Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research
Abstract
1. Introduction
2. Literature Search
3. Metabolomics as a Tool for Advancing Research in Metabolic Bariatric Surgery
3.1. Understanding the Dynamic Metabolic Landscape Through Metabolomics
3.2. Key Analytical Techniques for Metabolomic Studies in Metabolic Bariatric Surgery
4. Considerations Regarding Planning and Performing a Metabolomics Study
4.1. Study Design and Analytical Approaches in Metabolomics
4.2. Sample Handling, Extraction Protocols, and Quality Control
4.3. Data Acquisition, Processing, and Statistical Analysis
5. Metabolomic Changes Following Metabolic Bariatric Surgery
5.1. Amino Acid Derivatives
5.1.1. Role of Branched-Chain Amino Acids (BCAAs) in Obesity and Metabolic Disease
5.1.2. Aromatic Amino Acids (AAAs) and Their Link with Obesity and Glucose Dysregulation
5.1.3. Non-Classical Amino Acids and Their Metabolic Significance
5.2. Lipid Derivatives
5.2.1. Fatty Acids, Membrane Lipids, and Energy-Related Lipid Metabolites
5.2.2. Bile Acids
5.2.3. Endocannabinoids
5.3. The Interplay Between Intestinal Microbiota and Their Metabolic Outputs
6. Procedure-Specific Metabolomic Signatures in Metabolic Bariatric Surgery
7. Metabolic Bariatric Surgery Versus Incretin-Based Pharmacological Weight-Loss Interventions
8. Limitations, Challenges, and Research Gaps in Contemporary Metabolomic Studies Within Metabolic Bariatric Surgery
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Stratigou, T.; Voukali, M.; Karampela, I.; Dalamaga, M. The Kidney in Obesity: Current Evidence, Perspectives and Controversies. Curr. Obes. Rep. 2024, 13, 680–702. [Google Scholar] [CrossRef]
- Poirier, P.; Cornier, M.A.; Mazzone, T.; Stiles, S.; Cummings, S.; Klein, S.; McCullough, P.A.; Ren Fielding, C.; Franklin, B.A. Bariatric surgery and cardiovascular risk factors: A scientific statement from the American Heart Association. Circulation 2011, 123, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, S.; Carlsson, L.; De Gaetano, A.; Peltonen, M.; Rice, T.; Sjöström, L.; Mingrone, G.; Dixon, J.B. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care 2016, 39, 166–174. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e245. [Google Scholar] [CrossRef]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Meron-Eldar, S.; Brethauer, S.A.; Schauer, P.R.; Young, J.B. Effect of bariatric surgery on cardiovascular risk profile. Am. J. Cardiol. 2011, 108, 1499–1507. [Google Scholar] [CrossRef]
- van Veldhuisen, S.L.; Gorter, T.M.; van Woerden, G.; de Boer, R.A.; Rienstra, M.; Hazebroek, E.J.; van Veldhuisen, D.J. Bariatric surgery and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1955–1969. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, P.; Li, P.; Wang, G.; Yi, X.; Fu, Z.; Sun, X.; Cui, B.; Zhu, L.; Zhu, S. Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.-C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e1295. [Google Scholar] [CrossRef] [PubMed]
- Skubleny, D.; Switzer, N.J.; Gill, R.S.; Dykstra, M.; Shi, X.; Sagle, M.A.; de Gara, C.; Birch, D.W.; Karmali, S. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.V.; Pereira, T.V.; Aboud, C.M.; Petry, T.B.Z.; Lopes Correa, J.L.; Schiavon, C.A.; Pompílio, C.E.; Pechy, F.N.Q.; da Costa Silva, A.C.C.; de Melo, F.L.G.; et al. Effect of Gastric Bypass vs Best Medical Treatment on Early-Stage Chronic Kidney Disease in Patients With Type 2 Diabetes and Obesity: A Randomized Clinical Trial. JAMA Surg. 2020, 155, e200420. [Google Scholar] [CrossRef]
- Sandoval, D.A. Mechanisms for the metabolic success of bariatric surgery. J. Neuroendocrinol. 2019, 31, e12708. [Google Scholar] [CrossRef]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef]
- Attard, J.A.; Dunn, W.B.; Mergental, H.; Mirza, D.F.; Afford, S.C.; Perera, M. Systematic Review: Clinical Metabolomics to Forecast Outcomes in Liver Transplantation Surgery. Omics 2019, 23, 463–476. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, A.G. Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap. Metabolites 2022, 12, 458. [Google Scholar] [CrossRef]
- Tulipani, S.; Griffin, J.; Palau-Rodriguez, M.; Mora-Cubillos, X.; Bernal-Lopez, R.M.; Tinahones, F.J.; Corkey, B.E.; Andres-Lacueva, C. Metabolomics-guided insights on bariatric surgery versus behavioral interventions for weight loss. Obesity 2016, 24, 2451–2466. [Google Scholar] [CrossRef]
- Samczuk, P.; Ciborowski, M.; Kretowski, A. Application of Metabolomics to Study Effects of Bariatric Surgery. J. Diabetes Res. 2018, 2018, 6270875. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar]
- Akyol, S.; Ashrafi, N.; Yilmaz, A.; Turkoglu, O.; Graham, S.F. Metabolomics: An Emerging “Omics” Platform for Systems Biology and Its Implications for Huntington Disease Research. Metabolites 2023, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 2016, 12, 149. [Google Scholar] [CrossRef]
- Gonzalez-Covarrubias, V.; Martínez-Martínez, E.; Del Bosque-Plata, L. The Potential of Metabolomics in Biomedical Applications. Metabolites 2022, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Hajnajafi, K.; Iqbal, M.A. Mass-spectrometry based metabolomics: An overview of workflows, strategies, data analysis and applications. Proteome Sci. 2025, 23, 5. [Google Scholar] [CrossRef]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass spectrometry strategies in metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Magro, D.; Venezia, M.; Rita Balistreri, C. The omics technologies and liquid biopsies: Advantages, limitations, applications. Med. Omics 2024, 11, 100039. [Google Scholar] [CrossRef]
