Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication
Abstract
1. Introduction
2. Osteoporosis in Chronic Kidney Disease: A Rising Comorbidity in an Aging Population
3. Overview of Osteoporosis: Definition and Core Concepts
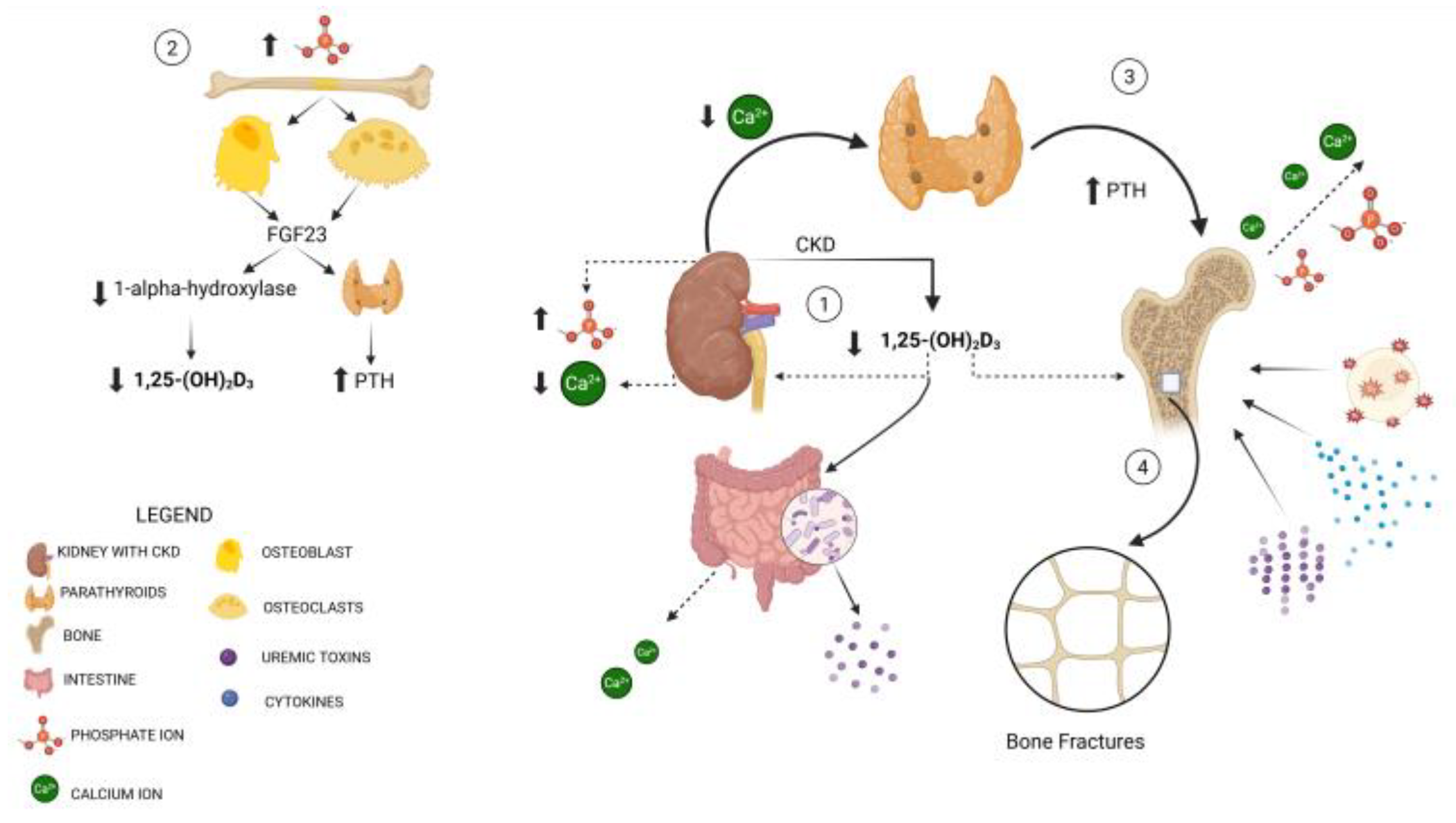
4. Pathophysiological Mechanisms Underlying Fragility Fractures in CKD Patients
5. Diagnostic Management
5.1. Bone Biomarkers
5.2. Fracture Risk Tools in CKD
6. Radiologic Imaging
6.1. Conventional Radiography
6.2. DXA
6.3. Computed Tomography (CT)
6.4. Magnetic Resonance Imaging (MRI)
6.5. Quantitative Ultrasound (QUS)
6.6. Nuclear Medicine
7. Therapeutic Strategies
7.1. Therapies Available for All CKD Stages
7.2. Patients with eGFR ≥ 30 mL/min/1.73 m2 (CKD Stages G1–G3)
- •
- Bisphosphonates: Oral bisphosphonates such as alendronate and risedronate are the primary agents for reducing fracture risk in CKD G1–G3 patients. Studies have confirmed the efficacy of bisphosphonates in improving BMD and preventing fractures in CKD patients with preserved renal function. However, the risk of renal complications mandates close monitoring [61].
- •
- Denosumab: Denosumab, a monoclonal antibody that inhibits RANKL, offers a promising alternative to bisphosphonates. It is safe in patients with an eGFR ≥ 30 mL/min/1.73 m2, as it is not excreted by the kidneys. However, the risk of hypocalcemia necessitates calcium monitoring, especially in patients with CKD, who may already have altered calcium metabolism [62]. Recent data suggest that renal function may also influence the skeletal response to denosumab therapy, underlining the importance of personalized monitoring. In an Italian study conducted in postmenopausal osteoporotic women, renal function levels were associated with significant changes in bone mineral density during treatment with denosumab [63].
