Increased Lyso-Gb1 Levels in an Obese Splenectomized Gaucher Disease Type 1 Patient Treated with Eliglustat: Unacknowledged Poor Compliance or Underlying Factors
Abstract
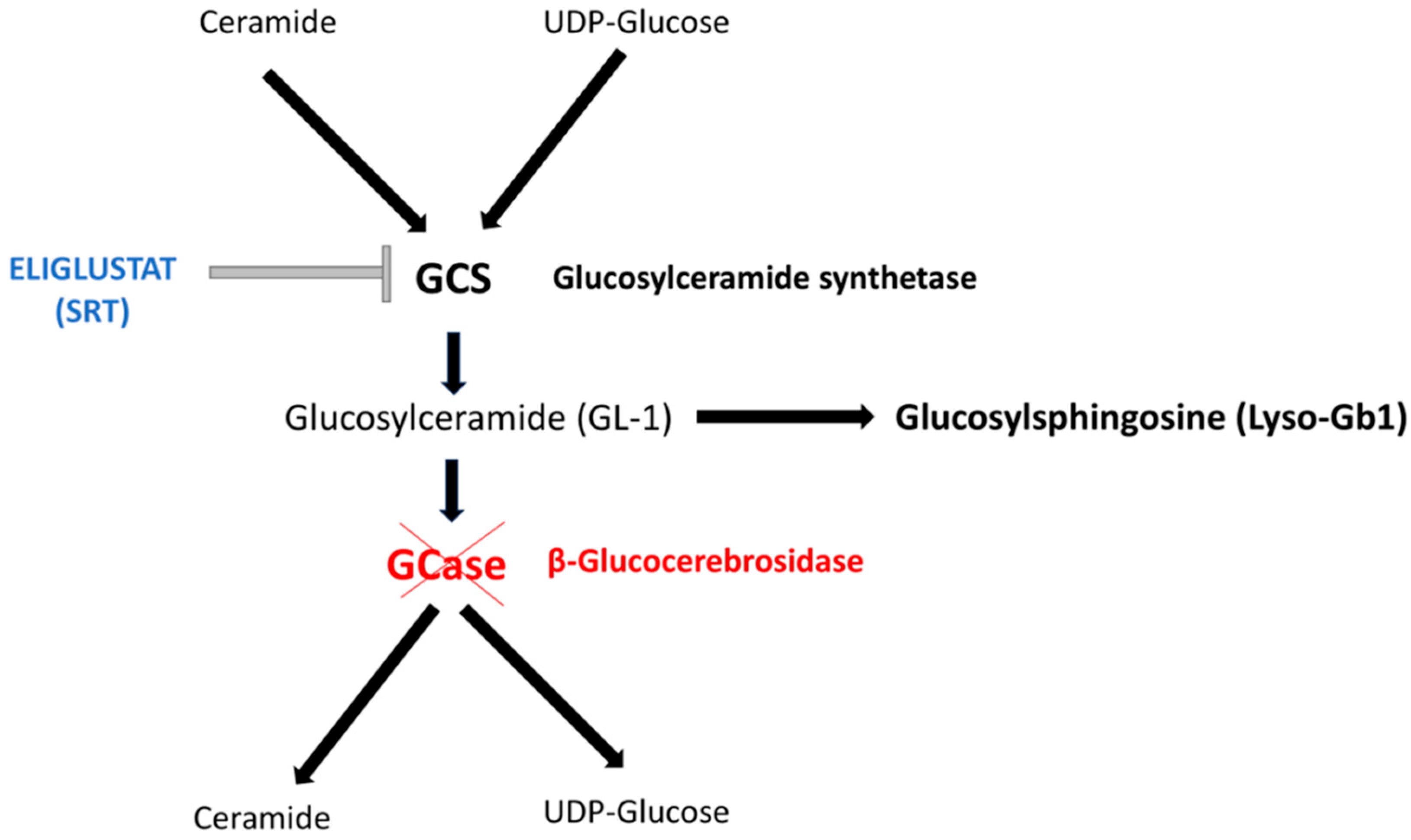
1. Background
2. Case Report
3. Discussion
4. Conclusions/Study Highlights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL18 | C-C Motif Chemokine Ligand 18 |
| CHITO | chitotriosidase enzymatic activity |
| CYP2D6 | cytochrome P450 2D6 |
| DBS | dried blood spot |
| ERT | enzymatic replacement therapy |
| GCase | acid beta-glucocerebrosidase |
| GCS | glucosylceramide synthase |
| GD1 | Gaucher disease type |
| GL-1 or GlcCer | glucosylceramide |
| Hb | hemoglobin |
| Lyso-Gb1 | glucosylsphingosine |
| MN | multiples of normal |
| MRI | magnetic resonance imaging |
| Plt | platelets |
| SRT | substrate reduction therapy |
References
- Peterschmitt, M.J.; Foster, M.C.; Ji, A.J.; Zajdel, M.B.; Cox, G.F. Plasma glucosylsphingosine correlations with baseline disease burden and response to eliglustat in two clinical trials of previously untreated adults with Gaucher disease type 1. Mol. Genet. Metab. 2023, 138, 107527. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, V.; Chuang, W.; Liu, J.; Lischuk, A.; Kacena, K.; Lin, H.; Pastores, G.M.; Yang, R.; Keutzer, J.; Zhang, K.; et al. Glucosylsphingosine is a key biomarker of Gaucher disease. Am. J. Hematol. 2016, 91, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Peterschmitt, J.; Foster, M.; Zhang, K.; Ji, A.; Cox, G. Correlations between glucosylsphingosine (lyso-GL-1) and baseline disease severity as well as response to treatment in two clinical trials of eliglustat in treatment-naïve adults with type 1 Gaucher disease. Mol. Genet. Metab. 2019, 126, S117. [Google Scholar] [CrossRef]
- Mistry, P.K.; Lukina, E.; Ben Turkia, H.; Shankar, S.P.; Baris Feldman, H.; Ghosn, M.; Mehta, A.; Packman, S.; Lau, H.; Petakov, M.; et al. Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: Phase 3 ENGAGE trial fnal results. Am. J. Hematol. 2021, 96, 1156–1165. [Google Scholar] [CrossRef]
- Lukina, E.; Watman, N.; Dragosky, M.; Lau, H.; Arreguin, E.A.; Rosenbaum, H.; Zimran, A.; Foster, M.C.; Gaemers, S.J.M.; Peterschmitt, M.J. Outcomes after 8 years of eliglustat therapy for Gaucher disease type 1: Final results from the Phase 2 trial. Am. J. Hematol. 2019, 94, 29–38. [Google Scholar] [CrossRef]
- Kleytman, N.; Ruan, J.; Ruan, A.; Zhang, B.; Murugesan, V.; Lin, H.; Guo, L.; Klinger, K.; Mistry, P.K. Incremental biomarker and clinical outcomes after switch from enzyme therapy to eliglustat substrate reduction therapy in Gaucher disease. Mol. Genet. Metab Rep. 2021, 29, 100798. [Google Scholar] [CrossRef]