- Wewer Albrechtsen, N.J.; Geyer, P.E.; Doll, S.; Treit, P.V.; Bojsen-Møller, K.N.; Martinussen, C.; Jørgensen, N.B.; Torekov, S.S.; Meier, F.; Niu, L.; et al. Plasma Proteome Profiling Reveals Dynamics of Inflammatory and Lipid Homeostasis Markers after Roux-En-Y Gastric Bypass Surgery. Cell Syst. 2018, 7, 601–612.e603. [Google Scholar] [CrossRef]
- Becker, S.; Kortz, L.; Helmschrodt, C.; Thiery, J.; Ceglarek, U. LC–MS-based metabolomics in the clinical laboratory. J. Chromatogr. B Biomed. Sci. Appl. 2012, 883–884, 68–75. [Google Scholar] [CrossRef]
- Rakusanova, S.; Cajka, T. Tips and tricks for LC–MS-based metabolomics and lipidomics analysis. TrAC Trends Anal. Chem. 2024, 180, 117940. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J. Current medical research with the application of coupled techniques with mass spectrometry. Med. Sci. Monit. 2011, 17, RA117–RA123. [Google Scholar] [CrossRef]
- Metz, T.O.; Zhang, Q.; Page, J.S.; Shen, Y.; Callister, S.J.; Jacobs, J.M.; Smith, R.D. The future of liquid chromatography-mass spectrometry (LC-MS) in metabolic profiling and metabolomic studies for biomarker discovery. Biomark. Med. 2007, 1, 159–185. [Google Scholar] [CrossRef] [PubMed]
- van der Gugten, J.G. Tandem mass spectrometry in the clinical laboratory: A tutorial overview. Clin. Mass Spectrom. 2020, 15, 36–43. [Google Scholar] [CrossRef]
- Rekhi, H.; Rani, S.; Sharma, N.; Malik, A.K. A Review on Recent Applications of High-Performance Liquid Chromatography in Metal Determination and Speciation Analysis. Crit. Rev. Anal. Chem. 2017, 47, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, Z.; Peterka, O.; Jirásko, R.; Holčapek, M. Reversed-Phase Ultrahigh-Performance Liquid Chromatography-Mass Spectrometry Method for High-Throughput Lipidomic Quantitation. Methods Mol. Biol. 2025, 2855, 185–194. [Google Scholar]
- Drouin, N.; Ramautar, R. Capillary Electrophoresis-Mass Spectrometry for Metabolomics: Possibilities and Perspectives. Adv. Exp. Med. Biol. 2021, 1336, 159–178. [Google Scholar]
- Delvaux, A.; Rathahao-Paris, E.; Alves, S. Different ion mobility-mass spectrometry coupling techniques to promote metabolomics. Mass Spectrom. Rev. 2022, 41, 695–721. [Google Scholar] [CrossRef]
- Ovbude, S.T.; Sharmeen, S.; Kyei, I.; Olupathage, H.; Jones, J.; Bell, R.J.; Powers, R.; Hage, D.S. Applications of chromatographic methods in metabolomics: A review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2024, 1239, 124124. [Google Scholar] [CrossRef]
- Kruk, J.; Doskocz, M.; Jodłowska, E.; Zacharzewska, A.; Łakomiec, J.; Czaja, K.; Kujawski, J. NMR Techniques in Metabolomic Studies: A Quick Overview on Examples of Utilization. Appl. Magn. Reson. 2017, 48, 1–21. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Balonov, I.; Kurlbaum, M.; Koschker, A.-C.; Stier, C.; Fassnacht, M.; Dischinger, U. Changes in Plasma Metabolomic Profile Following Bariatric Surgery, Lifestyle Intervention or Diet Restriction—Insights from Human and Rat Studies. Int. J. Mol. Sci. 2023, 24, 2354. [Google Scholar] [CrossRef]
- Gertsman, I.; Barshop, B.A. Promises and pitfalls of untargeted metabolomics. J. Inherit. Metab. 2018, 41, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Feng, Y.-Q. Mass spectrometry-based metabolomics for clinical study: Recent progresses and applications. TrAC Trends Anal. Chem. 2023, 158, 116896. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef]
- Han, W.; Li, L. Evaluating and minimizing batch effects in metabolomics. Mass Spectrom. Rev. 2022, 41, 421–442. [Google Scholar] [CrossRef]
- Hebels, D.G.A.J.; Georgiadis, P.; Keun, H.C.; Athersuch, T.J.; Vineis, P.; Vermeulen, R.; Portengen, L.; Bergdahl, I.A.; Hallmans, G.; Palli, D.; et al. Performance in Omics Analyses of Blood Samples in Long-Term Storage: Opportunities for the Exploitation of Existing Biobanks in Environmental Health Research. Environ. Health Perspect. 2013, 121, 480–487. [Google Scholar] [CrossRef]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef]
- Medina, J.; van der Velpen, V.; Teav, T.; Guitton, Y.; Gallart-Ayala, H.; Ivanisevic, J. Single-Step Extraction Coupled with Targeted HILIC-MS/MS Approach for Comprehensive Analysis of Human Plasma Lipidome and Polar Metabolome. Metabolites 2020, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Martin, S.A.; Mitsche, M.A.; Thompson, B.M.; Eckert, K.M.; McDonald, J.G. Three-phase liquid extraction: A simple and fast method for lipidomic workflows. J. Lipid Res. 2019, 60, 694–706. [Google Scholar] [CrossRef]
- Čuklina, J.; Lee, C.H.; Williams, E.G.; Sajic, T.; Collins, B.C.; Rodríguez Martínez, M.; Sharma, V.S.; Wendt, F.; Goetze, S.; Keele, G.R.; et al. Diagnostics and correction of batch effects in large-scale proteomic studies: A tutorial. Mol. Syst. Biol. 2021, 17, e10240. [Google Scholar] [CrossRef]
