complex II operation support glutamate catabolism through mtSLP in anoxia. Sci. Rep. 2024, 14, 1729. [Google Scholar] [CrossRef] [PubMed]
- Tomitsuka, E.; Kita, K.; Esumi, H. Regulation of succinate-ubiquinone reductase and fumarate reductase activities in human complex II by phosphorylation of its flavoprotein subunit. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bisbach, C.M.; Hass, D.T.; Robbings, B.M.; Rountree, A.M.; Sadilek, M.; Sweet, I.R.; Hurley, J.B. Succinate Can Shuttle Reducing Power from the Hypoxic Retina to the O2 Rich Pigment Epithelium. Cell Rep. 2020, 31, 107606. [Google Scholar] [CrossRef]
- Spencer, M.E.; Guest, J.R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J. Bacteriol. 1973, 114, 563–570. [Google Scholar] [CrossRef]
- Harada, S.; Inaoka, D.K.; Ohmori, J.; Kita, K. Diversity of parasite complex II. Biochim. Biophys. Acta 2013, 1827, 658–667. [Google Scholar] [CrossRef]
- Salinas, G.; Langelaan, D.N.; Shepherd, J.N. Rhodoquinone in bacteria and animals: Two distinct pathways for biosynthesis of this key electron transporter used in anaerobic bioenergetics. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148278. [Google Scholar] [CrossRef]
- Kita, K.; Takamiya, S.; Furushima, R.; Ma, Y.C.; Suzuki, H.; Ozawa, T.; Oya, H. Electron-transfer complexes of Ascaris suum muscle mitochondria. III. Composition and fumarate reductase activity of complex II. Biochim. Biophys. Acta 1988, 935, 130–140. [Google Scholar] [CrossRef]
- Takamiya, S.; Matsui, T.; Taka, H.; Murayama, K.; Matsuda, M.; Aoki, T. Free-living nematodes Caenorhabditis elegans possess in their mitochondria an additional rhodoquinone, an essential component of the eukaryotic fumarate reductase system. Arch. Biochem. Biophys. 1999, 371, 284–289. [Google Scholar] [CrossRef]
- Valeros, J.; Jerome, M.; Tseyang, T.; Vo, P.; Do, T.; Fajardo Palomino, D.; Grotehans, N.; Kunala, M.; Jerrett, A.E.; Hathiramani, N.R.; et al. Rhodoquinone carries electrons in the mammalian electron transport chain. Cell 2025, 188, 1084–1099. [Google Scholar] [CrossRef]
- Parmeggiani, A.; Bowman, R.H. Regulation of Phosphofructokinase Activity by Citrate in Normal and Diabetic Muscle. Biochem. Biophys. Res. Commun. 1963, 12, 268–273. [Google Scholar] [CrossRef]
- Stasi, E.; Sciascia, S.; Naretto, C.; Baldovino, S.; Roccatello, D. Lymphatic System and the Kidney: From Lymphangiogenesis to Renal Inflammation and Fibrosis Development. Int. J. Mol. Sci. 2024, 25, 2583. [Google Scholar] [CrossRef]



| Patient | [lactatefinal]/ [glucoseinitial] | Final Timepoint (Minutes) |
|---|---|---|
| Patient 1 | 2.2 | 60 |
| Patient 2 | 6.8 | 90 |
| Patient 3 | 8.9 | 150 |
| Patient 4 | 37.8 | 51 |
| Patient 5 | 2.9 | 27 |
| Patient 6 | 18.7 | 35 |
| Patient 7 | 11.9 | 120 |
| Patient 8 | 7.7 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arimilli, B.; On, T.A.; Srirama, V.S.; Yang, Y.; Asampille, G.; Brender, J.R.; Krishna, M.C.; Hseuh, J.Y.; Chegu, V.P.; Kozel, Z.; et al. Understanding the Metabolic Effects of Surgically Induced Renal Ischemia in Humans: A Temporal Approach. Metabolites 2025, 15, 462. https://doi.org/10.3390/metabo15070462
Arimilli B, On TA, Srirama VS, Yang Y, Asampille G, Brender JR, Krishna MC, Hseuh JY, Chegu VP, Kozel Z, et al. Understanding the Metabolic Effects of Surgically Induced Renal Ischemia in Humans: A Temporal Approach. Metabolites. 2025; 15(7):462. https://doi.org/10.3390/metabo15070462
Chicago/Turabian StyleArimilli, Bhargav, Tyler A. On, Vaishnavi S. Srirama, Ye Yang, Gitanjali Asampille, Jeffrey R. Brender, Murali C. Krishna, Jessica Y. Hseuh, Viraj P. Chegu, Zachary Kozel, and et al. 2025. "Understanding the Metabolic Effects of Surgically Induced Renal Ischemia in Humans: A Temporal Approach" Metabolites 15, no. 7: 462. https://doi.org/10.3390/metabo15070462
APA StyleArimilli, B., On, T. A., Srirama, V. S., Yang, Y., Asampille, G., Brender, J. R., Krishna, M. C., Hseuh, J. Y., Chegu, V. P., Kozel, Z., Gurram, S., Ball, M. W., Linehan, W. M., & Crooks, D. R. (2025). Understanding the Metabolic Effects of Surgically Induced Renal Ischemia in Humans: A Temporal Approach. Metabolites, 15(7), 462. https://doi.org/10.3390/metabo15070462