- A Study of Eliglustat Tartrate (Genz-112638) in Patients with Gaucher Disease Who Have Reached Therapeutic Goals with Enzyme Replacement Therapy (ENCORE). Available online: https://clinicaltrials.gov/ct2/show/NCT00943111?term=eliglustat&cond=Gaucher+Disease%2C+Type+1&draw=1&rank=2 (accessed on 8 November 2024).
- Gackowski, M.; Jasińska-Stroschein, M.; Osmałek, T.; Waszyk-Nowaczyk, M. Innovative Approaches to Enhance and Measure Medication Adherence in Chronic Disease Management: A Review. Med. Sci. Monit. 2024, 16, e944605. [Google Scholar] [CrossRef]
- Camou, F.; Lagadec, A.; Coutinho, A.; Berger, M.G.; Cador-Rousseau, B.; Gaches, F.; Belmatoug, N. Patient reported outcomes of patients with Gaucher disease type 1 treated with eliglustat in real-world settings: The ELIPRO study. Mol. Genet. Metab. 2023, 140, 107667. [Google Scholar] [CrossRef]
- Zimran, A.; Altarescu, G.; Elstein, D. Nonprecipitous changes upon withdrawal from imiglucerase for Gaucher disease because of a shortage in supply. Blood Cells Mol. Dis. 2011, 46, 111–114. [Google Scholar] [CrossRef]
- Giraldo, P.; Irún, P.; Alfonso, P.; Dalmau, J.; Fernández-Galán, M.A.; Figueredo, A.; Hernández-Rivas, J.M.; Julia, A.; Luño, E.; Marín-Jimenez, F.; et al. Evaluation of Spanish Gaucher disease patients after a 6-month imiglucerase shortage. Blood Cells Mol. Dis. 2011, 46, 115–118. [Google Scholar] [CrossRef]
- Stirnemann, J.; Rose, C.; Serratrice, C.; Dalbies, F.; Lidove, O.; Masseau, A.; Pers, Y.-M.; Baron, C.; Belmatoug, N. Impact of imiglucerase supply constraint on the therapeutic management and course of disease in French patients with Gaucher disease type 1. Orphanet J. Rare Dis. 2015, 10, 62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gayed, M.M.; Jung, S.-H.; Huggins, E.; Rodriguez-Rassi, E.; DeArmey, S.; Kishnani, P.S.; Stiles, A.R. Glucosylsphingosine (Lyso-Gb1): An Informative Biomarker in the Clinical Monitoring of Patients with Gaucher Disease. Int. J. Mol. Sci. 2022, 23, 14938. [Google Scholar] [CrossRef] [PubMed]
- Peterschmitt, M.J.; Burke, A.; Blankstein, L.; Smith, S.E.; Puga, A.C.; Kramer, W.G.; Harris, J.A.; Mathews, D.; Bonate, P.L. Safety, tolerability, and pharmacokinetics of eliglustat tartrate (Genz-112638) after single doses, multiple doses, and food in healthy volunteers. J. Clin. Pharmacol. 2011, 51, 695–705. [Google Scholar] [CrossRef] [PubMed]
- A Study of Eliglustat Tartrate (Genz-112638) in Patients with Gaucher Disease (ENGAGE). Available online: https://clinicaltrials.gov/ct2/show/NCT00891202?term=eliglustat&cond=Gaucher+Disease%2C+Type+1&draw=2&rank=1 (accessed on 8 November 2024).
- A Study of Eliglustat Tartrate (Genz-112638) in Patients with Gaucher Disease to Evaluate Once Daily Versus Twice Daily Dosing (EDGE). Available online: https://clinicaltrials.gov/ct2/show/NCT01074944?term=eliglustat&cond=Gaucher+Disease%2C+Type+1&draw=1&rank=5 (accessed on 8 November 2024).