- Defossez, E.; Bourquin, J.; von Reuss, S.; Rasmann, S.; Glauser, G. Eight key rules for successful data-dependent acquisition in mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2023, 42, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. New software tools, databases, and resources in metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Hohrenk, L.L.; Itzel, F.; Baetz, N.; Tuerk, J.; Vosough, M.; Schmidt, T.C. Comparison of Software Tools for Liquid Chromatography–High-Resolution Mass Spectrometry Data Processing in Nontarget Screening of Environmental Samples. Anal. Chem. 2020, 92, 1898–1907. [Google Scholar] [CrossRef]
- Krettler, C.A.; Thallinger, G.G. A map of mass spectrometry-based in silico fragmentation prediction and compound identification in metabolomics. Brief Bioinform. 2021, 22, bbab073. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.R.; Rezaei, M.; Abdollahi, A.; Gholi, Z.; Mokhber, S.; Mohammadi-Farsani, G.; Abdoli, D.; Mousavi, S.D.; Amini, H.; Ghandchi, M. Long-term systemic effects of metabolic bariatric surgery: A multidisciplinary perspective. Heliyon 2024, 10, e34339. [Google Scholar] [CrossRef]
- Santamaria, G.; Pinto, F.R. Bioinformatic Analysis of Metabolomic Data: From Raw Spectra to Biological Insight. BioChem 2024, 4, 90–114. [Google Scholar] [CrossRef]
- Ramadan, Z.; Jacobs, D.; Grigorov, M.; Kochhar, S. Metabolic Profiling Using Principal Component Analysis, Discriminant Partial Least Squares, and Genetic Algorithms. Talanta 2006, 68, 1683–1691. [Google Scholar] [CrossRef]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Ha, J.; Kwon, Y.; Park, S. Metabolomics in Bariatric Surgery: Towards Identification of Mechanisms and Biomarkers of Metabolic Outcomes. Obes. Surg. 2021, 31, 4564–4574. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Michaliszyn, S.F.; de las Heras, J.; Bacha, F.; Lee, S.; Chace, D.H.; DeJesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: Evidence for enhanced mitochondrial oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diabetes Rep. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Qi, Q.; Hruby, A.; Manson, J.E.; Willett, W.C.; Wolpin, B.M.; Hu, F.B.; Qi, L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int. J. Epidemiol. 2016, 45, 1482–1492. [Google Scholar] [CrossRef]
- Tan, H.C.; Hsu, J.W.; Kovalik, J.P.; Eng, A.; Chan, W.H.; Khoo, C.M.; Tai, E.S.; Chacko, S.; Jahoor, F. Branched-Chain Amino Acid Oxidation Is Elevated in Adults with Morbid Obesity and Decreases Significantly after Sleeve Gastrectomy. J. Nutr. 2020, 150, 3180–3189. [Google Scholar] [CrossRef]
- Bozadjieva Kramer, N.; Evers, S.S.; Shin, J.H.; Silverwood, S.; Wang, Y.; Burant, C.F.; Sandoval, D.A.; Seeley, R.J. The Role of Elevated Branched-Chain Amino Acids in the Effects of Vertical Sleeve Gastrectomy to Reduce Weight and Improve Glucose Regulation. Cell Rep. 2020, 33, 108239. [Google Scholar] [CrossRef]
- Shah, H.; Kramer, A.; Mullins, C.A.; Mattern, M.; Gannaban, R.B.; Townsend, R.L.; Campagna, S.R.; Morrison, C.D.; Berthoud, H.-R.; Shin, A.C. Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21. Nutrients 2023, 15, 1713. [Google Scholar] [CrossRef]
- Barati-Boldaji, R.; Esmaeilinezhad, Z.; Babajafari, S.; Kazemi, A.; Clark, C.C.T.; Mazidi, M.; Ofori-Asenso, R.; Haghighat, N.; Shafiee, M.; Mazloomi, S.M. Bariatric surgery reduces branched-chain amino acids’ levels: A systematic review and meta-analysis. Nutr. Res. 2021, 87, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.I.; Geloneze, B.; Pareja, J.C.; Calixto, A.R.; Ferreira, M.M.; Marsaioli, A.J. Blood Metabolome Changes Before and After Bariatric Surgery: A (1)H NMR-Based Clinical Investigation. Omics 2015, 19, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.I.B.; Geloneze, B.; Pareja, J.C.; Calixto, A.R.; Ferreira, M.M.C.; Marsaioli, A.J. “Omics” Prospective Monitoring of Bariatric Surgery: Roux-En-Y Gastric Bypass Outcomes Using Mixed-Meal Tolerance Test and Time-Resolved 1H NMR-Based Metabolomics. OMICS 2016, 20, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mutch, D.M.; Fuhrmann, J.C.; Rein, D.; Wiemer, J.C.; Bouillot, J.L.; Poitou, C.; Clément, K. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS ONE 2009, 4, e7905. [Google Scholar] [CrossRef]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Metabolomic Analysis of the Improvements in Insulin Secretion and Resistance After Sleeve Gastrectomy: Implications of the Novel Biomarkers. Obes. Surg. 2021, 31, 43–52. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Swierczynski, J.; Sledzinski, T.; Slominska, E.; Smolenski, R.; Sledzinski, Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes. Surg. 2009, 19, 883–889. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Verbanck, M.; Pigeyre, M.; Caiazzo, R.; Raverdy, V.; Verkindt, H.; Leloire, A.; Guillemin, G.J.; Yengo, L.; et al. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS ONE 2016, 11, e0158051. [Google Scholar] [CrossRef]
- Yeung, K.T.D.; Penney, N.; Whiley, L.; Ashrafian, H.; Lewis, M.R.; Purkayastha, S.; Darzi, A.; Holmes, E. The impact of bariatric surgery on serum tryptophan–kynurenine pathway metabolites. Sci. Rep. 2022, 12, 294. [Google Scholar] [CrossRef]
- Bernard, A.; Le May, C.; Dastugue, A.; Ayer, A.; Blanchard, C.; Martin, J.C.; Pais de Barros, J.P.; Delaby, P.; Le Bourgot, C.; Ledoux, S.; et al. The Tryptophan/Kynurenine Pathway: A Novel Cross-Talk between Nutritional Obesity, Bariatric Surgery and Taste of Fat. Nutrients 2021, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef]