- •
- Romosozumab: Approved by the FDA in April 2019, it is used to treat osteoporosis in postmenopausal women at a high risk of fractures. It functions by inhibiting sclerostin, both enhancing osteoblast and preventing osteoclast activity, and consequently promoting bone formation. Sclerostin does not only act on bone: it is also involved in vascular mechanisms, influencing vascular calcification and endothelial remodeling. Recent studies have highlighted its possible role as a mediator in cardiovascular physiopathology, suggesting a link between bone metabolism and vascular risk [64]. In chronic dialysis patients, osteoporosis remains inadequately managed despite the increased fracture risk. Romosozumab has been shown to increase bone mineral density in these patients without significant adverse effects, although hypocalcemia may occur, particularly when co-administered with calcium-sensing receptor agonists. This suggests that romosozumab may be a treatment option for severe osteoporosis in postmenopausal women on dialysis, but the careful monitoring of calcium levels is required. Its fracture prevention efficacy is evident in patients with moderate renal function (eGFR ≥ 30 mL/min/1.73 m2), while further studies are needed for those with more severe kidney impairment [65,66]. However, the use of romosozumab has raised concerns about a possible increase in cardiovascular risk, especially major ischemic events. Some clinical studies, such as the ARCH trial, have reported a higher incidence of myocardial infarction and stroke in patients treated with romosozumab compared to the control group. This has led regulatory agencies to contraindicate romosozumab in patients with previous major cardiovascular events. In light of this, the use of romosozumab in patients with CKD, already at high cardiovascular risk, must be carefully evaluated, favoring an individualized approach and a careful balance between skeletal benefits and cardiovascular risks [67].
- •
- Calcium and Vitamin D Supplementation: If there are no biochemical signs of CKD-MBD (such as hyperparathyroidism or hyperphosphatemia), calcium and vitamin D intake should be similar to that of people without CKD. Daily calcium requirements vary depending on age and specific conditions. Taking calcium alone may slightly reduce fracture risk, primarily in older individuals. However, a more significant reduction is observed when calcium is combined with vitamin D [58]. The goal of vitamin D deficiency treatment is to rapidly restore normal serum levels of 25(OH)D. For severe deficiency, 50,000 IU of cholecalciferol weekly for 2–3 months is recommended, followed by maintenance up to 2000 IU daily. This aims to improve bone density, decrease fracture incidence, and improve the quality of life [58]. In recent years, extended-release calcifediol has received increasing attention as a therapeutic strategy in patients with CKD and hypovitaminosis D, particularly in those with secondary hyperparathyroidism. Unlike cholecalciferol, calcifediol bypasses hepatic hydroxylation and is more effective in rapidly normalizing 25(OH)D levels, with a predictable and more stable effect on PTH. In CKD stages G3–G4, the early use of calcifediol may prevent the progression of hyperparathyroidism and improve bone and cardiovascular outcomes. Furthermore, compared to calcitriol, which is the active form 1,25(OH)2D and acts rapidly but with a higher risk of hypercalcemia and vascular calcifications, calcifediol offers a more favorable safety profile, especially in the pre-dialysis phase. In addition to its well-known action on bone metabolism, vitamin D also exerts pleiotropic effects at the renal level, modulating tubular inflammation and contributing to the protection of tubular structure and function, as demonstrated in patients with tubulointerstitial nephropathies and chronic renal diseases [68]. A cost-effectiveness analysis evaluated early vitamin D therapy in CKD patients with vitamin D insufficiency and secondary hyperparathyroidism (SHPT) to prevent fractures and other complications [69]. Extended-release calcifediol has been shown to effectively increase 25-hydroxyvitamin D levels and reduce PTH levels in both clinical trials and real-world studies, regardless of CKD stage or baseline PTH levels. Initiating treatment in CKD stages G3 or G4, rather than waiting until stage G5/G5d, was found to be more cost-effective, reducing both cardiovascular events [70] and fractures [71].
7.3. Patients with eGFR < 30 mL/min/1.73 m2 (CKD Stages G4–G5 Non-Dialysis)
- •
- Bisphosphonates are typically not recommended for patients with an eGFR below 30 mL/min/1.73 m2 (for alendronate, risedronate, and ibandronate) or below 35 mL/min/1.73 m2 (for zoledronic acid). Their routine use in patients with an eGFR < 30 mL/min/1.73 m2 is discouraged and should only be considered by clinicians specialized in CKD-MBD management, following comprehensive biochemical testing and/or bone biopsy to exclude ROD, especially in those with an eGFR < 15 mL/min/1.73 m2. In stage G4-G5 CKD patients, however, bisphosphonate use is typically avoided due to the risk of acute renal failure and atypical fractures [72].
- •
- Denosumab: In advanced CKD, denosumab remains a viable option due to its non-renal clearance. Denosumab has been explored as a treatment option for bone loss in patients with CKD, particularly those with advanced stages [73]. Initial studies, such as the FREEDOM trial [74], demonstrated its efficacy in reducing fracture risk in osteoporotic women, with subsequent post hoc analyses showing similar benefits across varying levels of kidney function [75]. A retrospective study evaluated the effectiveness of denosumab in improving BMD in patients previously treated with bisphosphonates while also assessing the impact of CKD on treatment response. Among 134 patients, denosumab significantly increased BMD at the lumbar spine, total hip, and femoral neck. However, patients with an eGFR below 35 mL/min showed a lower response at the total hip and femoral neck compared to those with an eGFR above 35 mL/min, which was associated with PTH levels [76].
7.4. Patients on Hemodialysis (CKD Stage G5D)
- •
- Non-Pharmacologic Measures: In addition to pharmacologic therapy, patients should follow non-pharmacologic strategies such a balanced diet, weight loss exercises [59], and fall prevention strategies. These measures are crucial for reducing fracture risk and supporting bone health, especially in patients with severe CKD. Moreover, it is recommended to aim for a total calcium intake (from diet and supplements) of 1200 mg/day, with no more than 500 mg/day coming from calcium supplements [58]. The remaining calcium should be obtained through dietary sources, such as calcium-fortified orange juice, soy products, and vegetables, especially for patients who need to limit dairy due to its phosphorus content. Additionally, a daily intake of 800 international units of vitamin D (either cholecalciferol or ergocalciferol) is suggested. In CKD-MBD patients with low BMD and fragility fractures, vitamin D supplementation can prevent secondary hyperparathyroidism, activate osteoblasts, relieve muscle weakness and myalgia, and reduce vascular calcification [80].
- •
- The Management of Secondary Hyperparathyroidism: Controlling secondary hyperparathyroidism (SHPT) is critical for maintaining bone health in HD patients. Elevated PTH levels can lead to high bone turnover and increased fracture risk. Calcimimetics and vitamin D analogs are essential in managing secondary hyperparathyroidism and reducing bone resorption [81]. The efficacy of cinacalcet remains evident even in patients undergoing chronic HD with severe secondary hyperparathyroidism. Notably, improvements in bone markers, reductions in fibroblast growth factor 23 levels, and the stabilization of vascular calcification have been observed. Consequently, cinacalcet offers beneficial effects on chronic kidney disease-related mineral and bone disorder in severe cases of secondary hyperparathyroidism. It may serve as an effective initial therapy to lower PTH levels before considering surgical parathyroidectomy and as an alternative option for patients who are not suitable candidates for surgery [82]. Abnormalities in vitamin D metabolism are common, contributing to both bone fragility and vascular calcifications. Calcitriol, the active form of vitamin D, plays a key role in managing these imbalances. It was found that treatment with oral calcitriol significantly reduced the prevalence of vertebral fractures, without increasing the burden of aortic or iliac calcifications. This suggests that calcitriol may help mitigate fracture risk in CKD patients while maintaining a stable VC profile, though further research is needed to solidify these findings [83].