- Smid, B.E.; Ferraz, M.J.; Verhoek, M.; Mirzaian, M.; Wisse, P.; Overkleeft, H.S.; Hollak, C.E.; Johannes, M. Aerts Biochemical response to substrate reduction therapy versus enzyme replacement therapy in Gaucher disease type 1 patients. Orphanet J. Rare Dis. 2016, 11, 28. [Google Scholar] [CrossRef]
- Annex I. Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2015/20150119130463/anx_130463_en.pdf (accessed on 8 November 2024).
- May, M.; Schindler, C.; Engeli, S. Modern pharmacological treatment of obese patients. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018819897527. [Google Scholar] [CrossRef]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef]
- Brill, M.J.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Cox, T.M.; Aerts, J.M.F.G.; Belmatoug, N.; Cappellini, M.D.; Vom Dahl, S.; Goldblatt, J.; Maas, M.; Martins, A.M.; Mistry, P.K.; Pastores, G.M.; et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J. Inherit. Metab. Dis. 2008, 31, 319–336. [Google Scholar] [CrossRef]
- Belmatoug, N.; Di Rocco, M.; Fraga, C.; Giraldo, P.; Hughes, D.; Lukina, E.; Maison-Blanche, P.; Merkel, M.; Niederau, C.; Plöckinger, U.; et al. Management and monitoring recommendations for the use of eliglustat in adults with type 1 Gaucher disease in Europe. Eur. J. Intern. Med. 2017, 37, 25–32. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Allison, A.C.; Eugui, E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000, 47, 85–118. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, X.; Tian, W.; Li, C.; Li, P.; Zhao, J.; Yang, S.; Li, S. The role of redox-mediated lysosomal dysfunction and therapeutic strategies. Biomed. Pharmacother. 2023, 165, 115121. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.D.; Nelsonb, P.; Sharp, P.C.; Bindloss, C.A.; Deana, C.; Ravenscroft, E.M.; Fong, B.A.; Fietz, M.J.; Hopwood, J.J.; Meikle, P.J. Correlation among genotype, phenotype, and biochemical markers in Gaucher disease: Implications for the prediction of disease severity. Mol. Genet. Metab. 2002, 75, 46–55. [Google Scholar] [CrossRef]
- Hurvitz, N.; Dinur, T.; Becker-Cohen, M.; Cozma, C.; Hovakimyan, M.; Oppermann, S.; Demuth, L.; Rolfs, A.; Abramov, A.; Zimran, A.; et al. Glucosylsphingosine (lyso-Gb1) as a Biomarker for Monitoring Treated and Untreated Children with Gaucher Disease. Int. J. Mol. Sci. 2019, 20, 3033. [Google Scholar] [CrossRef]
- Grabowski, G.A. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 2008, 372, 1263–1271. [Google Scholar] [CrossRef]
- Kim, I.W.; Han, N.; Burckart, G.J.; Oh, J.M. Epigenetic changes in gene expression for drug-metabolizing enzymes and transporters. Pharmacotherapy 2014, 34, 140–150. [Google Scholar] [CrossRef]
- Kane, M.; Dean, L. Eliglustat Therapy and CYP2D6 Genotype. In Medical Genetics Summaries [Internet]; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565950 (accessed on 4 December 2024).