- Chaudhry, S.; Bernardes, M.; Harris, P.E.; Maffei, A. Gastrointestinal dopamine as an anti-incretin and its possible role in bypass surgery as therapy for type 2 diabetes with associated obesity. Minerva Endocrinol. 2016, 41, 43–56. [Google Scholar] [PubMed]
- Korner, J.; Cline, G.W.; Slifstein, M.; Barba, P.; Rayat, G.R.; Febres, G.; Leibel, R.L.; Maffei, A.; Harris, P.E. A role for foregut tyrosine metabolism in glucose tolerance. Mol. Metab. 2019, 23, 37–50. [Google Scholar] [CrossRef]
- Ha, J.; Jang, M.; Kwon, Y.; Park, Y.S.; Park, D.J.; Lee, J.H.; Lee, H.J.; Ha, T.K.; Kim, Y.J.; Han, S.M.; et al. Metabolomic Profiles Predict Diabetes Remission after Bariatric Surgery. J. Clin. Med. 2020, 9, 3897. [Google Scholar] [CrossRef]
- Almheiri, R.T.; Hajjar, B.; Alkhaaldi, S.M.I.; Rabeh, N.; Aljoudi, S.; Abd-Elrahman, K.S.; Hamdan, H. Beyond weight loss: Exploring the neurological ramifications of altered gut microbiota post-bariatric surgery. J. Trans. Med. 2025, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef]
- Hamilton, J.; Swenson, S.; Hajnal, A.; Thanos, P.K. Roux-en-Y gastric bypass surgery normalizes dopamine D1, D2, and DAT levels. Synapse 2018. online ahead of print. [Google Scholar] [CrossRef]
- Tan, H.C.; Hsu, J.W.; Tai, E.S.; Chacko, S.; Wu, V.; Lee, C.F.; Kovalik, J.P.; Jahoor, F. De Novo Glycine Synthesis Is Reduced in Adults With Morbid Obesity and Increases Following Bariatric Surgery. Front. Endocrinol. 2022, 13, 900343. [Google Scholar] [CrossRef]
- Tan, H.C.; Hsu, J.W.; Tai, E.S.; Chacko, S.; Kovalik, J.P.; Jahoor, F. The Impact of Bariatric Surgery on Glutathione Synthesis in Individuals with Severe Obesity. Antioxidants 2024, 13, 967. [Google Scholar] [CrossRef]
- Li, Q.-R.; Wang, Z.-M.; Wewer Albrechtsen, N.J.; Wang, D.-D.; Su, Z.-D.; Gao, X.-F.; Wu, Q.-Q.; Zhang, H.-P.; Zhu, L.; Li, R.-X.; et al. Systems Signatures Reveal Unique Remission-path of Type 2 Diabetes Following Roux-en-Y Gastric Bypass Surgery. EBioMedicine 2018, 28, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.C.; Hsu, J.W.; Tai, E.S.; Chacko, S.; Kovalik, J.P.; Jahoor, F. The impact of obesity-associated glycine deficiency on the elimination of endogenous and exogenous metabolites via the glycine conjugation pathway. Front. Endocrinol. 2024, 15, 1343738. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, F.; Czernichow, S.; Carette, C.; Levy, R.; Ravaud, P.; Cynober, L.; Neveux, N.; Rives-Lange, C.; Eustache, F.; Coupaye, M.; et al. Behaviour of plasma citrulline after bariatric surgery in the BARIASPERM cohort. Clin. Nutr. 2021, 40, 505–510. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Molina, B.; Queipo-Ortuño, M.I.; Lambertos, A.; Tinahones, F.J.; Peñafiel, R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front. Nutr. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Wilhelmi, L.; Cardona, F.; Garrido-Sanchez, L.; Fernandez-Garcia, D.; Tinahones, F.J.; Ramos-Molina, B. Change in serum polyamine metabolome pattern after bariatric surgery in obese patients with metabolic syndrome. Surg. Obes. Relat. Dis. 2020, 16, 306–311. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Kokkinos, A.; Sanoudou, D.; Connelly, M.A.; Alexandrou, A.; Mingrone, G.; Mantzoros, C.S. Early metabolomic, lipid and lipoprotein changes in response to medical and surgical therapeutic approaches to obesity. Metabolism 2023, 138, 155346. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 2011, 3, 80re82. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.M.; Muehlbauer, M.J.; Stevens, R.D.; Pamuklar, Z.; Chen, J.; Newgard, C.B.; Torquati, A. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann. Surg. 2014, 259, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Molina, B.; Castellano-Castillo, D.; Alcaide-Torres, J.; Pastor, Ó.; de Luna Díaz, R.; Salas-Salvadó, J.; López-Moreno, J.; Fernández-García, J.C.; Macías-González, M.; Cardona, F.; et al. Differential effects of restrictive and malabsorptive bariatric surgery procedures on the serum lipidome in obese subjects. J. Clin. Lipidol. 2018, 12, 1502–1512. [Google Scholar] [CrossRef]
- Benaiges, D.; Flores-Le-Roux, J.A.; Pedro-Botet, J.; Ramon, J.M.; Parri, A.; Villatoro, M.; Carrera, M.J.; Pera, M.; Sagarra, E.; Grande, L.; et al. Impact of restrictive (sleeve gastrectomy) vs hybrid bariatric surgery (Roux-en-Y gastric bypass) on lipid profile. Obes. Surg. 2012, 22, 1268–1275. [Google Scholar] [CrossRef]
- Benetti, A.; Del Puppo, M.; Crosignani, A.; Veronelli, A.; Masci, E.; Frigè, F.; Micheletto, G.; Panizzo, V.; Pontiroli, A.E. Cholesterol metabolism after bariatric surgery in grade 3 obesity: Differences between malabsorptive and restrictive procedures. Diabetes Care 2013, 36, 1443–1447. [Google Scholar] [CrossRef]
- Wijayatunga, N.N.; Sams, V.G.; Dawson, J.A.; Mancini, M.L.; Mancini, G.J.; Moustaid-Moussa, N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab. Res. Rev. 2018, 34, e3045. [Google Scholar] [CrossRef]
- Fiamoncini, J.; Fernandes Barbosa, C.; Arnoni Junior, J.R.; Araújo Junior, J.C.; Taglieri, C.; Szego, T.; Gelhaus, B.; Possolo de Souza, H.; Daniel, H.; Martins de Lima, T. Roux-en-Y Gastric Bypass Surgery Induces Distinct but Frequently Transient Effects on Acylcarnitine, Bile Acid and Phospholipid Levels. Metabolites 2018, 8, 83. [Google Scholar] [CrossRef]
