Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars
Abstract
1. Introduction
2. Methods
2.1. Breeding Boars and Sample Collection
2.2. Assessment of Sperm Motility and Quality
2.3. Fecal Sample Pretreatment
2.4. Microbial Community Sequencing of Breeding Boar Feces
2.5. LC-MS Analysis
2.6. Data Analysis
3. Results
3.1. Semen Parameters of Breeding Boars
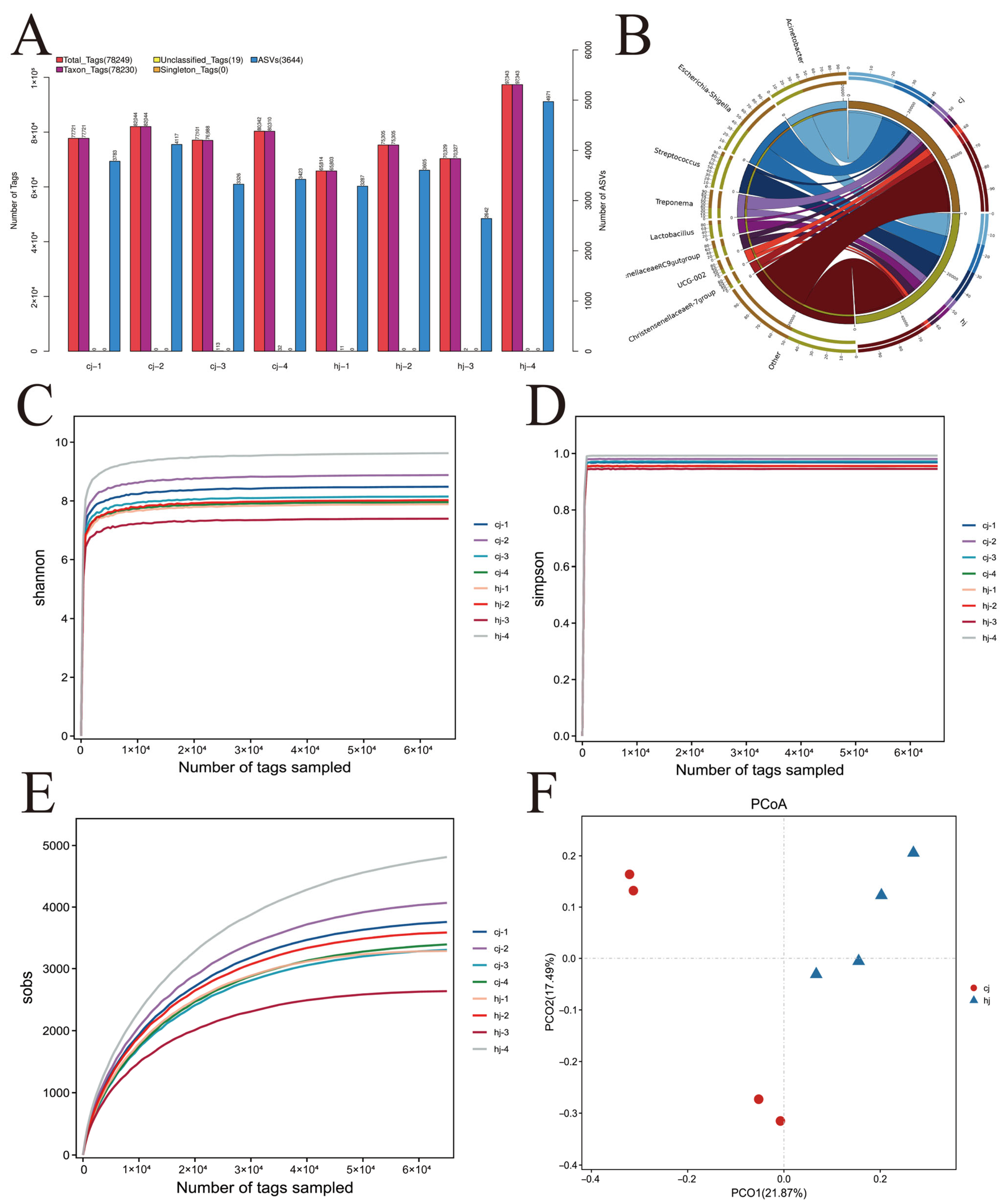
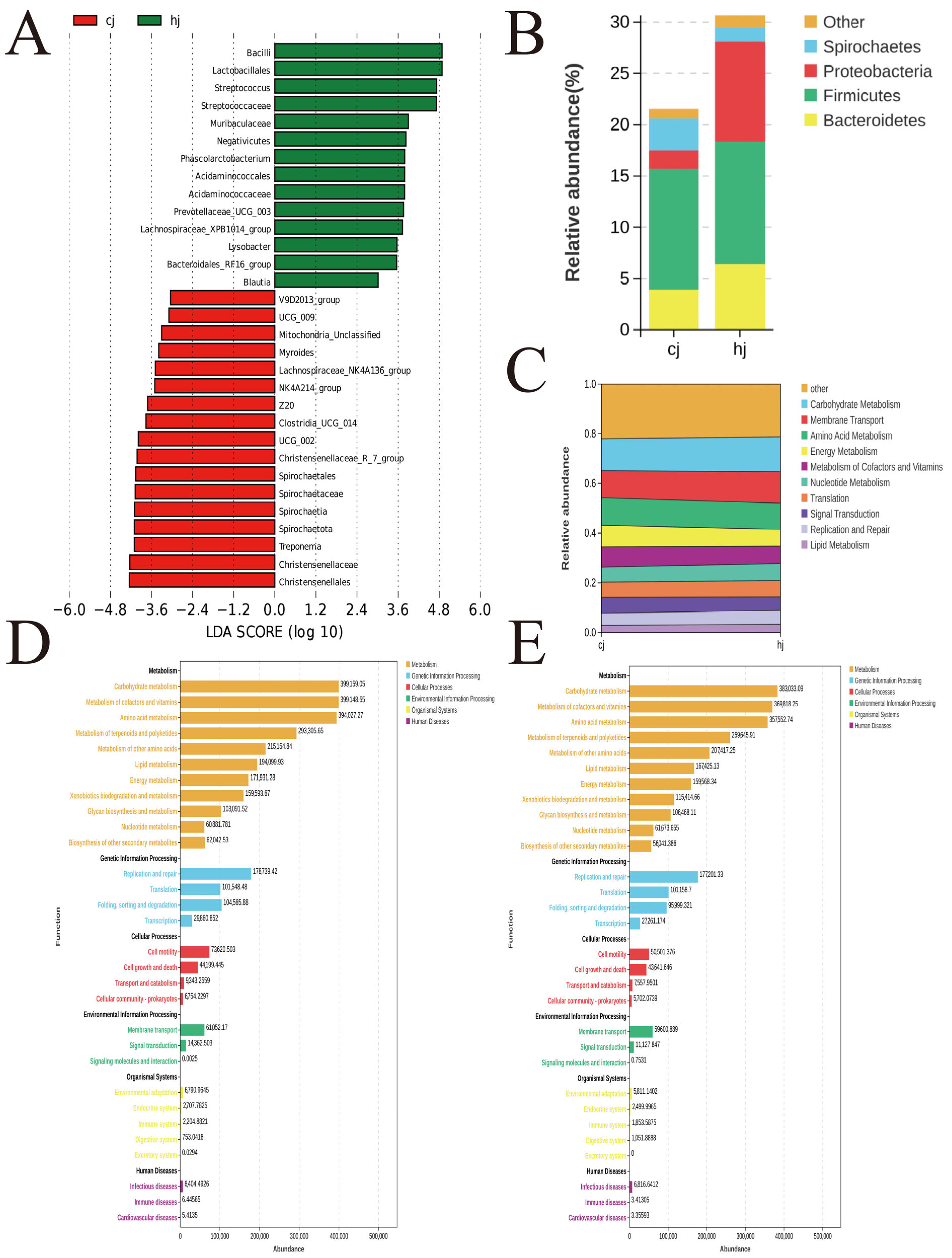
3.2. Gut Microbial Community Composition
3.3. Functional Prediction of Gut Microbiota
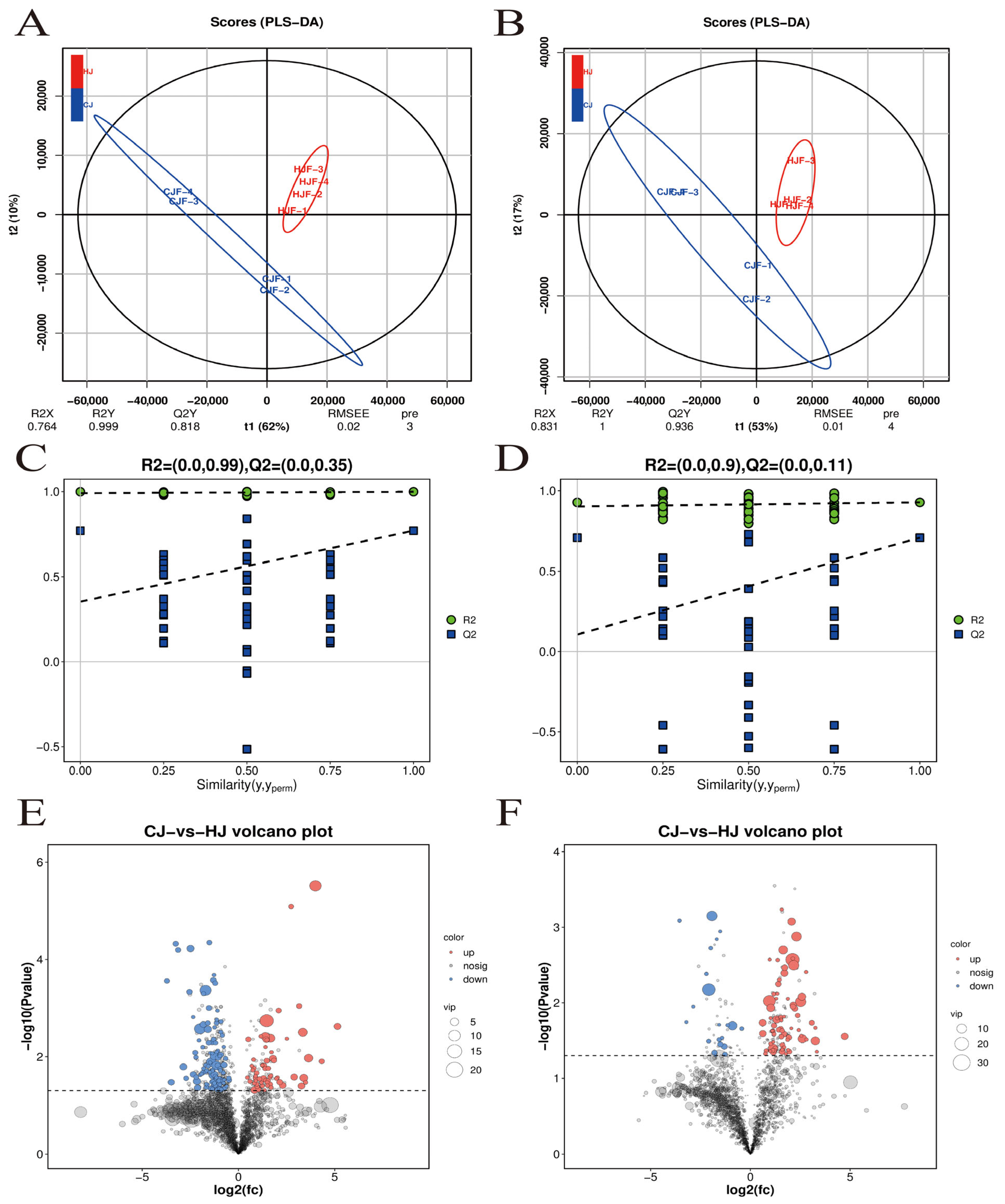
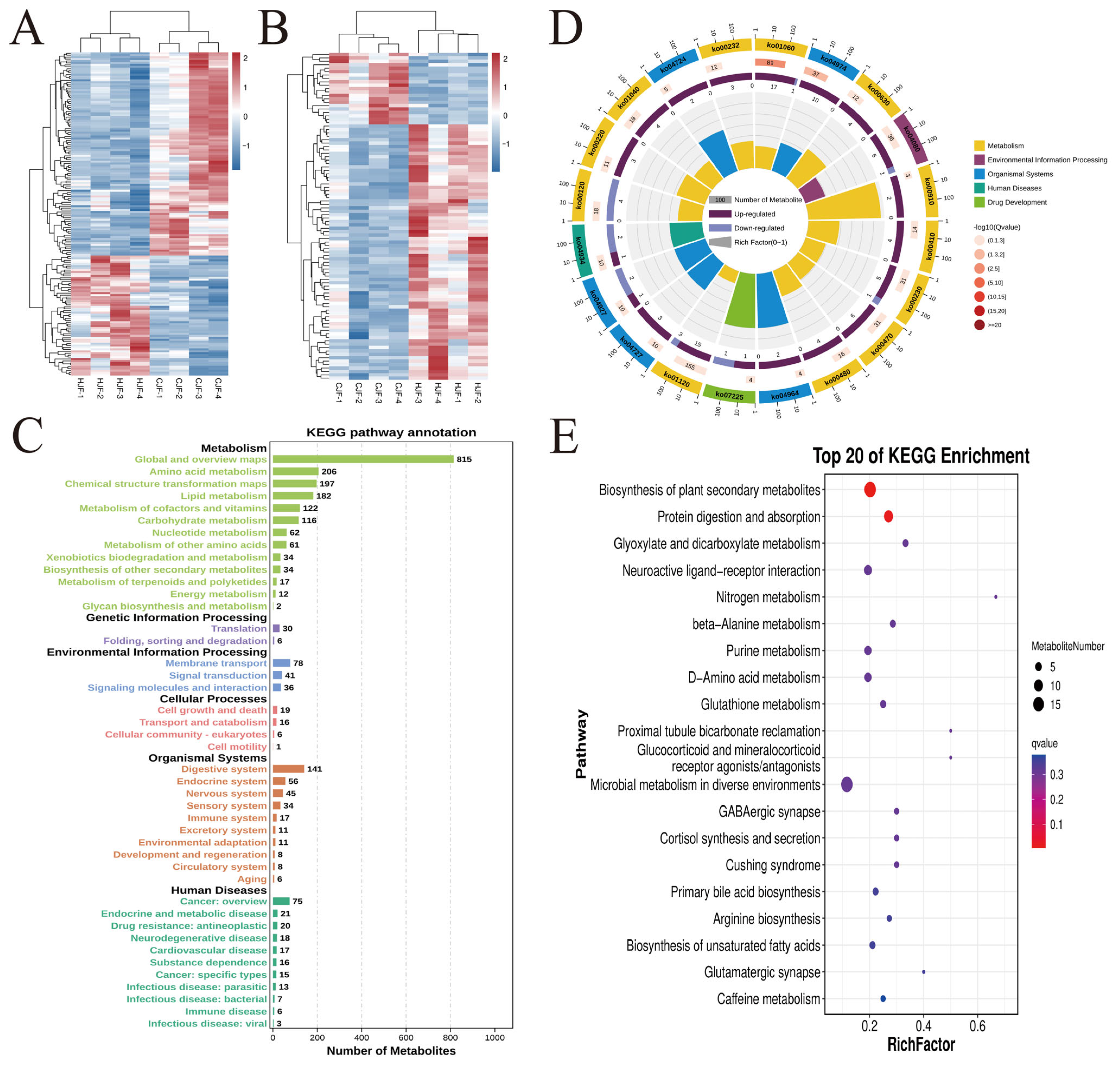
3.4. Fecal Metabolite Profiles in Breeding Boars
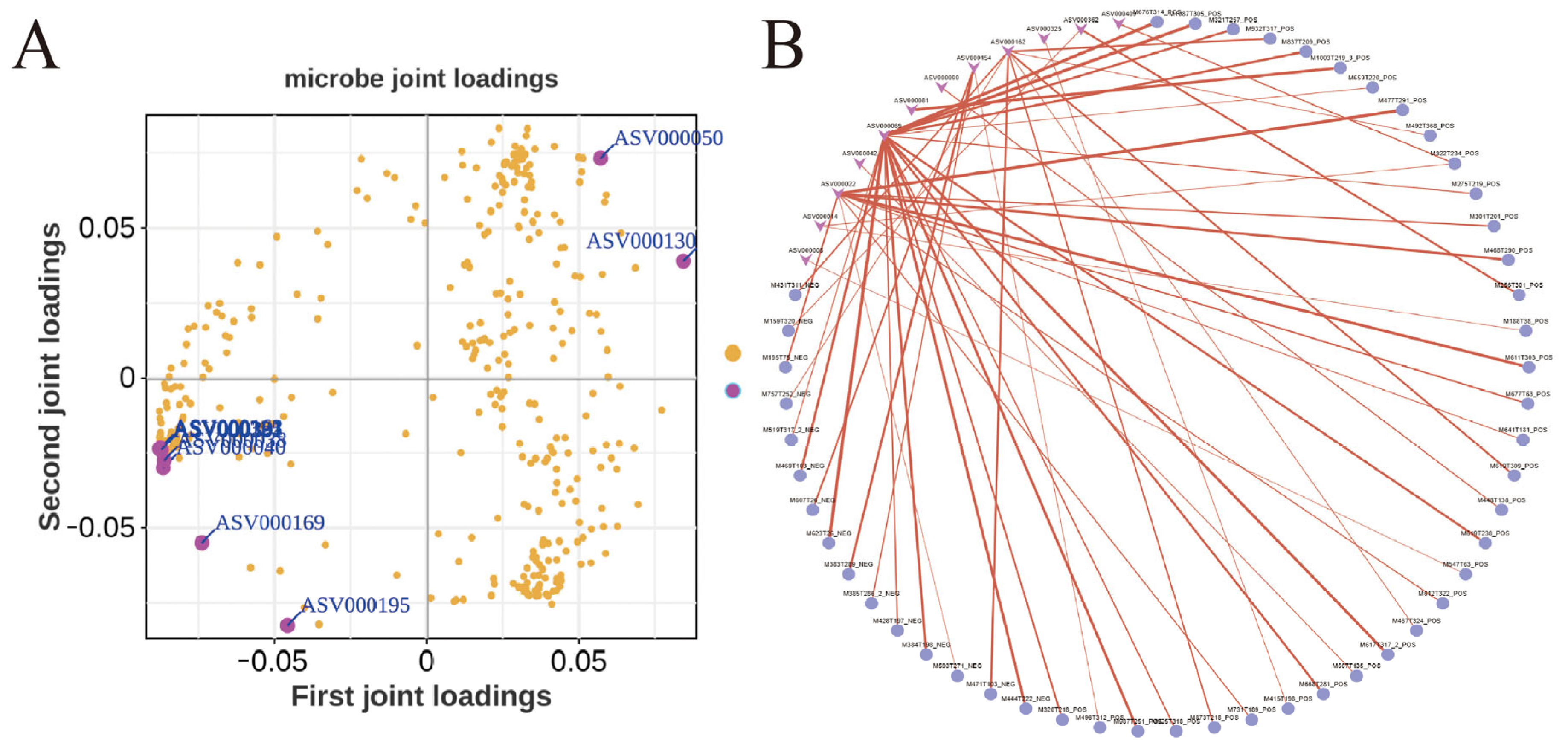
3.5. Microbiota-Metabolite Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Tian, M.; Cheng, Z.; Wang, J.; Ren, Z. DNA Methylation may be a testicular plateau adaptation in Tibetan pig. J. Appl. Anim. Res. 2021, 49, 62–67. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, X.; Cheng, Z.; Tian, M.; Qiangba, Y.; Fu, Q.; Ren, Z. Comparative proteomic analysis of Tibetan pig spermatozoa at high and low altitudes. BMC Genom. 2019, 20, 569. [Google Scholar]
- Waberski, D.; Suarez, S.S.; Henning, H. Assessment of sperm motility in livestock: Perspectives based on sperm swimming conditions in vivo. Anim. Reprod. Sci. 2022, 246, 106849. [Google Scholar] [CrossRef] [PubMed]
- Pipan, M.Z.; Mrkun, J.; Strajn, B.J.; Vrta, K.P.; Kos, J.; Pišlar, A.; Zrimšek, P. The influence of macro- and microelements in seminal plasma on diluted boar sperm quality. Acta Vet. Scand. 2017, 59, 1–9. [Google Scholar] [CrossRef]
- Khan, M.Z.; Chen, W.; Naz, S.; Liu, X.; Liang, H.; Chen, Y.; Kou, X.; Liu, Y.; Ashraf, I.; Han, Y.; et al. Determinant genetic markers of semen quality in livestock. Front. Endocrinol. 2024, 15, 1456305. [Google Scholar] [CrossRef]
- Morrell, J.M.; Mayer, I. Reproduction biotechnologies in germplasm banking of livestock species: A review. Zygote 2017, 25, 545–557. [Google Scholar] [CrossRef]
- Llavanera, M. Evaluation of sperm quality and male fertility: The use of molecular markers in boar sperm and seminal plasma. Anim. Reprod. Sci. 2024, 269, 107545. [Google Scholar] [CrossRef]
- García-Vázquez, F.A. Artificial intelligence and porcine breeding. Anim. Reprod. Sci. 2024, 269, 107545. [Google Scholar] [CrossRef]
