Periodontitis Frequently Exists in Patients with Colorectal Carcinoma and Causes Supplementary Impairment of Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Clinical Data and Laboratory Methods
2.4. Periodontology Profile
2.5. Ethical Approval
2.6. Statistical Analyses
3. Results
3.1. Patients Sample
3.2. Metabolic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dekker, E.; Tanis, J.P.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal cancer epidemiology: Recent trends and impact on outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar]
- Weitz, J.; Koch, M.; Debus, J.; Hohler, T.; Galle, P.R.; Buchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.; Chapman, W., Jr.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jin, C.; Guan, Q. Causal effects of overall and abdominal obesity on insulin resistance and the risk of type 2 diabetes mellitus: A two-sample Mendelian randomization study. Front. Genet. 2020, 11, 603. [Google Scholar] [CrossRef]
- Erbach, M.; Mehnert, H.; Schnell, O. Diabetes and the risk for colorectal cancer. J. Diabetes Complic. 2012, 26, 50–55. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Ito, Y.; Hada, Y.; Maki, T.; Takekawa, S.; Kamon, J.; Kobayashi, M.; Suzuki, R.; Hara, K.; et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J. Biol. Chem. 2004, 279, 30817–30822. [Google Scholar] [CrossRef]
- Lira-Junior, R.; Akerman, S.; Gustafsson, A.; Klinge, B.; Bostrom, E.A. Colony stimulating factor-1 in saliva in relation to age, smoking, and oral and systemic diseases. Sci. Rep. 2017, 7, 7280. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, L.; Wei, X.; Jin, D.; Qian, T.; Yu, A.; Sun, J.; Cui, J.; Yang, Z. The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine 2016, 51, 456–468. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Sanchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Pouliou, C.; Piperi, C. Advances of Oxidative Stress Impact in Periodontitis: Biomarkers and Effective Targeting Options. Curr. Med. Chem. 2024, 31, 6187–6203. [Google Scholar] [CrossRef]
- Page, R.C.; Beck, J.D. Risk assessment for periodontal diseases. Int. Dent. J. 1997, 47, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Triebl, Z.; Bencze, B.; Banyai, D.; Rozsa, N.; Hermann, P.; Vegh, D. Poor glycemic control impairs oral health in children with type 1 diabetes mellitus—A systematic review and meta-analysis. BMC Oral Health 2024, 24, 748. [Google Scholar] [CrossRef]