- •
- Denosumab in Hemodialysis: Denosumab has shown significant efficacy in improving BMD in patients with advanced CKD and those on dialysis (G5/G5D) [85]. Studies report progressive increases in both cortical and trabecular BMD over 2–3 years of treatment, particularly in key areas such as the hip region [86,87]. These improvements make denosumab a promising option for enhancing bone strength in this population, despite the lack of direct evidence for fracture risk reduction. However, the risk of hypocalcemia is a critical concern, especially in patients with CKD and those undergoing dialysis. Denosumab is neither metabolized nor excreted by the kidneys and is not dialyzable. Calcium and vitamin D supplementation are necessary during treatment to prevent severe hypocalcemia, a frequent complication in these patients due to their impaired renal function [86]. The close monitoring of calcium levels is essential to mitigate this risk and ensure the safety of denosumab therapy. Moreover, denosumab’s discontinuation poses additional challenges. Rapid and significant bone loss has been observed within one year after stopping treatment, particularly in the greater trochanter, highlighting the need for continued surveillance even after therapy cessation [88]. This underscores the importance of managing treatment carefully, especially considering the potential for BMD decline following discontinuation. While some authors support denosumab’s efficacy and safety in treating osteoporosis in HD patients, potentially reducing fracture risk, these risks—severe hypocalcemia during treatment and rapid bone loss after the discontinuation of treatment—should be considered with caution in clinical practice [89].
- •
- Anabolic Therapy: Teriparatide, a recombinant 1-34 N-terminal sequence of human PTH, is an anabolic treatment for osteoporosis that may be cautiously used in patients with severe CKD. Although data on its safety are limited, recent studies indicate that teriparatide can effectively increase BMD and improve bone formation markers, even in patients undergoing dialysis. A Japanese post hoc analysis of elderly patients with advanced CKD demonstrated increased BMD and procollagen type 1 N-terminal propeptide (P1NP) levels without serious adverse events, highlighting its potential use in this high-risk group, though hypercalcemia needs careful monitoring [90]. Teriparatide may also be beneficial for patients with adynamic bone disease as it can inhibit sclerostin and promote bone formation. Although teriparatide has shown efficacy in improving BMD, it is contraindicated in those with a history of nephrolithiasis and can lead to adverse effects like hyperuricemia, hypercalcemia, and hypercalciuria. Compared to antiresorptive therapies like denosumab, which also improve BMD but carry a risk of severe hypocalcemia, teriparatide presents an anabolic alternative that could be particularly beneficial in CKD patients with osteoporosis. However, further large-scale studies are needed to confirm its long-term safety and effectiveness, especially in complex CKD–mineral and bone disorder cases [91].
8. Physical Activity for Bone Health in CKD
| Exercise Type | Duration/Frequency/Intensity | CKD Stage/ Setting | Bone Outcomes | Key References |
|---|---|---|---|---|
| Aerobic training | 30–45 min, 3×/week, moderate intensity | CKD stages 3–5, hemodialysis (HD), transplant recipients | Higher Irisin, potential osteoanabolic signaling; preserved femoral neck BMD | [94,95,96] |
| Resistance training | 30–40 min, 2–3×/week, moderate-to-high intensity (e.g., cluster sets) | Mainly HD and post-transplant patients | ↑ BMD (total, femoral, L3–L4), ↑ bone turnover markers | [97,98] |
| Combined (aerobic + resistance) | 45–60 min, 2–3×/week, alternating modalities | Primarily HD; some data in CKD 3–4 and transplant | ↑ Physical performance (6MWT, SPPB); indirect fall prevention | [93,96] |
| Balance training | 20–30 min, 2×/week, progressive difficulty | CKD stages 3–5 (non-dialysis) | Preserved total BMD; ↑ T-score and Z-score | [99] |
9. Clinical Considerations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jørgensen, H.S.; Lloret, M.J.; Lalayiannis, A.D.; Shroff , R.; Evenepoel, P. European Renal Osteodystrophy (EUROD) initiative of the CKD-MBD working group of the European Renal Association (ERA), and the CKD-MBD and Dialysis working groups of the European Society of Pediatric Nephrology. Ten tips on how to assess bone health in patients with chronic kidney disease. Clin. Kidney J. 2024, 17, sfae093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bover, J.; Bailone, L.; López-Báez, V. Osteoporosis, bone mineral density and CKD-MBD: Treatment considerations. J. Nephrol. 2017, 30, 677–687. [Google Scholar] [CrossRef] [PubMed]
- McNerny, E.M.B.; Nickolas, T.L. Bone Quality in Chronic Kidney Disease: Definitions and Diagnostics. Curr. Osteoporos. Rep. 2017, 15, 207–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fusaro, M.; Aghi, A.; Mereu, M.C.; Giusti, A. Fratture da fragilità nella Malattia Renale Cronica (MRC) [Fragility fracture in the Chronic Kidney Disease (CKD)]. G. Ital. Nefrol. 2017, 34. [Google Scholar] [PubMed]
- Iseri, K.; Carrero, J.J.; Evans, M. Incidence of Fractures Before and After Dialysis Initiation. J. Bone Miner. Res. 2020, 35, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pazianas, M.; Miller, P.D. Osteoporosis and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Back to Basics. Am. J. Kidney Dis. 2021, 78, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Harhay, M.N.; Ong, A.C.M. American Society of Nephrology; European Renal Association; International Society of Nephrology. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowski, S.; Liu, S.; Montez-Rath, M.E.; Denburg, M.; Winkelmayer, W.C.; Chertow, G.; O’Shaughnessy, M.M. Association between cause of kidney failure and fracture incidence in a national US dialysis population cohort study. Clin. Kidney J. 2022, 15, 2245–2257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanis, J.A.; Odén, A.; McCloskey, E.V. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballane, G.; Cauley, J.A.; Luckey, M.M.; El-Hajj Fuleihan, G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 2017, 28, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Galeano, D.; Alessandrello, I.; Aprile, G.; Distefano, G.; Ficara, V.; Giglio, E.; Musumeci, S.; Pocorobba, B.; Zuppardo, C. Osteoporosis and chronic kidney disease: Review and new therapeutic strategies. G. Ital. Nefrol. 2019, 36. [Google Scholar] [PubMed]