- Du Toit, E.; Lategan-Potgieter, R. Environmental, Lifestyle and Dietary Factors that Influence Semen Parameters. J. Acad. Nutr. Diet. 2019, 119, A46. [Google Scholar] [CrossRef]
- Petrelli, S.; Buglione, M.; Maselli, V.; Troiano, C.; Larson, G.; Frantz, L.; Manin, A.; Ricca, E.; Baccigalupi, L.; Wright, D.; et al. Population genomic, olfactory, dietary, and gut microbiota analyses demonstrate the unique evolutionary trajectory of feral pigs. Mol. Ecol. 2022, 31, 220–237. [Google Scholar] [CrossRef]
- El-Jurdi, N.; Ghannoum, M.A.; Heitman, J. The Mycobiome: Impact on Health and Disease States. Microbiol. Spectr. 2017, 5, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Chae, J.P.; Pajarillo, E.A.B.; Kim, S.H.; Kwak, M.; Eun, J.; Chee, S.W.; Whang, K.; Kim, S.; Kang, D. Association between the body weight of growing pigs and the functional capacity of their gut microbiota. Anim. Sci. J. 2020, 91, e13418. [Google Scholar] [CrossRef]
- Feng, G.; Deng, M.; Li, R.; Hou, G.; Ouyang, Q.; Jiang, X.; Liu, X.; Tang, H.; Chen, F.; Pu, S.; et al. Gastrointestinal microbiota and metabolites responses to dietary cereal grains in an adult pig model. Front. Microbiol. 2024, 15, 1442077. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Luo, Z.; Yang, X.; Zhang, Y.; Li, H.; Wang, C.; Zou, Z.; Ma, Y.; Ma, J.; Yue, D.; et al. Protective effects and antioxidant mechanisms of lactobacillus combined with carnosine on the stomach and small intestine. J. Funct. Foods 2025, 125, 106687. [Google Scholar] [CrossRef]
- Shimizu, H.; Arai, K.; Asahara, T.; Takahashi, T.; Tsuji, H.; Matsumoto, S.; Takeuchi, I.; Kyodo, R.; Yamashiro, Y. Stool preparation under anaerobic conditions contributes to retention of obligate anaerobes: Potential improvement for fecal microbiota transplantation. BMC Microbiol. 2021, 21, 275. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Mego, M. Exploring the microbiome-gut-testis axis in testicular germ cell tumors. Front. Cell. Infect. Microbiol. 2025, 14, 1529871. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The potential influence and intervention measures of gut microbiota on sperm: It is time to focus on testis-gut microbiota axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Cao, F.; Song, X. Gut microbiota-derived fatty acid and sterol metabolites: Biotransformation and immunomodulatory functions. Gut Microbes 2024, 16, 2382336. [Google Scholar] [CrossRef]