- Meikle, P.J.; Summers, S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Samczuk, P.; Luba, M.; Godzien, J.; Mastrangelo, A.; Hady, H.R.; Dadan, J.; Barbas, C.; Gorska, M.; Kretowski, A.; Ciborowski, M. “Gear mechanism” of bariatric interventions revealed by untargeted metabolomics. J. Pharm. Biomed. Anal. 2018, 151, 219–226. [Google Scholar] [CrossRef]
- Oberbach, A.; von Bergen, M.; Blüher, S.; Lehmann, S.; Till, H. Combined serum proteomic and metabonomic profiling after laparoscopic sleeve gastrectomy in children and adolescents. J. Laparoendosc. Adv. Surg. Tech. A 2012, 22, 184–188. [Google Scholar] [CrossRef]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kasumov, T.; Gatmaitan, P.; Heneghan, H.M.; Kashyap, S.R.; Schauer, P.R.; Brethauer, S.A.; Kirwan, J.P. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity 2011, 19, 2235–2240. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Huang, H.; Kashyap, S.R.; Gornik, H.L.; McCullough, A.J.; Schauer, P.R.; Brethauer, S.A.; Kirwan, J.P.; Kasumov, T. Reduced cardiovascular risk after bariatric surgery is linked to plasma ceramides, apolipoprotein-B100, and ApoB100/A1 ratio. Surg. Obes. Relat. Dis. 2013, 9, 100–107. [Google Scholar] [CrossRef]
- Vice, E.; Privette, J.D.; Hickner, R.C.; Barakat, H.A. Ketone body metabolism in lean and obese women. Metabolism 2005, 54, 1542–1545. [Google Scholar] [CrossRef]
- Penney, N.C.; Yeung, D.K.T.; Garcia-Perez, I.; Posma, J.M.; Kopytek, A.; Garratt, B.; Ashrafian, H.; Frost, G.; Marchesi, J.R.; Purkayastha, S.; et al. Multi-omic phenotyping reveals host-microbe responses to bariatric surgery, glycaemic control and obesity. Commun. Med. 2022, 2, 127. [Google Scholar] [CrossRef]
- Haran, A.; Bergel, M.; Kleiman, D.; Hefetz, L.; Israeli, H.; Weksler-Zangen, S.; Agranovich, B.; Abramovich, I.; Ben-Haroush Schyr, R.; Gottlieb, E.; et al. Differential effects of bariatric surgery and caloric restriction on hepatic one-carbon and fatty acid metabolism. iScience 2023, 26, 107046. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Wang, Y.; Dai, Y.; Zhang, X.; Hu, S. Role of Bile Acids in Bariatric Surgery. Front. Physiol. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, D.J.; Glicksman, C.; Vincent, R.P.; Kuganolipava, S.; Alaghband-Zadeh, J.; Mahon, D.; Bekker, J.H.; Ghatei, M.A.; Bloom, S.R.; Walters, J.R.; et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012, 153, 3613–3619. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Blüher, M.; Wirth, H.; Till, H.; Kovacs, P.; Kullnick, Y.; Schlichting, N.; Tomm, J.M.; Rolle-Kampczyk, U.; Murugaiyan, J.; et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J. Proteome Res. 2011, 10, 4769–4788. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Risstad, H.; Kristinsson, J.A.; Fagerland, M.W.; le Roux, C.W.; Birkeland, K.I.; Gulseth, H.L.; Thorsby, P.M.; Vincent, R.P.; Engström, M.; Olbers, T.; et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: Results from a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, V.; Lalloyer, F.; Baud, G.; Osto, E.; Kouach, M.; Daoudi, M.; Vallez, E.; Raverdy, V.; Goossens, J.F.; Descat, A.; et al. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: A comparative study between rats, pigs and humans. Int. J. Obes. 2016, 40, 1260–1267. [Google Scholar] [CrossRef]
- Patti, M.E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Maratos-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef]
- Bozadjieva, N.; Heppner, K.M.; Seeley, R.J. Targeting FXR and FGF19 to Treat Metabolic Diseases-Lessons Learned From Bariatric Surgery. Diabetes 2018, 67, 1720–1728. [Google Scholar] [CrossRef]
- Chaudhari, S.N.; Harris, D.A.; Aliakbarian, H.; Luo, J.N.; Henke, M.T.; Subramaniam, R.; Vernon, A.H.; Tavakkoli, A.; Sheu, E.G.; Devlin, A.S. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol. 2021, 17, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Aydin, Ö.; Olsson, L.M.; Sjöland, W.; Henricsson, M.; Lundqvist, A.; Marschall, H.-U.; Franken, R.; van de Laar, A.; Gerdes, V.; et al. Alterations in bile acid kinetics after bariatric surgery in patients with obesity with or without type 2 diabetes. eBioMedicine 2024, 106, 105265. [Google Scholar] [CrossRef]
- So, S.S.Y.; Yeung, C.H.C.; Schooling, C.M.; El-Nezami, H. Targeting bile acid metabolism in obesity reduction: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13017. [Google Scholar] [CrossRef]