- Haarhaus, M.; Aaltonen, L.; Cejka, D.; Cozzolino, M.; de Jong , R.T.; D’Haese, P.; Evenepoel , P.; Lafage-Proust, M.H.; Mazzaferro, S.; McCloskey , E.; et al. Management of fracture risk in CKD-traditional and novel approaches. Clin. Kidney J. 2022, 16, 456–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G.; et al. Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Salusky, I.B.; Malluche, H.; Nickolas, T.L. Renal osteodystrophy: Something old, something new, something needed. Curr. Opin. Nephrol. Hypertens. 2023, 32, 559–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carbonara, C.E.M.; Reis, L.M.D.; Quadros, K.R.D.S.; Vieira Roza , N.A.; Sano, R.; Carvalho, A.B.; Jorgetti, V.; de Oliveira, R.B. Renal osteodystrophy and clinical outcomes: Data from the Brazilian Registry of Bone Biopsies—REBRABO. J. Bras. Nefrol. 2020, 42, 138–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jha, S.; Chapman, M.; Roszko, K. When Low Bone Mineral Density and Fractures Is Not Osteoporosis. Curr. Osteoporos. Rep. 2019, 17, 324–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen-Solal, M.; Funck-Brentano, T.; Ureña Torres, P. Bone fragility in patients with chronic kidney disease. Endocr. Connect. 2020, 9, R93–R101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ong, T.; Yong, B.K.A.; Shouter, T.; Shahrokhi, N.; Sahota, O. Optimising bone health among older people with hip fractures and co-existing advanced chronic kidney disease. Eur. Geriatr. Med. 2020, 11, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Keung, L.; Perwad, F. Vitamin D and kidney disease. Bone Rep. 2018, 9, 93–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguilar, A.; Gifre, L.; Ureña-Torres, P.; Carrillo-López , N.; Rodriguez-García, M.; Massó, E.; da Silva, I.; López-Báez, V.; Sánchez-Bayá, M.; Prior-Español, A.; et al. Pathophysiology of bone disease in chronic kidney disease: From basics to renal osteodystrophy and osteoporosis. Front. Physiol. 2023, 14, 1177829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sessa, C.; Granata, A.; Gaudio, A.; Xourafa, A.; Malatino, L.; Lentini, P.; Fatuzzo, P.; Rapisarda, F.; Castellino, P.; Zanoli, L. Vascular dysfunction in Cardiorenal Syndrome type 4. G. Ital. Nefrol. 2020, 37. [Google Scholar] [PubMed]
- Bellone, F.; Cinquegrani, M.; Nicotera, R.; Carullo, N.; Casarella, A.; Presta, P.; Andreucci, M.; Squadrito, G.; Mandraffino, G.; Prunesti, M.; et al. Role of Vitamin K in Chronic Kidney Disease: A Focus on Bone and Cardiovascular Health. Int. J. Mol. Sci. 2022, 23, 5282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabatino, A.; Cuppari, L.; Stenvinkel Pet, a.l.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fusaro, M.; Pereira, L.; Bover, J. Current and Emerging Markers and Tools Used in the Diagnosis and Management of Chronic Kidney Disease-Mineral and Bone Disorder in Non-Dialysis Adult Patients. J. Clin. Med. 2023, 12, 6306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vervloet, M.G.; Brandenburg, V.M.; CKD-MBD working group of ERA-EDTA. Circulating markers of bone turnover. J. Nephrol. 2017, 30, 663–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bover, J.; Ureña-Torres, P.; Cozzolino, M.; Rodríguez-García, M.; Gómez-Alonso, C. The Non-invasive Diagnosis of Bone Disorders in CKD. Calcif. Tissue Int. 2021, 108, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2024, 177, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Morabito, N.; Basile, G.; Fusco, S.; Castagna, G.; Reitano, F.; Albanese, R.C.; Lasco, A. Fracture risk assessment in postmenopausal women referred to an Italian center for osteoporosis: A single day experience in Messina. Clin. Cases Miner. Bone Metab. 2013, 10, 191–194. [Google Scholar] [PubMed] [PubMed Central]
- Przedlacki, J.; Buczyńska-Chyl, J.; Koźmiński, P.; Niemczyk, E.; Wojtaszek, E.; Gieglis , E.; Żebrowski , P.; Podgórzak , A.; Wściślak, J.; Wieliczko, M.; et al. FRAX prognostic and intervention thresholds in the management of major bone fractures in hemodialysis patients: A two-year prospective multicenter cohort study. Bone 2020, 133, 115188. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Anwar, S.; Kour, K.; Sanjoy, S.; Goyal, K.; Prasad, B. T Scores, FRAX, Frailty Phenotype, Falls, and Its Relationship to Fractures in Patients on Maintenance Hemodialysis. Can. J. Kidney Health Dis. 2021, 8, 20543581211041184. [Google Scholar] [CrossRef]
- Tan, T.H.A.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.; Schini, M. Assessment of fracture risk with FRAX and FRAXplus. Gac. Med. Mex. 2024, 160, 363–373. (In English) [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Bianchi, G.; Brandi, M.L.; Di Munno, O.; Frediani, B.; Gatti, D.; Giannini, S.; Girasole, G.; Minosola, G.; Minosola, S.; et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: Project rationale and description. Clin. Exp. Rheumatol. 2010, 28, 561–570. [Google Scholar] [PubMed]
- Catalano, A.; Gaudio, A.; Bellone, F.; La Fauci, M.M.; Xourafa, A.; Gembillo, G.; Basile, G.; Natale, G.; Squadrito, G.; Corica, F.; et al. Trabecular bone score and phalangeal quantitative ultrasound are associated with muscle strength and fracture risk in hemodialysis patients. Front. Endocrinol. 2022, 13, 940040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pimentel, A.; Bover, J.; Elder , G.; Cohen-Solal , M.; Ureña-Torres, P.A. The Use of Imaging Techniques in Chronic Kidney Disease-Mineral and Bone Disorders (CKD-MBD)-A Systematic Review. Diagnostics 2021, 11, 772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, C.Y.; Ong, K.O. Various musculoskeletal manifestations of chronic renal insufficiency. Clin. Radiol. 2013, 68, e397–e411. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Chew, F.S. Tumoral calcinosis: Pearls, polemics, and alternative possibilities. Radiographics 2006, 26, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M. Osteoporosis imaging: State of the art and advanced imaging. Radiology 2012, 263, 3–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Gerges, M.; Raynor, W.Y.; Uk Park, P.S.; Nguyen, E.; Chan, D.H.; Gholamrezanezhad, A. State of the Art Imaging of Osteoporosis. Semin. Nucl. Med. 2024, 54, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, S1–S130. [CrossRef] [PubMed]