- Xu, B.; Qin, W.; Chen, Y.; Huang, J.; Ma, L.; Yan, X. Dietary Short-chain Fatty Acids Supplementation Improves Reproductive Performance and Gut Microbiota in Gilts. J. Nutr. 2025, 155, 1089–1098. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Wang, C.; Wei, H.; Tan, J.; Sun, H.; Jiang, S.; Peng, J. Gut Microbiological Disorders Reduce Semen Utilization Rate in Duroc Boars. Front. Microbiol. 2020, 11, 581926. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Di Zhang, X.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Komninos, D.; Ramos, L.; Van der Heijden, G.W.; Morrison, M.C.; Kleemann, R.; Van Herwaarden, A.E.; Kiliaan, A.J.; Arnoldussen, I.A.C. High fat diet-induced obesity prolongs critical stages of the spermatogenic cycle in a Ldlr. Sci. Rep. 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M.; Cantore, S. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. BioMed Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhou, H.; Wang, S.; Chen, X.; Xiao, H. Gut microbiota, microbiota-derived metabolites, and graft-versus-host disease. Cancer Med. 2024, 13, 25. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.U.; Kweon, M.N. Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 2024, 46, 2. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, K.; Li, Z.F.; Wang, Z.Y.; Wang, L. Glyphosate-induced gut microbiota dysbiosis facilitates male reproductive toxicity in rats. Sci. Total Environ. 2022, 805, 150368. [Google Scholar] [CrossRef]
- Wang, M.; Ren, C.; Wang, P.; Cheng, X.; Chen, Y.; Huang, Y.; Chen, J.; Sun, Z.; Wang, Q.; Zhang, Z. Microbiome–Metabolome Reveals the Contribution of the Gut–Testis Axis to Sperm Motility in Sheep (Ovis aries). Animals 2023, 13, 996. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Shen, H.; Jiang, Y.; Yang, Q.; Su, S.; Wu, L.; Fan, X.; Gao, M.; Wu, Y.; et al. Mixed probiotics modulated gut microbiota to improve spermatogenesis in bisphenol A-exposed male mice. Ecotoxicol. Environ. Saf. 2024, 270, 115922. [Google Scholar] [CrossRef]
- Bagga, R.; Arora, P. Genital Micro-Organisms in Pregnancy. Front. Public Health 2020, 8, 225. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Zhou, F.; Wu, Z.; Zhang, L.; Xie, R.; Jiang, F.; Tian, Q.; Huang, X. Ningxiang Pig-Derived Microbiota Affects the Growth Performance, Gut Microbiota, and Serum Metabolome of Nursery Pigs. Animals 2024, 14, 2450. [Google Scholar] [CrossRef]