- Ramos Pena, D.E.; Pillet, S.; Grupioni Lourenco, A.; Pozzetto, B.; Bourlet, T.; Motta, A.C.F. Human immunodeficiency virus and oral microbiota: Mutual influence on the establishment of a viral gingival reservoir in individuals under antiretroviral therapy. Front. Cell. Infect. Microbiol. 2024, 14, 1364002. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Zhang, R.; Li, Y.; Wang, J.; Zhang, X.; Lin, L. Is periodontal disease a risk indicator for colorectal cancer? A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 336–347. [Google Scholar] [CrossRef]
- Hu, J.M.; Shen, C.J.; Chou, Y.C.; Hung, C.F.; Tian, Y.F.; You, S.L.; Chen, C.Y.; Hsu, C.H.; Hsiao, C.W.; Lin, C.Y.; et al. Risk of colorectal cancer in patients with periodontal disease severity: A nationwide, population-based cohort study. Int. J. Colorectal Dis. 2018, 33, 349–352. [Google Scholar] [CrossRef]
- Arjunan, P.; Meghil, M.M.; Pi, W.; Xu, J.; Lang, L.; El-Awady, A.; Sullivan, W.; Rajendran, M.; Rabelo, M.S.; Wang, T.; et al. Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic Cell Proliferation. Sci. Rep. 2018, 8, 16607. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Deng, J.; Donati, V.; Merali, N.; Frampton, A.E.; Giovannetti, E.; Deng, D. The Roles and Interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in Oral and Gastrointestinal Carcinogenesis: A Narrative Review. Pathogens 2024, 13, 93. [Google Scholar] [CrossRef]
- Negrut, R.L.; Cote, A.; Maghiar, A.M. Exploring the Potential of Oral Microbiome Biomarkers for Colorectal Cancer Diagnosis and Prognosis: A Systematic Review. Microorganisms 2023, 11, 1586. [Google Scholar] [CrossRef]
- Idrissi Janati, A.; Karp, I.; Latulippe, J.F.; Charlebois, P.; Emami, E. Periodontal disease as a risk factor for sporadic colorectal cancer: Results from COLDENT study. Cancer Causes Control 2022, 33, 463–472. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal disease and diabetes mellitus: The role of tumor necrosis factor-alpha in a 2-way relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H.; Sammalkorpi, K.; Koivisto, V.A.; Nikkila, E.A. Severity, duration, and mechanisms of insulin resistance during acute infections. J. Clin. Endocrinol. Metab. 1989, 69, 317–323. [Google Scholar] [CrossRef]