- Cailleaux, P.E.; Ostertag, A.; Metzger, M.; Stengel, B.; Boucquemont, J.; Houillier, P.; Flamant, M.; Ureña-Torres, P.; Cohen-Solal, M. NephroTest Study group. Longitudinal Bone Loss Occurs at the Radius in CKD. Kidney Int. Rep. 2021, 6, 1525–1536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Gómez-Alonso, C.; Casado, E.; Rodríguez-García, M.; Lloret, M.J.; Castro-Alonso, C.; Gifre, L.; Henríquez-Palop, F.; Prior-Español , A.; de la Manzanara, V.L.; et al. Osteoporosis management in patients with chronic kidney disease (ERCOS Study): A challenge in nephrological care. Nefrologia 2024, 44, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Ferguson, T.; Tangri, N.; Yong Ng, C.; Nickolas, T.L. Association of Bone Mineral Density with Fractures Across the Spectrum of Chronic Kidney Disease: The Regina CKD-MBD Study. Can. J. Kidney Health Dis. 2019, 6, 2054358119870539. [Google Scholar] [CrossRef]
- Jones, B.C.; Lee, H.; Cheng, C.C.; Al Mukaddam, M.; Song, H.K.; Snyder , P.J.; Kamona, N.; Rajapakse, C.; Wehrli, F.W. MRI Quantification of Cortical Bone Porosity, Mineralization, and Morphologic Structure in Postmenopausal Osteoporosis. Radiology 2023, 307, e221810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, W.C. Advances and Shortfalls in MRI Evaluation of Osteoporosis. Radiology 2023, 307, e223144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moayyeri, A.; Adams, J.E.; Adler, R.A.; Krieg, M.A.; Hans, D.; Compston, J.; Lewiecki, E.M. Quantitative ultrasound of the heel and fracture risk assessment: An updated meta-analysis. Osteoporos. Int. 2012, 23, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wittich, A.; Vega, E.; Casco, C.; Marini, A.; Forlano, C.; Segovia, F.; Nadal, M.; Mautalen, C. Ultrasound velocity of the tibia in patients on hemodialysis. J. Clin. Densitom. 1998, 1, 157–163. [Google Scholar] [CrossRef]
- Park, P.S.U.; Werner, T.J.; Alavi, A. PET/CT for the Opportunistic Screening of Osteoporosis and Fractures in Cancer Patients. Curr. Osteoporos. Rep. 2024, 22, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Nakajima, H.; Miyazaki, T.; Yayama, T.; Kawahara, H.; Kobayashi, S.; Tsuchida, T.; Okazawa, H.; Fujibayashi, Y.; Baba, H. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: A prospective study. J. Nucl. Med. 2009, 50, 1808–1814. [Google Scholar] [CrossRef]
- Evenepoel, P.; D’Haese, P.; Bacchetta, J.; Cannata-Andia, J.; Ferreira, A.; Haarhaus, M.; Mazzaferro, S.; Proust, M.H.L.; Salam, S.; Spasovski, G.; et al. Bone biopsy practice patterns across Europe: The European renal osteodystrophy initiative-a position paper. Nephrol. Dial. Transplant. 2017, 32, 1608–1613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heaney, R.P.; Dowell, M.S.; Barger-Lux, M.J. Absorption of calcium as the carbonate and citrate salts, with some observations on method. Osteoporos. Int. 1999, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Esche, J.; Johner, S.; Shi, L.; Schönau, E.; Remer, T. Urinary Citrate, an Index of Acid-Base Status, Predicts Bone Strength in Youths and Fracture Risk in Adult Females. J. Clin. Endocrinol. Metab. 2016, 101, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Kommer, A.; Kostev, K.; Schleicher, E.M.; Weinmann-Menke , J.; Labenz, C. Proton pump inhibitor use and bone fractures in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2024, 40, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster , J.Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin. Exp. Res. 2019, 31, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; David, K.; Salam, S.; Evenepoel 6, P. Traditional and Non-traditional Risk Factors for Osteoporosis in CKD. Calcif. Tissue Int. 2021, 108, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alonso, C.; D’Marco, L.; Pomes, J.; Del Amo Conill, M.; García-Diez, A.I.; Molina, P.; Puchades, M.J.; Valdivielso, J.M.; Escudero , V.; Bover, J.; et al. Prevalence of Vertebral Fractures and Their Prognostic Significance in the Survival in Patients with Chronic Kidney Disease Stages 3–5 Not on Dialysis. J. Clin. Med. 2020, 9, 1604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alarkawi, D.; Ali, M.S.; Bliuc, D.; Pallares, N.; Tebe, C.; Elhussein, L.; Caskey, F.J.; Arden, N.K.; Ben-Shlomo, Y.; Abrahamsen, B.; et al. Oral Bisphosphonate Use and All-Cause Mortality in Patients with Moderate-Severe (Grade 3B-5D) Chronic Kidney Disease: A Population-Based Cohort Study. J. Bone Miner. Res. 2020, 35, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Mizobuchi, M.; Winzenrieth, R.; Humbert, L.; Saitou, T.; Kato, T.; Nakajima, Y.; Wakasa, M.; Shishido, K.; Honda, H.; et al. Long-Term Effect of Denosumab on Bone Disease in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2023, 18, 1195–1203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catalano, A.; Oliveri, C.; Natale, G.; Agostino, R.M.; Squadrito, G.; Gaudio, A.; Gembillo, G.; Marina, D.; Cernaro, V.; Longhitano, E.; et al. Renal Function Is Associated with Changes in Bone Mineral Density in Postmenopausal Osteoporotic Women Treated with Denosumab: Data from a Retrospective Cohort Study. J. Clin. Med. 2024, 13, 6239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catalano, A.; Bellone, F.; Morabito, N.; Corica, F. Sclerostin and Vascular Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyauchi, A.; Hamaya, E.; Nishi, K.; Tolman, C.; Shimauchi, J. Efficacy and safety of romosozumab among Japanese postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J. Bone Miner. Metab. 