- Dong, H.J.; Wu, D.; Xu, S.Y.; Li, Q.; Fang, Z.F.; Che, L.Q.; Wu, C.M.; Xu, X.Y.; Lin, Y. Effect of dietary supplementation with amino acids on boar sperm quality and fertility. Anim. Reprod. Sci. 2016, 172, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Dutta, S.; Sengupta, P.; Khorshid, H.R.K.; Esteves, S.; Gilany, K.; Hedayati, M.; et al. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J. Assist. Reprod. Genet. 2019, 36, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Gualdoni, G.S.; Jacobo, P.V.; Sobarzo, C.M.; Pérez, C.V.; Matzkin, M.E.; Höcht, C.; Frungieri, M.B.; Hill, M.; Anegon, I.; Lustig, L.; et al. Role of indoleamine 2,3-dioxygenase in testicular immune-privilege. Sci. Rep. 2019, 9, 15919. [Google Scholar] [CrossRef]
- Van der Horst, G.; Maree, L. Assessment of Sperm Motility with the Use of Computer-Aided Sperm Analysis (CASA). Methods Mol. Biol. 2025, 2897, 219–234. [Google Scholar]
- Hackerova, L.; Pilsova, A.; Pilsova, Z.; Zelenkova, N.; Tymich Hegrova, P.; Klusackova, B.; Chmelikova, E.; Sedmikova, M.; Simonik, O.; Postlerova, P. Boar Sperm Motility Assessment Using Computer-Assisted Sperm Analysis: Current Practices, Limitations, and Methodological Challenges. Animals 2025, 15, 305. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Liu, F.; Luo, L.; Wei, M.; Ye, Y.; Yangzom, C.; Shang, P. Effects of Different Feed Additives on Intestinal Metabolite Composition of Weaned Piglets. Metabolites 2024, 14, 138. [Google Scholar] [CrossRef]
- Singh, V.; Choi, S.D.; Mahra, K.; Son, H.; Lee, H.; Lee, Y.J.; Kim, E.S.; Shin, J.H. Cultured fecal microbial community and its impact as fecal microbiota transplantation treatment in mice gut inflammation. Appl. Microbiol. Biotechnol. 2024, 108, 463. [Google Scholar] [CrossRef]
- Huang, N.; Liu, X.; Pei, X.; Peng, J.; Wei, H. The quantitative profiling of oxylipins from arachidonic acid by LC-MS/MS in feces at birth 3 days and 21 days of piglets. Metabolites 2022, 12, 702. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, Y.; Guo, C.; Li, C.; Wei, H.; Li, L.; Meng, L.; Zhang, S. Analysis of differentially abundant proteins related to boar fertility in seminal plasma using iTRAQ-based quantitative proteomics. J. Proteom. 2021, 236, 104120. [Google Scholar] [CrossRef]