- Sobotka, L.; Sobotka, O. The predominant role of glucose as a building block and precursor of reducing equivalents. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Petrides, A.S.; Luzi, L.; Reuben, A.; Riely, C.; DeFronzo, R.A. Effect of insulin and plasma amino acid concentration on leucine metabolism in cirrhosis. Hepatology 1991, 14, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Kaga, H.; Someya, Y.; Tabata, H.; Kakehi, S.; Tajima, T.; Ito, N.; Yamasaki, N.; Sato, M.; Kadowaki, S.; et al. Fat Accumulation and Elevated Free Fatty Acid Are Associated With Age-Related Glucose Intolerance: Bunkyo Health Study. J. Endocr. Soc. 2024, 8, bvad164. [Google Scholar] [CrossRef]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005, 76 (Suppl. 11), 2075–2084. [Google Scholar] [CrossRef]
- Tortora, S.C.; Bodiwala, V.M.; Quinn, A.; Martello, L.A.; Vignesh, S. Microbiome and colorectal carcinogenesis: Linked mechanisms and racial differences. World J. Gastrointest. Oncol. 2022, 14, 375–395. [Google Scholar] [CrossRef]
| Mean ± SD | Mann–Whitney | p * | Spearman’s Rho (p) | |
|---|---|---|---|---|
| Controls | Periodontitis | |||
| N = 21 | N = 58 | |||
| Age (years) | 58.71 ± 14.07 | 68.22 ± 10.76 | 0.006 | 0.299 (0.022) |
| Metabolic years | 55.57 ± 18.36 | 63.35 ± 13.44 | 0.044 | 0.167 (0.211) |
| Body weight (kg) | 78.79 ± 17.96 | 82.46 ± 18.56 | 0.427 | −0.144 (0.281) |
| Body mass index-BMI (kg/m2) | 26.24 ± 5.41 | 28.27 ± 4.82 | 0.111 | −0.059 (0.662) |
| Adipose tissue weight (kg) | 23.35 ± 12.16 | 26.11 ± 10.84 | 0.297 | −0.086 (0.519) |
| Muscle tissue weight (kg) | 52.65 ± 9.79 | 53.56 ± 12.02 | 0.894 | −0.126 (0.347) |
| Skeletal muscle tissue weight (kg) | 31.29 ± 5.87 | 31.91 ± 7.14 | 0.881 | −0.126 (0.347) |
| Bone tissue weight (kg) | 2.79 ± 0.49 | 2.82 ± 0.59 | 0.885 | −0.139 (0.297) |
| Vitamin D (nmol/L) | 47.29 ± 24.26 | 45.62 ± 23.59 | 0.824 | 0.187 (0.159) |
| Human growth hormone (ng/mL) | 1.23 ± 1.78 | 1.09 ± 1.29 | 0.786 | 0.059 (0.662) |
| IGF-I (ng/mL) | 135.95 ± 38.51 | 128.91 ± 44.39 | 0.289 | −0.093 (0.486) |
| Hemoglobin (g/L) | 127.76 ± 18.23 | 122.38 ± 22.02 | 0.444 | −0.296 (0.024) |
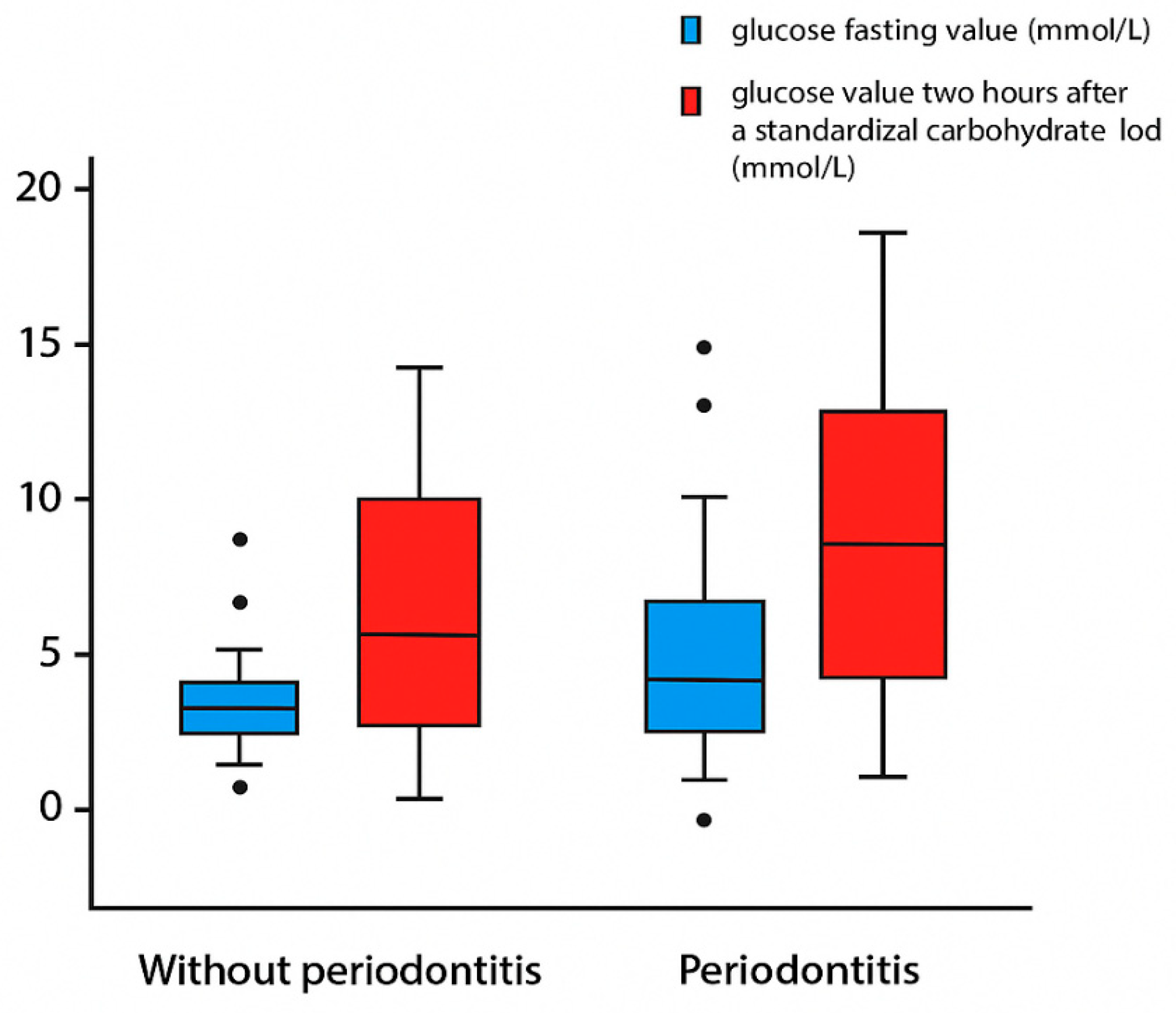