2022, 40, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Mizobuchi, M.; Kato, T.; Suzuki, T.; Fujiwara, Y.; Kanamori, N.; Makuuchi, M.; Honda, H. One-Year Romosozumab Treatment Followed by One-Year Denosumab Treatment for Osteoporosis in Patients on Hemodialysis: An Observational Study. Calcif. Tissue Int. 2023, 112, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Cernaro, V.; Siligato, R.; Curreri, F.; Catalano, A.; Santoro, D. Protective Role of Vitamin D in Renal Tubulopathies. Metabolites 2020, 10, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snyder, S.; Hollenbeak, C.S.; Kalantar-Zadeh, K.; Gitlin, M.; Ashfaq, A. Cost-Effectiveness and Estimated Health Benefits of Treating Patients with Vitamin D in Pre-Dialysis. Forum Health Econ. Policy 2020, 23. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.; Ketteler, M. Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future. Nutrients 2022, 14, 3009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merante, D.; Schou, H.; Morin, I.; Manu, M.; Ashfaq, A.; Bishop, C.; Strugnell, S. Extended-Release Calcifediol: A Data Journey from Phase 3 Studies to Real-World Evidence Highlights the Importance of Early Treatment of Secondary Hyperparathyroidism. Nephron 2024, 148, 657–666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abrahamsen, B.; Ernst, M.T.; Smith, C.D.; Nybo, M.; Rubin, K.H.; Prieto-Alhambra, D.; Hermann, A.P. The association between renal function and BMD response to bisphosphonate treatment: Real-world cohort study using linked national registers. Bone 2020, 137, 115371. [Google Scholar] [CrossRef] [PubMed]
- Gopaul, A.; Kanagalingam, T.; Thain, J.; Khan, T.; Cowan, A.; Sultan, N.; Clemens, K. Denosumab in chronic kidney disease: A narrative review of treatment efficacy and safety. Arch. Osteoporos. 2021, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Chines, A.; Brandi, M.L.; Papapoulos, S.; Lewiecki, E.M.; Reginster, J.Y.; Torres , M.M.; Wang, A.; Bone, H.G. The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: Results from the FREEDOM and FREEDOM Extension studies. Osteoporos. Int. 2019, 30, 71–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gronskaya, S.; Belaya, Z.; Rozhinskaya, L.; Mamedova, E.; Vorontsova, M.; Solodovnikov, S.; Golounina, O.; Melnichenko, G. Denosumab for osteoporosis in patients with primary hyperparathyroidism and mild-to-moderate renal insufficiency. Endocrine 2023, 81, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.R.; Flogaitis, I.; Moore, A.E.; Hampson, G. The effect of previous treatment with bisphosphonate and renal impairment on the response to denosumab in osteoporosis: A ‘real-life’ study. J. Endocrinol. Investig. 2020, 43, 469–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, S.; Fukagawa, M. Uremic Toxicity and Bone in CKD. J. Nephrol. 2017, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinopoulou, K.; Sofianos, I. Risk of falls in chronic kidney disease. J. Frailty Sarcopenia Falls. 2017, 2, 33–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davenport, A. Frailty, appendicular lean mass, osteoporosis and osteosarcopenia in peritoneal dialysis patients. J. Nephrol. 2022, 35, 2333–2340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y. Role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder: A narrative review. Medicine 2023, 102, e33477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salam, S.N.; Khwaja, A.; Wilkie, M.E. Pharmacological Management of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease. Drugs 2016, 76, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Vadcharavivad, S.; Susomboon, T.; Singhan, W.; Dumrongpisutikul , N.; Jakchairoongruang, K.; Eiam-Ong, S.; Praditpornsilpa, K. The effectiveness of cinacalcet: A randomized, open label study in chronic hemodialysis patients with severe secondary hyperparathyroidism. Ren. Fail. 2019, 41, 326–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fusaro, M.; Cianciolo, G.; Tripepi, G.; Plebani, M.; Aghi, A.; Politi, C.; Zaninotto, M.; Nickolas, T.L.; Ferrari, S.; Ketteler, M.; et al. Oral Calcitriol Use, Vertebral Fractures, and Vitamin K in Hemodialysis Patients: A Cross-Sectional Study. J. Bone Miner. Res. 2021, 36, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Cherasard, J.; Sung, J.; Agarwal, S.; Aponte, M.S.; Bucovsky, M.; Fusaro, M.; Silberzweig, J.; Frumkin, G.N.; El Hachem, K.; et al. Changes in Bone Quality after Treatment with Etelcalcetide. Clin. J. Am. Soc. Nephrol. 2023, 18, 1456–1465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hori, M.; Yasuda, K.; Takahashi, H.; Kondo , C.; Shirasawa, Y.; Ishimaru, Y.; Sekiya, Y.; Morozumi, K.; Maruyama, S. Effects of bone turnover status on the efficacy and safety of denosumab among haemodialysis patients. Sci. Rep. 2022, 12, 7781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, C.T.; Deng, Y.L.; Chung, M.C.; Tsai, S.F.; Lin, S.Y.; Chen, C.H. Integrated Osteoporosis Care to Reduce Denosumab-Associated Hypocalcemia for Patients with Advanced Chronic Kidney Disease and End-Stage Renal Disease. Healthcare 2023, 11, 313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.; Lee, E.J.; Woo, S.; Rho, S.; Jung, J.Y. Effect of Denosumab on Bone Health, Vascular Calcification, and Health-Related Quality of Life in Hemodialysis Patients with Osteoporosis: A Prospective Observational Study. J. Clin. Med. 2024, 13, 1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simonini, M.; Bologna, A.; Vezzoli, G. Is denosumab an efficient and safe drug for osteoporosis in dialysis patients? Considerations and state of the art about its use in this setting. Int. Urol. Nephrol. 2024, 56, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, F.; Jafari, M.T.; Moioli, A.; Fofi, C.; Barberi, S.; Amendola, S.; Sciacchiano, S.; Punzo, G.; Menè, P. Safety and efficacy of denosumab in osteoporotic hemodialysed patients. J. Nephrol. 2017, 30, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Tanaka, S.; Kuroda, T.; Hagino, H.; Mori, S.; Soen, S. Association between renal function and fracture incidence during treatment with teriparatide or alendronate: An exploratory subgroup analysis of the Japanese Osteoporosis Intervention Trial-05. Osteoporos. Int. 2024, 35, 2175–2182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishikawa, A.; Yoshiki, F.; Taketsuna, M.; Kajimoto, K.; Enomoto, H. Safety and effectiveness of daily teriparatide for osteoporosis in patients with severe stages of chronic kidney disease: Post hoc analysis of a postmarketing observational study. Clin. Interv. Aging 2016, 11, 1653–1659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.Y.; Harris, A.G.; et al. Effect of Abaloparatide vs. Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2017, 317, 442. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, Y.; Baciga, F.; Bulighin, F.; Amicone, M.; Mosconi, G.; Storari, A.; Brugnano, R.; Pozzato, M.; Motta, D.; D’alessandro, C.; et al. Physical activity and exercise in chronic kidney disease: Consensus statements from the Physical Exercise Working Group of the Italian Society of Nephrology. J. Nephrol. 2024, 37, 1735–1765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, D.F.; Marques, E.A.; Leal, D.; Ferreira , A.; Baker, L.A.; Smith, A.C.; Viana, J.L. Impact of physical activity and exercise on bone health in patients with chronic kidney disease: A systematic review of observational and experimental studies. BMC Nephrol. 2020, 21, 334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Li, H.; Sun, X.; Zhong, R.; Cai, L.; Chen, R.; Madeniyet, M.; Ren, K.; Peng, Z.; Yang, Y.; et al. Aerobic exercise prevents renal osteodystrophy via irisin-activated osteoblasts. JCI Insight 2025, 10, e184468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valenzuela, P.L.; Castillo-García, A.; Saco-Ledo, G.; Santos-Lozano, A.; Lucia, A. Physical exercise: A polypill against chronic kidney disease. Nephrol. Dial. Transplant. 2024, 39, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Heiwe, S.; Jacobson, S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2011, 2011, CD003236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magalhães de Castro, B.; Dos Santos Rosa, T.; de Araújo, T.B.; de Luca Corrêa, H.; de Deus, L.A.; Neves, R.V.P.; Reis, A.L.; Dos Santos, R.L.; da Silva Barbosa, J.M.; de Sousa Honorato, F.; et al. Effects of cluster set resistance training on bone mineral density and markers of bone metabolism in older hemodialysis subjects: A pilot study. Bone 2024, 189, 117240. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.M.; Moraes, C.; Barbosa, J.E.; Carraro Eduardo, J.C.; Fouque, D.; Pelletier, S.; Mafra, D. Exercise Training Alters the Bone Mineral Density of Hemodialysis Patients. J. Strength Cond. Res. 2016, 30, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Petrauskiene, V.; Hellberg, M.; Svensson, P.; Zhou, Y.; Clyne, N. Bone mineral density after exercise training in patients with chronic kidney disease stages 3 to 5: A sub-study of RENEXC—A randomized controlled trial. Clin Kidney J. 2023, 17, sfad287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castillo, R.F.; Pérez, R.G.; González, A.L. Beneficial effects of physical exercise on the osteo-renal Klotho-FGF-23 axis in Chronic Kidney Disease: A systematic review with meta-analysis. Int. J. Med. Sci. 2024, 21, 332–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishop, N.C.; Burton, J.O.; Graham-Brown, M.P.M.; Stensel, D.J.; Viana, J.L.; Watson, E.L. Exercise and chronic kidney disease: Potential mechanisms underlying the physiological benefits. Nat. Rev. Nephrol. 2023, 19, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hashmi, M.F.; Aeddula, N.R. Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD). 3 April 2024. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
| Mechanism | Pathophysiological Features | Clinical Implications |
|---|---|---|
| Mineral Metabolism Imbalance | Impaired P excretion and ↓ Ca reabsorption due to declining renal function. | Predisposes individuals to osteomalacia and poor bone quality; necessitates correction of the imbalance. |
| Secondary Hyperparathyroidism | Higher PTH levels increase osteoclastic activity, leading to excessive bone resorption and lower strength. | Associated with osteitis fibrosa cystica; requires PTH monitoring and control. |
| Vitamin D deficiency | ↓ synthesis of 1,25(OH)2D due to loss of 1-α-hydroxylaze activity leads to ↓ Ca absorption and impaired bone formation. | ↑ susceptibility to fractures; requires vitamin D replacement and monitoring. |
| Uremic toxin accumulation | Retention of toxins like indoxyl sulfate and AGEs inhibits osteoblast function and enhances osteoclast activity. | Linked to skeletal resistance to PTH and reduced bone quality; highlights need for toxin reduction strategies. |
| Bone remodeling disruption | Disorganized or suppressed bone turnover, often due to suppressed PTH, leads to structurally weak bone. | Associated with adynamic bone disease and fracture risk; necessitates tailored treatment decisions. |
| Vascular calcification | Deposition of calcium in blood vessels limits calcium availability for bone. | Increases risk of cardiovascular events and skeletal fragility; complicates treatment planning. |
| Inflammation and oxidative stress | Chronic inflammation and ROS damage osteocyte and impair bone healing, contributing to cortical porosity. | Exacerbates bone loss and increases fracture risk; anti-inflammatory interventions may be beneficial. |
| Sarcopenia and muscle weakness | Reduced muscle mass and strength increase fall risk and lead to reduced mechanical loading on bone, weakening bone structure. | Important contributor to falls and fracture risk in older CKD patients; requires exercise and nutrition intervention. |
| Diagnostic Tool | Purpose | CKD-Specific Considerations |
|---|---|---|
| Serum Biochemical Markers | Calcium, phosphate, vitamin D, and FGF-23 levels are useful to understand mineral metabolism. | Interpretation must account for CKD stage and concurrent therapies. |
| PTH | Reflects bone turnover status; essential in distinguishing high vs. low turnover disease. | PTH targets are CKD stage-specific; extreme values indicate altered turnover. |
| BSAP | Marker of osteoblastic activity; helps evaluate bone formation. | May be elevated in high turnover states; needs correlation with other markers. |
| BMD through DXA | Quantifies bone mineral content; commonly used for osteoporosis diagnosis. | May overestimate bone strength due to vascular calcifications or soft tissue changes |