- Tan, M.; Zhao, Y.; Ren, L.; Li, C.; Cai, J.; He, B. Methionine Improves Boar Sperm Quality by Promoting Mitochondrial Translation during Liquid Storage. Animals 2024, 14, 2227. [Google Scholar] [CrossRef]
- Zheng, X.; Nie, K.; Xu, Y.; Zhang, H.; Xie, F.; Xu, L.; Zhang, Z.; Ding, Y.; Yin, Z.; Zhang, X. Fecal Microbial Structure and Metabolic Profile in Post-Weaning Diarrheic Piglets. Genes 2023, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Luise, D.; Modesto, M.; Correa, F.; Bosi, P.; Mattarelli, P.; Trevisi, P. Assessment of Biolog EcoplateTM method for functional metabolic diversity of aerotolerant pig fecal microbiota. Appl. Microbiol. Biotechnol. 2021, 105, 6033–6045. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cui, X.; Wang, X.; Zhai, F.; Wang, L.; Zhu, L. Multi-omics analysis of gut microbiota and metabolites reveals contrasting profiles in domestic pigs and wild boars across urban environments. Front. Microbiol. 2024, 15, 1450306. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Azulai, D.; Dueker, M.E.; Vos, M.; Perron, G.G. Triclosan Alters Microbial Communities in Freshwater Microcosms. Water 2019, 11, 961. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Wang, L.; Zheng, Y.; Hoque, S.A.M.; Lv, Y.; Zeng, W. Glycogen Synthase Kinase-3 Regulates Sperm Motility and Acrosome Reaction via Affecting Energy Metabolism in Goats. Front. Physiol. 2019, 10, 968. [Google Scholar] [CrossRef]
- Ye, L.; Huang, W.; Liu, S.; Cai, S.; Hong, L.; Xiao, W.; Thiele, K.; Zeng, Y.; Song, M.; Diao, L.J. Impacts of Immunometabolism on Male Reproduction. Front. Immunol. 2021, 12, 658432. [Google Scholar] [CrossRef]
- Ferramosca, A.; Moscatelli, N.; di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Varela, E.; Rojas, M.; Restrepo, G. Membrane stability and mitochondrial activity of bovine sperm frozen with low-density lipoproteins and trehalose. Reprod. Domest. Anim. 2020, 55, 146–153. [Google Scholar] [CrossRef]
- Torres, M.A.; Ravagnani, G.M.; Leal, D.F.; Martins, S.M.M.K.; Muro, B.B.D.; Meirelles, F.V.; Papa, F.O.; Dell’aqua, J.A.; Alvarenga, M.A.; Moretti, A.S.; et al. Seminal plasma arising from the whole boar sperm-rich fraction increases the stability of sperm membrane after thawing. J. Anim. Sci. 2016, 94, 1906–1912. [Google Scholar] [CrossRef]