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2008, 51, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Mukhopadhyay, P.; Haskó, G.; Pacher, P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012, 180, 432–442. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Engeli, S.; Klöting, N.; Berndt, J.; Fasshauer, M.; Bátkai, S.; Pacher, P.; Schön, M.R.; Jordan, J.; Stumvoll, M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006, 55, 3053–3060. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Sipe, J.C.; Scott, T.M.; Murray, S.; Harismendy, O.; Simon, G.M.; Cravatt, B.F.; Waalen, J. Biomarkers of endocannabinoid system activation in severe obesity. PLoS ONE 2010, 5, e8792. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Hayashi, M.; Sameshima, M.; Amitani, H.; Kojima, S.; Fujimiya, M.; Inui, A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol. Psychiatry 2009, 65, 748–759. [Google Scholar] [CrossRef]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, A.; Montecucco, F.; Pataky, Z.; Thomas, A.; Ambrosio, G.; Staub, C.; Di Marzo, V.; Ratib, O.; Mach, F.; Golay, A.; et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: Relation to alterations in endocannabinoids and adipocytokines. Eur. Heart J. 2013, 34, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, L.; Kantonen, T.; Oikonen, V.; Haaparanta-Solin, M.; Aarnio, R.; Dickens, A.M.; von Eyken, A.; Latva-Rasku, A.; Dadson, P.; Kirjavainen, A.K.; et al. Lower abdominal adipose tissue cannabinoid type 1 receptor availability in young men with overweight. Obesity 2023, 31, 1844–1858. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Trøseid, M.; Hov, J.R.; Nestvold, T.K.; Thoresen, H.; Berge, R.K.; Svardal, A.; Lappegård, K.T. Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 197–201. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Chen, Y.; Lian, G.; Wang, J.; Zhang, J.; Shan, K.; Shang, L.; Tian, F.; Jing, C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes. Surg. 2021, 31, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Caparrós-Martín, J.A.; Lee, S.; O’Gara, F.; Yeap, B.B.; Green, D.J.; Ballal, M.; Ward, N.C.; Dwivedi, G. Gut Microbiome and Associated Metabolites Following Bariatric Surgery and Comparison to Healthy Controls. Microorganisms 2023, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Hernandez, M.M.; Vital, M.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Circulating levels of the anti-oxidant indoleproprionic acid are associated with higher gut microbiome diversity. Gut Microbes 2019, 10, 688–695. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Hallowell, P.T.; Slack-Davis, J.K.; Michalek, R.D.; Atkins, K.A.; Kelley, S.L.; Arapovic, S.; Shupnik, M.A.; Hoehn, K. Women at extreme risk for obesity-related carcinogenesis: Baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol. Oncol. 2015, 138, 238–245. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Shakhshir, M.; Barqawi, A.; Abushanab, A.S.; Koni, A.; Khilfeh, S.; Shahwan, M.; Jairoun, A.A.; Taha, A.A.; Abushamma, F.; et al. Comprehensive visualization of bariatric surgery and gut microbiota research: A global analysis. Transl. Med. Commun. 2024, 9, 13. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Garrido-Sánchez, L.; Alcaide-Torres, J.; Cornejo-Pareja, I.; Ocaña-Wilhelmi, L.; García-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Predictive Role of Gut Microbiota in Weight Loss Achievement after Bariatric Surgery. J. Am. Coll. Surg. 2022, 234, 861–871. [Google Scholar] [CrossRef]
- Mika, A.; Janczy, A.; Waleron, K.; Szymanski, M.; Kaska, L.; Sledzinski, T. The impact of the interplay of the intestinal microbiome and diet on the metabolomic and health outcomes of bariatric surgery. Obes. Rev. 2022, 23, e13455. [Google Scholar] [CrossRef]
- Luijten, J.; Vugts, G.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. The Importance of the Microbiome in Bariatric Surgery: A Systematic Review. Obes. Surg. 2019, 29, 2338–2349. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; Tinahones, F.J. Shifts in gut microbiota and their metabolites induced by bariatric surgery. Impact of factors shaping gut microbiota on bariatric surgery outcomes. Rev. Endocr. Metab. Disord. 2021, 22, 1137–1156. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Pereira, S.S.; Monteiro, M.P. Metabolomic signatures after bariatric surgery—A systematic review. Rev. Endocr. Metab. Disord. 2022, 23, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef]
- Jarak, I.; Pereira, S.S.; Carvalho, R.A.; Oliveira, P.F.; Alves, M.G.; Guimarães, M.; Wewer Albrechtsen, N.J.; Holst, J.J.; Nora, M.; Monteiro, M.P. Gastric Bypass with Different Biliopancreatic Limb Lengths Results in Similar Post-absorptive Metabolomics Profiles. Obes. Surg. 2020, 30, 1068–1078. [Google Scholar] [CrossRef]
- Brolin, R.E.; Bradley, L.J.; Wilson, A.C.; Cody, R.P. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J. Gastrointest. Surg. 2000, 4, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, C.; Vandermeulen, G.; Luypaerts, A.; Vermeulen, D.; Lannoo, M.; Van der Schueren, B.; Buyse, J.; Verbeke, K. The impact of bariatric surgery on macronutrient malabsorption depends on the type of procedure. Front. Nutr. 2023, 9, 1028881. [Google Scholar] [CrossRef]
- Pakiet, A.; Haliński, Ł.P.; Rostkowska, O.; Kaska, Ł.; Proczko-Stepaniak, M.; Śledziński, T.; Mika, A. The Effects of One-Anastomosis Gastric Bypass on Fatty Acids in the Serum of Patients with Morbid Obesity. Obes. Surg. 2021, 31, 4264–4271. [Google Scholar] [CrossRef]
- Pereira, S.S.; Jarak, I.; Carvalho, R.A.; Oliveira, P.F.; Alves, M.G.; Guimaraes, M.; Almeida, R.; Pereira, A.M.; Wewer Albrechtsen, N.J.; Holst, J.J.; et al. Different Malabsorptive Obesity Surgery Interventions Result in Distinct Postprandial Amino Acid Metabolomic Signatures. Obes. Surg. 2020, 30, 4019–4028. [Google Scholar] [CrossRef]