| Glucose fasting (mmol/L) | 6.06 ± 1.10 | 7.31 ± 2.32 | 0.016 | 0.326 (0.013) |
| Glucose 2 h after standardized carbohydrate load (mmol/L) | 8.02 ± 3.48 | 10.78 ± 4.28 | 0.005 | 0.327 (0.012) |
| Insulin fasting (mU/L) | 12.61 ± 7.01 | 19.08 ± 17.22 | 0.053 | 0.195 (0.142) |
| Insulin 2 h after standardized carbohydrate load (mU/L) | 77.00 ± 65.15 | 127.05 ± 143.88 | 0.058 | 0.161 (0.226) |
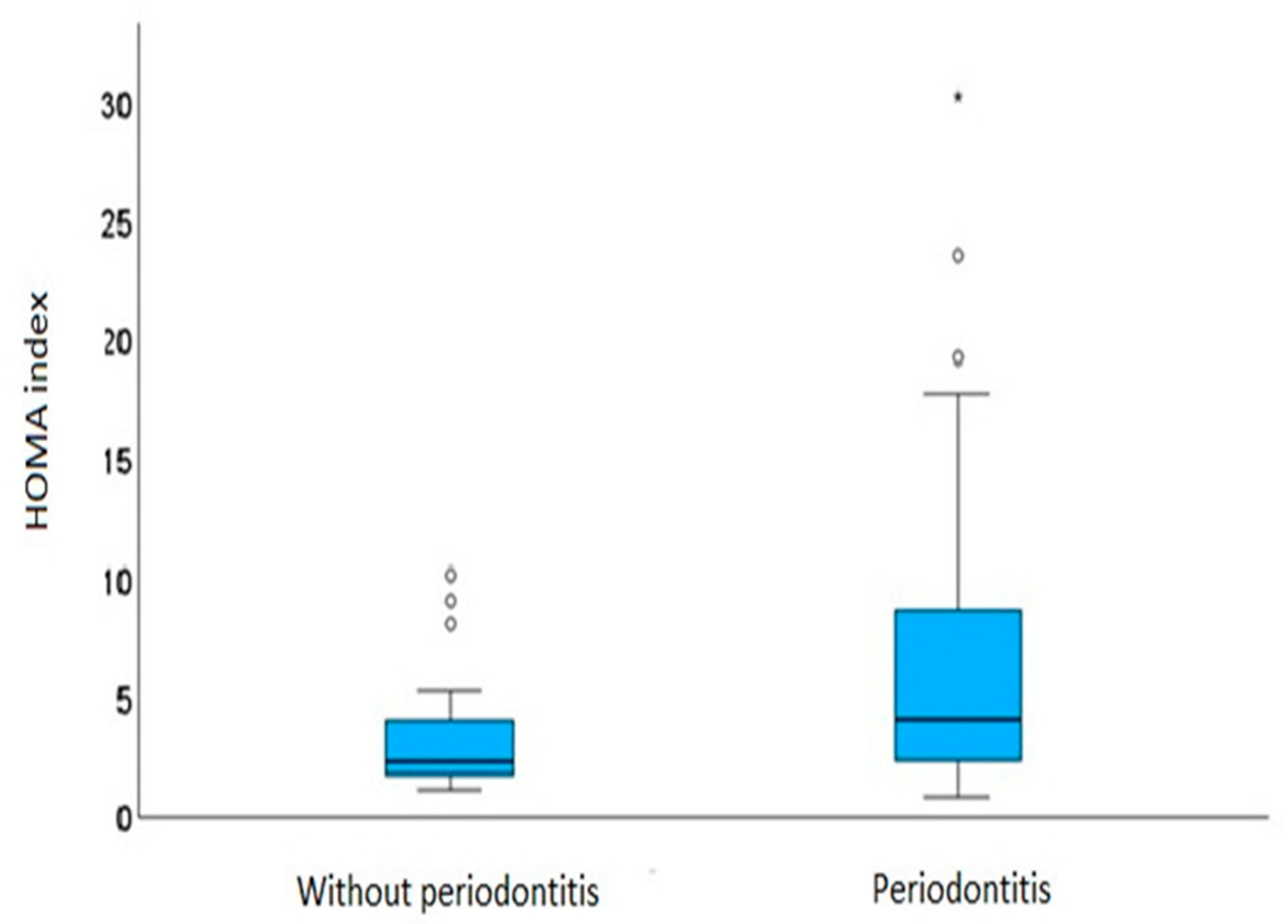
| Homa index | 3.58 ± 2.61 | 6.38 ± 5.74 | 0.012 | 0.271 (0.039) |
| Albumin (g/L) | 44.73 ± 2.78 | 43.69 ± 2.00 | 0.128 | −0.118 (0.378) |
| Cholesterol (mmol/L) | 5.34 ± 1.19 | 4.92 ± 1.06 | 0.269 | −0.021 (0.874) |
| Triglycerides (mmol/L) | 1.23 ± 0.59 | 1.23 ± 0.41 | 0.663 | −0.007 (0.96) |
| LDL (mmol/L) | 3.25 ± 1.07 | 3.02 ± 0.87 | 0.582 | −0.032 (0.811) |
| HDL (mmol/L) | 1.41 ± 0.28 | 1.23 ± 0.35 | 0.016 | 0.045 (0.738) |
| Creatinine (umol/L) | 72.05 ± 9.43 | 78.78 ± 18.46 | 0.124 | −0.031 (0.815) |
| Urea (mmol/L) | 4.96 ± 1.61 | 5.77 ± 1.93 | 0.114 | 0.369 (0.004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, M.; Boban, M.; Vrazic, D.; Vulic, I.; Budimir, I.; Blazevic, N.; Vcev, A.; Nikolic, M. Periodontitis Frequently Exists in Patients with Colorectal Carcinoma and Causes Supplementary Impairment of Insulin Resistance. Metabolites 2025, 15, 414. https://doi.org/10.3390/metabo15060414
Zivkovic M, Boban M, Vrazic D, Vulic I, Budimir I, Blazevic N, Vcev A, Nikolic M. Periodontitis Frequently Exists in Patients with Colorectal Carcinoma and Causes Supplementary Impairment of Insulin Resistance. Metabolites. 2025; 15(6):414. https://doi.org/10.3390/metabo15060414
Chicago/Turabian StyleZivkovic, Mario, Marko Boban, Domagoj Vrazic, Ivan Vulic, Ivan Budimir, Nina Blazevic, Aleksandar Vcev, and Marko Nikolic. 2025. "Periodontitis Frequently Exists in Patients with Colorectal Carcinoma and Causes Supplementary Impairment of Insulin Resistance" Metabolites 15, no. 6: 414. https://doi.org/10.3390/metabo15060414
APA StyleZivkovic, M., Boban, M., Vrazic, D., Vulic, I., Budimir, I., Blazevic, N., Vcev, A., & Nikolic, M. (2025). Periodontitis Frequently Exists in Patients with Colorectal Carcinoma and Causes Supplementary Impairment of Insulin Resistance. Metabolites, 15(6), 414. https://doi.org/10.3390/metabo15060414