- Hashem, N.; González-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Wang, M.; Yue, J.; Lv, G.; Wang, Y.; Guo, A.; Liu, Z.; Yu, T.; Yang, G. Effects of Interactions between Feeding Patterns and the Gut Microbiota on Pig Reproductive Performance. Animals 2024, 14, 2714. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Y.; Huang, A.; Ni, Q.; Zeng, C. Phenylbutyrate and Dichloroacetate Enhance the Liquid-Stored Boar Sperm Quality via PDK1 and PDK3. Int. J. Mol. Sci. 2023, 24, 17091. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Cheng, M.; Ye, F.; Li, W.; Wang, C.; Huang, Y.; Wu, Y.; Xuan, R.; Liu, G.; et al. Gut microbial diversity among Yorkshire, Landrace and Duroc boars and its impact on semen quality. AMB Express 2022, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, M.; Xu, Z.; Guo, J.; Chen, H.; Yang, X.; Chen, P.; Liu, G.; Liu, B.M. Association between semen microbiome disorder and sperm DNA damage. Microbiol. Spectr. 2024, 12, e0075924. [Google Scholar] [CrossRef]
- Maha, A.A.; Jan-Bernd, S.; Ahmed, R.; Farhana, A.; Mona-Lisa, S.; Lars, H.; Sven, P.; Olle, S.D.; Gray, C.M. The Gut Microbiota and Developmental Programming of the Testis in Mice. PLoS ONE 2014, 9, e103809. [Google Scholar]
- Alhaj, H.W.; Li, Z.; Shan, T.; Dai, P.; Zhu, P.; Li, Y.; Alsiddig, M.A.; Abdelghani, E.; Li, C. Effects of dietary sodium butyrate on reproduction in adult breeder roosters. Anim. Reprod. Sci. 2018, 196, 111–119. [Google Scholar] [CrossRef]
- Ma, S.; Li, J.; Ye, H.; Huang, S.; Huang, Z.; Wu, D.; Ma, K.; Xie, J.; Yin, Y.; Tan, C. Effects of dietary supplementation of different levels of gamma-aminobutyric acid on reproductive performance, glucose intolerance, and placental development of gilts. J. Anim. Sci. 2024, 102, skad405. [Google Scholar] [CrossRef]
- Li, T.; Qin, Z.; Wang, D.; Xia, X.; Zhou, X.; Hu, G. Coenzyme self-sufficiency system-recent advances in microbial production of high-value chemical phenyllactic acid. World J. Microbiol. Biotechnol. 2023, 39, 36. [Google Scholar] [CrossRef]
- Van-Wehle, T.; Vital, M. Investigating the response of the butyrate production potential to major fibers in dietary intervention studies. npj Biofilms Microbiomes 2024, 10, 63. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rnning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef]