- Lauti, M.; Kularatna, M.; Hill, A.G.; MacCormick, A.D. Weight Regain Following Sleeve Gastrectomy-a Systematic Review. Obes. Surg. 2016, 26, 1326–1334. [Google Scholar] [CrossRef]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; de Gara, C.; Birch, D.W. Weight recidivism post-bariatric surgery: A systematic review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Amino Acid Metabolites and Slow Weight Loss in the Early Postoperative Period after Sleeve Gastrectomy. J. Clin. Med. 2020, 9, 2348. [Google Scholar] [CrossRef]
- Abidi, W.; Nestoridi, E.; Feldman, H.; Stefater, M.; Clish, C.; Thompson, C.C.; Stylopoulos, N. Differential Metabolomic Signatures in Patients with Weight Regain and Sustained Weight Loss After Gastric Bypass Surgery: A Pilot Study. Dig. Dis. Sci. 2020, 65, 1144–1154. [Google Scholar] [CrossRef]
- Hanvold, S.E.; Vinknes, K.J.; Bastani, N.E.; Turner, C.; Løken, E.B.; Mala, T.; Refsum, H.; Aas, A.M. Plasma amino acids, adiposity, and weight change after gastric bypass surgery: Are amino acids associated with weight regain? Eur. J. Nutr. 2018, 57, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Turkistani, Y. Glucagon-like peptide-1 receptor agonists: A review from a cardiovascular perspective. Front. Cardiovasc. Med. 2025, 12, 1535134. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.J.; Goodwin Cartwright, B.M.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Semaglutide vs Tirzepatide for Weight Loss in Adults With Overweight or Obesity. JAMA Intern. Med. 2024, 184, 1056–1064. [Google Scholar] [CrossRef]
- Sarma, S.; Palcu, P. Weight loss between glucagon-like peptide-1 receptor agonists and bariatric surgery in adults with obesity: A systematic review and meta-analysis. Obesity 2022, 30, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Saeed, L.; Sharif, G.; Eda, S.; Raju Tullimalli, I.; Amin, A.; Riyalat, A.A.; Alrashid, F.F.; Abdelrahim, A.A. Comparative Effectiveness of Bariatric Metabolic Surgery Versus Glucagon-Like Peptide-1 Receptor Agonists on Cardiovascular Outcomes and Mortality: A Meta-Analysis. Cureus 2024, 16, e71684. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chan, S.E.; Hsu, W.H.; Kuo, C.C.; Tsai, Y.W.; Liu, T.H.; Huang, P.Y.; Chuang, M.H.; Yu, T.; Lai, C.C. Comparing clinical outcomes of adults with obesity receiving tirzepatide versus bariatric metabolic surgery: A multi-institutional propensity score-matched study. Diabetes Obes. Metab. 2025, 27, 3357–3366. [Google Scholar] [CrossRef]
- Pratama, K.G.; Nugroho, H.; Hengky, A.; Tandry, M.; Pauliana, P. Glucagon-like peptide-1 receptor agonists for post-bariatric surgery weight regain and insufficient weight loss: A systematic review. Obes. Med. 2024, 46, 100533. [Google Scholar] [CrossRef]
- Jones, B.; Sands, C.; Alexiadou, K.; Minnion, J.; Tharakan, G.; Behary, P.; Ahmed, A.R.; Purkayastha, S.; Lewis, M.R.; Bloom, S.; et al. The Metabolomic Effects of Tripeptide Gut Hormone Infusion Compared to Roux-en-Y Gastric Bypass and Caloric Restriction. J. Clin. Endocrinol. Metab. 2021, 107, e767–e782. [Google Scholar] [CrossRef]
- Anastasiou, I.; Argyrakopoulou, G.; Dalamaga, M.; Kokkinos, A. Dual and Triple Gut Peptide Agonists on the Horizon for the Treatment of Type 2 Diabetes and Obesity. An Overview of Preclinical and Clinical Data. Curr. Obes. Rep. 2025, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, C.L.; Hari, A.; Dantas, W.S.; Kashyap, S.R.; Schauer, P.R.; Kirwan, J.P. Metabolomic Fingerprints of Medical Therapy Versus Bariatric Surgery in Patients With Obesity and Type 2 Diabetes: The STAMPEDE Trial. Diabetes Care 2024, 47, 2024–2032. [Google Scholar] [CrossRef]
- Jendoubi, T. Approaches to Integrating Metabolomics and Multi-Omics Data: A Primer. Metabolites 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.A.; Rosati, G.; Moguet, C.; Fuentes, C.; Marrugo-Ramírez, J.; Lefebvre, T.; Volland, H.; Merkoçi, A.; Simon, S.; Fenaille, F.; et al. Metabolomics for personalized medicine: The input of analytical chemistry from biomarker discovery to point-of-care tests. Anal. Bioanal. Chem. 2022, 414, 759–789. [Google Scholar] [CrossRef]
- Dalamaga, M. Clinical metabolomics: Useful insights, perspectives and challenges. Metabol. Open 2024, 22, 100290. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, F.; Al-Absi, H.R.H.; Yousri, N.A.; El Hajj, N.; Shah, Z. A scoping review of artificial intelligence-based methods for diabetes risk prediction. Npj Digit. Med. 2023, 6, 197. [Google Scholar] [CrossRef]
- Yagin, F.H.; Al-Hashem, F.; Ahmad, I.; Ahmad, F.; Alkhateeb, A. Pilot-Study to Explore Metabolic Signature of Type 2 Diabetes: A Pipeline of Tree-Based Machine Learning and Bioinformatics Techniques for Biomarkers Discovery. Nutrients 2024, 16, 1537. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef]
- Ahotupa, M. Lipid Oxidation Products and the Risk of Cardiovascular Diseases: Role of Lipoprotein Transport. Antioxidants 2024, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, W.; Zhang, Y.; Dong, X.; Liu, Y. Diverse Physiological Roles of Kynurenine Pathway Metabolites: Updated Implications for Health and Disease. Metabolites 2025, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, Z.; Ye, J.; Lv, Y.; Zhang, C.; Liang, T.; Wang, Y. Emerging roles of exosomes in the diagnosis and treatment of kidney diseases. Front. Pharmacol. 2025, 16, 1525314. [Google Scholar] [CrossRef]