| TBS | Provides information on bone microarchitecture and fracture risk beyond BMD. | Complementary to DXA; useful in CKD stages where BMD is less predictive. |
| FRAX, Adapted | Estimates fracture probability, though not validated for CKD (requires clinical adaptation). | Not fully validated in CKD; clinical judgment required to adjust risk estimates. |
| Vascular Imaging (e.g., CT, X-ray) | Identifies vascular calcifications often associated with CKD-MBD. | Helpful in advanced disease; calcifications may influence treatment choices. |
| Bone Biopsy | Gold standard for diagnosing renal osteodystrophy and differentiating bone turnover types. | Invasive and rarely used clinically; reserved for complex cases or research. |
| CKD Stages | Drug | Mechanism | Pros and Cons | Key Considerations |
|---|---|---|---|---|
| All | Calcium and vitamin D (cholecalciferol, calcitriol) | Supports bone mineralization, suppresses PTH | Essential for bone health; risk of hypercalcemia and vascular calcifications | Monitor levels, avoid overload |
| G1-G3 (eGFR ≥ 30 mL/min/1.73 m2) | Biphosphonates (alendronate, risedronate) | Inhibit osteoclast-mediated bone resorption | Pros: improve BMD, reduce fracture risk; cons: risk of renal complications, gastrointestinal side effects | Avoid in severe CKD, monitor renal function |
| Denosumab | RANKL inhibitor, reduces osteoclast activity | Pros: not renally cleared, improves BMD; cons: risk of hypocalcemia, rebound bone loss after discontinuation | Monitor calcium, continue supplementation | |
| Romosozumab | Sclerostin inhibitor, promotes bone formation | Pros: increases BMD, reduces fractures; cons: potential cardiovascular risk | Avoid in patients with cardiovascular disease history | |
| G4-G5 (eGFR < 30 mL/min/1.73 m2) | Denosumab | RANKL inhibitor | Pros: effective in advanced CKD; cons: higher hypocalcemia risk | Close calcium monitoring required |
| G5D (dialysis) | Cinacalcet, etelcalcetide | Calcimimetics, decrease PTH | Pros: control secondary hyperparathyroidism; cons: may reduce bone turnover | Monitor bone biomarkers, adjust dose |
| Teriparatide | Anabolic, stimulates osteoblasts | Pros: improves BMD and bone markers; cons: hypercalcemia, contraindicated in nephrolithiasis | Cautious use, monitor closely | |
| G3-G5 | Extended-release calcifediol | Vitamin D prohormone, increases 25 (OH)D and suppresses PTH | Pros: effective in SHPT, fewer side effects; cons: less effective in late CKD | Use early in CKD; monitor 25(OH)D and PTH |
| Category | Practical Points | Details |
|---|---|---|
| Epidemiology and Risk | High fracture risk in CKD | CKD G3–G5D patients have 2.5 increased fracture risk. Hip fractures are particularly morbid. |
| Osteoporosis is prevalent in CKD | 18–32% prevalence in CKD; especially common in G4–G5 and dialysis patients. | |
| Pathophysiology | CKD-MBD overlap | Includes phosphate retention, low vitamin D, secondary hyperparathyroidism, and vascular calcifications. |
| Bone quality degradation | Low turnover diseases (e.g., adynamic bone), osteitis fibrosa, and osteomalacia all contribute. | |
| Vitamin K deficiency | Leads to vascular calcification and bone fragility. | |
| Sarcopenia impact | Muscle wasting in CKD increases fall and fracture risk, especially in elderly. | |
| Diagnosis | BMD (via DXA) | Recommended in CKD G3–G5D with fracture risk factors per 2017 KDIGO. Interpret cautiously in G4–G5D due to vascular calcification. |
| Trabecular bone score (TBS) | Additive to DXA; may identify fragility not detected by BMD alone. | |
| Biomarkers | Assess PTH, calcium, phosphate, BSAP, 25(OH)D, and FGF23 to understand turnover type. | |
| Risk tools | Use FRAX/FRAXplus, DeFRA, and K-DeFRA to estimate risk. | |
| HR-pQCT/QUS | Consider where available for advanced structural insight. | |
| Treatment Strategy | Multidisciplinary care | Include nephrologists, endocrinologists, rheumatologists, geriatricians. |
| Treat based on turnover status | High turnover: avoid anabolic agents; low turnover: avoid antiresorptives. | |
| Calcium and vitamin D | Supplement carefully to avoid vascular calcification. Use active vitamin D in later CKD. | |
| Antiresorptive therapy | Denosumab may be used, even in dialysis; monitor for hypocalcemia. Bisphosphonates are controversial in G4–G5D. | |
| Anabolic therapy | Teriparatide or romosozumab may be considered in low turnover disease; use with caution and specialist input. | |
| Non-pharmacologic measures | Fall prevention, resistance exercise, sarcopenia management, smoking/alcohol cessation. | |
| Special Considerations | Dialysis patients | Higher mortality post fracture; consider tailored tools (e.g., K-DeFRA). |
| FRAX limitations | May underestimate or overestimate fracture risk; combine with biomarkers and clinical judgment. | |
| Biopsy/histomorphometry | Gold standard for turnover diagnosis but rarely performed. Reserved for unclear or refractory cases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Sessa, C.; Morale, W.; Zanoli, L.; Catalano, A.; Silipigni, S.; Soraci, L.; Corsonello, A.; Princiotto, M.; Lomonte, C.; et al. Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication. Metabolites 2025, 15, 460. https://doi.org/10.3390/metabo15070460
Gembillo G, Sessa C, Morale W, Zanoli L, Catalano A, Silipigni S, Soraci L, Corsonello A, Princiotto M, Lomonte C, et al. Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication. Metabolites. 2025; 15(7):460. https://doi.org/10.3390/metabo15070460
Chicago/Turabian StyleGembillo, Guido, Concetto Sessa, Walter Morale, Luca Zanoli, Antonino Catalano, Salvatore Silipigni, Luca Soraci, Andrea Corsonello, Maria Princiotto, Carlo Lomonte, and et al. 2025. "Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication" Metabolites 15, no. 7: 460. https://doi.org/10.3390/metabo15070460
APA StyleGembillo, G., Sessa, C., Morale, W., Zanoli, L., Catalano, A., Silipigni, S., Soraci, L., Corsonello, A., Princiotto, M., Lomonte, C., & Santoro, D. (2025). Fracture Risk in Chronic Kidney Disease: Addressing an Overlooked Complication. Metabolites, 15(7), 460. https://doi.org/10.3390/metabo15070460