- Santamaria, R.; Michelakakis, H.; Moraitou, M.; Dimitriou, E.; Dominissini, S.; Grossi, S.; Sánchez-Ollé, G.; Chabás, A.; Pittis, M.G.; Filocamo, M.; et al. Haplotype analysis suggests a single Balkan origin for the Gaucher disease [D409H;H255Q] double mutant allele. Hum. Mutat. 2008, 29, E58–E67. [Google Scholar] [CrossRef]
- Reczek, D.; Schwake, M.; Schröder, J.; Hughes, H.; Blanz, J.; Jin, X.; Brondyk, W.; Van Patten, S.; Edmunds, T.; Saftig, P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 2007, 131, 770–783. [Google Scholar] [CrossRef]
- Vithayathil, J.; Gibney, G.; Baxevanis, A.D.; Stubblefield, B.K.; Sidransky, E.; Tayebi, N. Glucocerebrosidase mutation H255Q appears to be exclusively in cis with D409H: Structural implications. Clin. Genet. 2009, 75, 503–504. [Google Scholar] [CrossRef]
- Nawrocki, A.R.; Scherer, P.E. The delicate balance between fat and muscle: Adipokines in metabolic disease and musculoskeletal inflammation. Curr. Opin. Pharmacol. 2004, 4, 281–289. [Google Scholar] [CrossRef]
- Fleshner, P.R.; Aufses, A.H., Jr.; Grabowski, G.A.; Elias, R. A 27-year experience with splenectomy for Gaucher’s disease. Am. J. Surg. 1991, 161, 69–75. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maines, E.; Franceschi, R.; Luppi, G.; Marchi, G.; Piccoli, G.; Vitturi, N.; Soffiati, M.; Campomori, A.; Urru, S.A.M. Increased Lyso-Gb1 Levels in an Obese Splenectomized Gaucher Disease Type 1 Patient Treated with Eliglustat: Unacknowledged Poor Compliance or Underlying Factors. Metabolites 2025, 15, 427. https://doi.org/10.3390/metabo15070427
Maines E, Franceschi R, Luppi G, Marchi G, Piccoli G, Vitturi N, Soffiati M, Campomori A, Urru SAM. Increased Lyso-Gb1 Levels in an Obese Splenectomized Gaucher Disease Type 1 Patient Treated with Eliglustat: Unacknowledged Poor Compliance or Underlying Factors. Metabolites. 2025; 15(7):427. https://doi.org/10.3390/metabo15070427
Chicago/Turabian StyleMaines, Evelina, Roberto Franceschi, Giacomo Luppi, Giacomo Marchi, Giovanni Piccoli, Nicola Vitturi, Massimo Soffiati, Annalisa Campomori, and Silvana Anna Maria Urru. 2025. "Increased Lyso-Gb1 Levels in an Obese Splenectomized Gaucher Disease Type 1 Patient Treated with Eliglustat: Unacknowledged Poor Compliance or Underlying Factors" Metabolites 15, no. 7: 427. https://doi.org/10.3390/metabo15070427
APA StyleMaines, E., Franceschi, R., Luppi, G., Marchi, G., Piccoli, G., Vitturi, N., Soffiati, M., Campomori, A., & Urru, S. A. M. (2025). Increased Lyso-Gb1 Levels in an Obese Splenectomized Gaucher Disease Type 1 Patient Treated with Eliglustat: Unacknowledged Poor Compliance or Underlying Factors. Metabolites, 15(7), 427. https://doi.org/10.3390/metabo15070427