- Saka, O.M.; Dora, D.D.; Kibar, G.; Tevlek, A. Expanding the role of exosomes in drug, biomolecule, and nanoparticle delivery. Life Sci. 2025, 368, 123499. [Google Scholar] [CrossRef]
| Technique | Advantages | Disadvantages |
|---|---|---|
| Nuclear Magnetic Resonance (NMR) Spectroscopy [48,49] | - High reproducibility - Simple and minimal sample preparation - Broad metabolite coverage (both polar and non-polar) - Straightforward metabolite identification | - Low sensitivity - Limited resolution |
| Gas Chromatography–Mass Spectrometry (GC-MS) [41,49] | - Excellent sensitivity - Superior resolution compared to NMR - Reliable metabolite identification using spectral libraries - Detects volatile compounds (both polar and non-polar) | - Requires extensive sample preparation - Lower reproducibility compared to NMR |
| Capillary Electrophoresis–Mass Spectrometry (CE-MS) [45,49] | - Higher resolution than NMR - Good sensitivity - Suitable for polar metabolites | - Medium level of sample preparation needed - Less reproducible than NMR - Difficult metabolite identification |
| High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS) [44,49] | - High sensitivity - Improved resolution compared to NMR - Can separate both polar and non-polar metabolites depending on column type | - Requires moderate sample preparation - Lower reproducibility than NMR - Challenging metabolite identification due to incomplete databases |
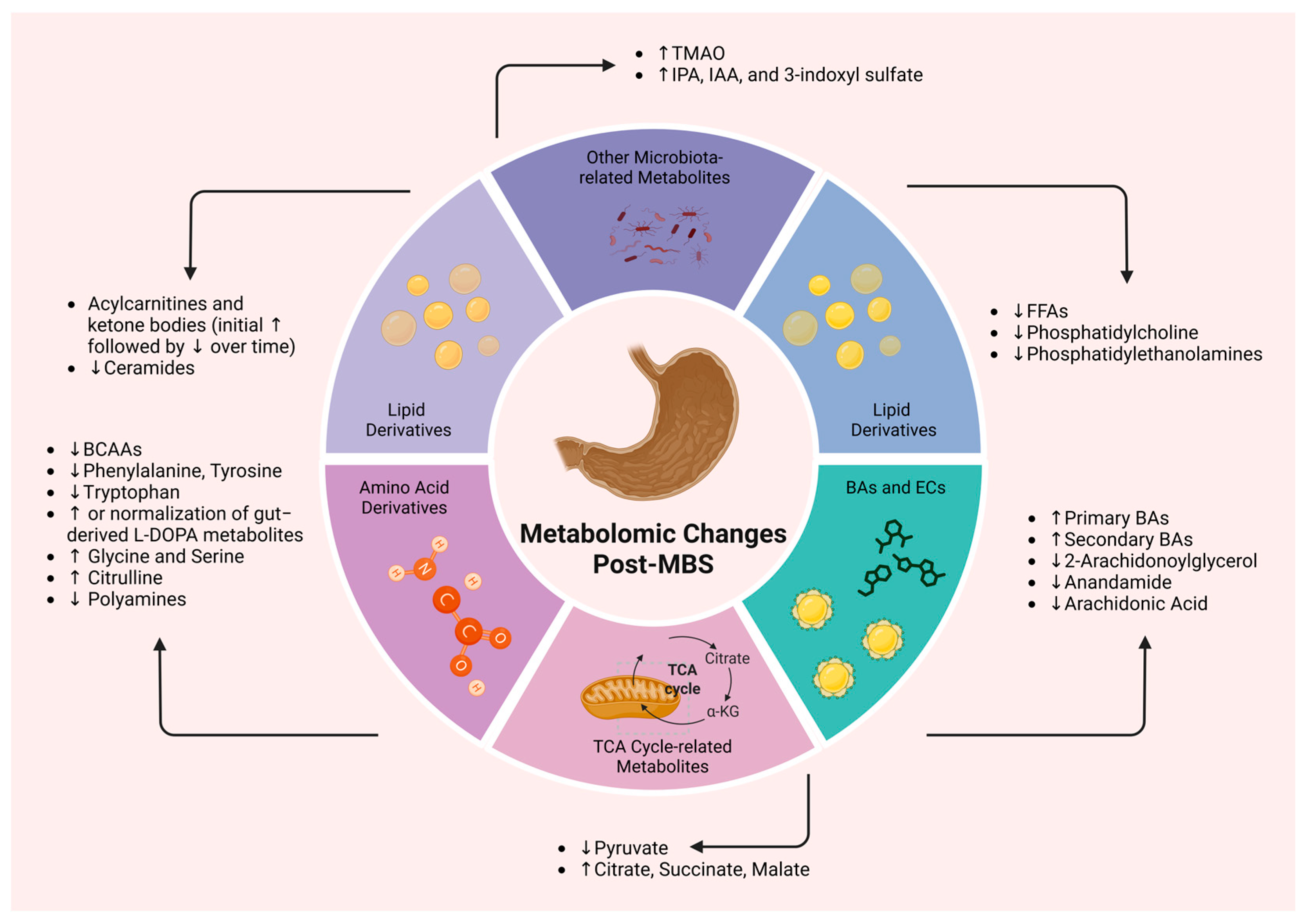
| Amino Acids | Post-MBS Change | Impact and Outcomes |
|---|---|---|
| Branched-Chain Amino Acids [73,74,75,76,77,78,79,80] | ↓ | Reduction correlates with improved mitochondrial function, insulin sensitivity, and metabolic health; dietary intake linked to metabolic disorders |
| Phenylalanine and Tyrosine [70,84,85,86,87,88] | ↓ | Associated with improved hepatic function, reduced inflammation, and better glucose regulation; early biomarkers of metabolic decline |
| Tryptophan Pathway Metabolites [89,90,91,92] | ↓ | Leads to reduced systemic inflammation and improved insulin sensitivity, aiding metabolic recovery |
| Dopamine Precursors (L-DOPA) [93,94,95,96] | ↑ or normalized | Restores receptor levels, potentially improving reward processing and glucose regulation, contributing to T2D remission |
| Glycine and Serine [104] | ↑ | Enhances antioxidant capacity, reduces oxidative stress, and alleviates IR |
| Citrulline [107] | ↑ | Indicates improved intestinal function, with positive effects on metabolic health |
| Category | Post-MBS Changes | Impact and Outcomes |
|---|---|---|
| Acylcarnitines and Fatty Acid Oxidation [112,113,114,115,116,117,118,119,120] | - ↑ acylcarnitine profiles - ↑ postprandial acylcarnitine response - ↑ substrate utilization - ↓ in acylcarnitine levels over time | - ↑ mitochondrial flexibility and function - ↑ fatty acid oxidation - ↓ metabolic stress - ↑ glycemic control and insulin sensitivity |
| Phospholipids [121,122,123] | - ↓ phosphatidylcholines and phosphatidylethanolamines (particularly after RYGB and SG) | - May reflect membrane composition and lipid remodeling - Possible role in insulin resistance regulation - Clinical significance still unclear |
| Ceramides [129,130,131] | - ↓ plasma ceramide subspecies | - ↑ insulin signaling and sensitivity - ↓ lipotoxicity - ↓ ApoB100/ApoA1 ratio |
| Ketone Bodies and TCA Cycle [44,129,130,131] | - ↑ β-hydroxybutyrate, acetoacetate, and acetone (although they tend to ↓ over time) - ↑ citrate, succinate, and malate - ↓ pyruvate | - ↑ metabolic flexibility - ↑ mitochondrial oxidative capacity - ↑ insulin sensitivity |
| Bile Acids [138,139,140,141,142,143,144] | - ↑ fasting and postprandial circulating BAs - ↓ fecal BA excretion - ↑ hyodeoxycholic acid - ↓ C4 levels | - Enhanced lipid and mitochondrial metabolism - Improved glucose metabolism - T2D remission in some individuals |
| Endocannabinoids [152,153,154] | - ↓ circulating ECs - Modulation of EC system activity | - ↓ inflammation and fat accumulation - Improved energy balance and metabolic homeostasis - Enhanced coronary circulatory function - Potential early detection and intervention target for metabolic dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiou, I.A.; Kounatidis, D.; Honka, M.-J.; Vallianou, N.G.; Rebelos, E.; Karamanolis, N.N.; Dalamaga, M.; Pantos, C.; Mourouzis, I. Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research. Metabolites 2025, 15, 434. https://doi.org/10.3390/metabo15070434
Anastasiou IA, Kounatidis D, Honka M-J, Vallianou NG, Rebelos E, Karamanolis NN, Dalamaga M, Pantos C, Mourouzis I. Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research. Metabolites. 2025; 15(7):434. https://doi.org/10.3390/metabo15070434
Chicago/Turabian StyleAnastasiou, Ioanna A., Dimitris Kounatidis, Miikka-Juhani Honka, Natalia G. Vallianou, Eleni Rebelos, Nikolaos Nektarios Karamanolis, Maria Dalamaga, Constantinos Pantos, and Iordanis Mourouzis. 2025. "Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research" Metabolites 15, no. 7: 434. https://doi.org/10.3390/metabo15070434
APA StyleAnastasiou, I. A., Kounatidis, D., Honka, M.-J., Vallianou, N. G., Rebelos, E., Karamanolis, N. N., Dalamaga, M., Pantos, C., & Mourouzis, I. (2025). Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research. Metabolites, 15(7), 434. https://doi.org/10.3390/metabo15070434