- Su, S.H.; Wu, Y.F.; Lin, Q.; Zhang, L.; Wang, D.P.; Hai, J. Fecal microbiota transplantation and replenishment of short-chain fatty acids protect against chronic cerebral hypoperfusion-induced colonic dysfunction by regulating gut microbiota, differentiation of Th17 cells, and mitochondrial energy metabolism. J. Neuroinflammation 2022, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Ayewoh, E.N.; Czuba, L.C.; Nguyen, T.T.; Swaan, P.W. S-acylation status of bile acid transporter hASBT regulates its function, metabolic stability, membrane expression, and phosphorylation state. Biochim. Biophys. Acta Biomembr. 2020, 1863, 183510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Rodionov, D.A.; Leyn, S.A.; Tran, D.; Iablokov, S.N.; Ding, H.; Peterson, D.A.; Osterman, A.L.; Peterson, S.N. B-Vitamin Sharing Promotes Stability of Gut Microbial Communities. Front. Microbiol. 2019, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.S. Vitamin B12 and Semen Quality. Biomolecules 2017, 7, 42. [Google Scholar] [CrossRef]
- Hassani-Bafrani, H.; Tavalaee, M.; Arbabian, M.; Arbabian, M.; Dattilo, M.; Andrologia, M.H. The effect of vitamin E & vitamin B on sperm function in rat varicocele model. Andrologia 2019, 51, e13429. [Google Scholar]
- Beltrame, F.L.; Santi, F.D.; Vendramini, V.; Cabral, R.E.L.; Miraglia, S.M.; Cerri, P.S.; Sasso-Cerri, E. Vitamin B12 Prevents Cimetidine-Induced Androgenic Failure and Damage to Sperm Quality in Rats. Front. Endocrinol. 2019, 10, 309. [Google Scholar] [CrossRef]
- Han, H.; Zhong, R.; Zhou, Y.; Xiong, B.; Chen, L.; Jiang, Y.; Liu, L.; Sun, H.; Tan, J.; Tao, F.; et al. Hydroxytyrosol Benefits Boar Semen Quality via Improving Gut Microbiota and Blood Metabolome. Front. Nutr. 2021, 8, 815922. [Google Scholar] [CrossRef]
- Martinot, E.; Baptissart, M.; Véga, A.; Sèdes, L.; Rouaisnel, B.; Vaz, F.; Saru, J.P.; de Haze, A.; Baron, S.; Caira, F.; et al. Bile acid homeostasis controls CAR signaling pathways in mouse testis through FXRalpha. Sci. Rep. 2017, 7, 42182. [Google Scholar] [CrossRef]
| Items | Proportion/% |
|---|---|
| Corn | 45.00 |
| Wheat bran | 35.00 |
| Soybean meal | 16.00 |
| Additive premixes 2624 | 4.00 |
| DE (Digestible Energy Mcal/kg) | 2.8487 |
| CP (Crude Protein %) | 15.89 |
| CF (Crude Fat %) | 3.94 |
| Ca (Calcium %) | 0.70 |
| TP (Total Phosphorus %) | 0.66 |
| NPP (Non-Phytate Phosphorus %) | 0.30 |
| Lys (Lysine %) | 0.79 |
| Met + Cys (Methionine + Cystine %) | 0.53 |
| Thr (Threonine %) | 0.58 |
| Trp (Tryptophan %) | 0.21 |
| Target | Amplified Region | Primer Name | Primer Sequence (5′ → 3′) | Amplicon Length (bp) |
|---|---|---|---|---|
| 16S Bacteria | V3-V4 | 341F | CCTACGGGNGGCWGCAG | ~466 |
| 806R | GGACTACHVGGGTATCTAAT | |||
| 16S Bacteria | V5-V7 | 799F | AACMGGATTAGATACCCKG | ~414 |
| 1193R | ACGTCATCCCCACCTTCC | |||
| 16S Archaea | V4-V5 | Arch519F | CAGCMGCCGCGGTAA | ~397 |
| Arch915R | GTGCTCCCCCGCCAATTCCT | |||
| 18S | V4 | 528F | GCGGTAATTCCAGCTCCAA | ~179 |
| 706R | AATCCRAGAATTTCACCTCT | |||
| ITS | ITS2 | ITS3_KYO2 | GATGAAGAACGYAGYRAA | ~381 |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| ITS | ITS1 | ITS1_F_KYO2 | TAGAGGAAGTAAAAGTCGTAA | ~366 |
| ITS86R | TTCAAAGATTCGATGATTCAC |
| Semen Quality Parameter | High Utilization Group | Low Utilization Group | p Value |
|---|---|---|---|
| Semen utilization rate % | 88.8025 ± 2.31535 | 73.2025 ± 18.62576 | 0.044 |
| Sperm motility % | 83.945 ± 1.69632 | 55.575 ± 24.74639 | 0.033 |
| Sperm density 109/ML | 1.515 ± 0.07141 | 1.18 ± 0.55516 | 0.024 |
| Progressive motility % | 49.6425 ± 2.85477 | 42.2325 ± 13.48129 | 0.018 |
| Metabolite/Pathway | Mechanism | Associated Taxa |
|---|---|---|
| Butyrate | Energy provision/Anti-inflammation | Faecalibacterium, Ruminococcus |
| 6-Hydroxyhexanoate | Xenobiotic detoxification/ATP synthesis | Streptococcaceae |
| Lithocholic acid (LCA) | Bile acid homeostasis/Membrane stability | Clostridium sensu stricto 3 |
| Phenyllactic acid (PLA) | Antimicrobial/ROS scavenging | Lactobacillus |
| Vitamin B6 metabolism | DNA protection/Antioxidant | Rikenellaceae_RC9_gut_group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Han, M.; Zhang, H.; Wang, X.; Yin, Y.; Zhang, J.; Shang, P. Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars. Metabolites 2025, 15, 447. https://doi.org/10.3390/metabo15070447
Zhao M, Han M, Zhang H, Wang X, Yin Y, Zhang J, Shang P. Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars. Metabolites. 2025; 15(7):447. https://doi.org/10.3390/metabo15070447
Chicago/Turabian StyleZhao, Mingxuan, Mengjia Han, Hongliang Zhang, Xiangdong Wang, Yikai Yin, Jian Zhang, and Peng Shang. 2025. "Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars" Metabolites 15, no. 7: 447. https://doi.org/10.3390/metabo15070447
APA StyleZhao, M., Han, M., Zhang, H., Wang, X., Yin, Y., Zhang, J., & Shang, P. (2025). Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars. Metabolites, 15(7), 447. https://doi.org/10.3390/metabo15070447





