Geopropolis from Melipona fasciculata Smith Accelerates Wound Healing in Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Melipona fasciculata Smith Geopropolis Samples
2.2. Hydroalcoholic Extract of Geopropolis (HEG) Preparation
2.3. Geopropolis Cream Preparation
2.4. Animals
2.5. Wound Model
2.6. Macroscopic and Microscopic Evaluation
2.7. Cytokine Assay
2.8. In Silico Assay
2.8.1. Ligand and Target Preparations
2.8.2. Molecular Docking
2.9. Statistical Analysis
3. Results
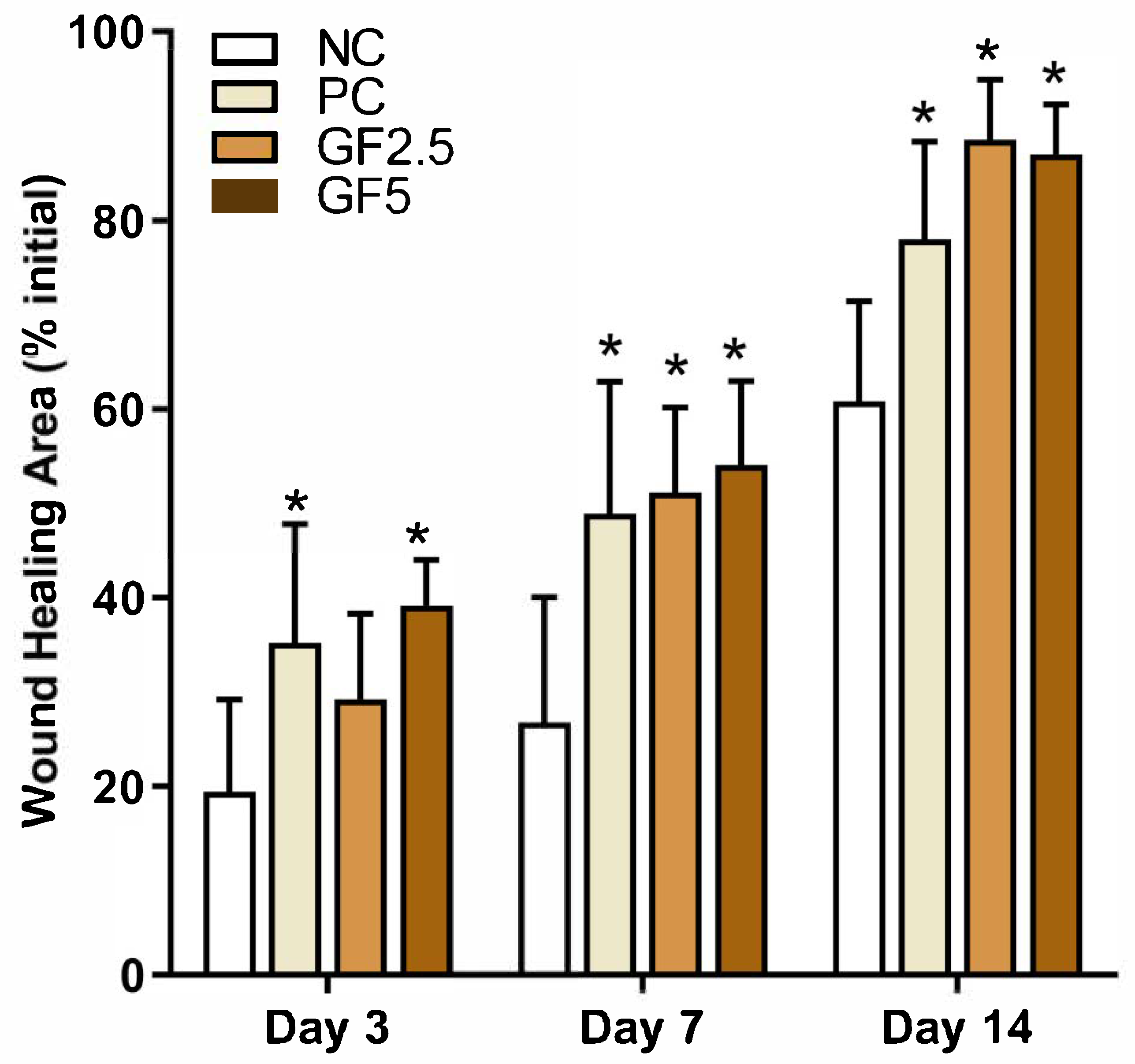
3.1. M. fasciculata Geopropolis Cream Accelerates Wound Closure
3.2. M. fasciculata Geopropolis Cream Alters Histopathological Parameters of Lesions
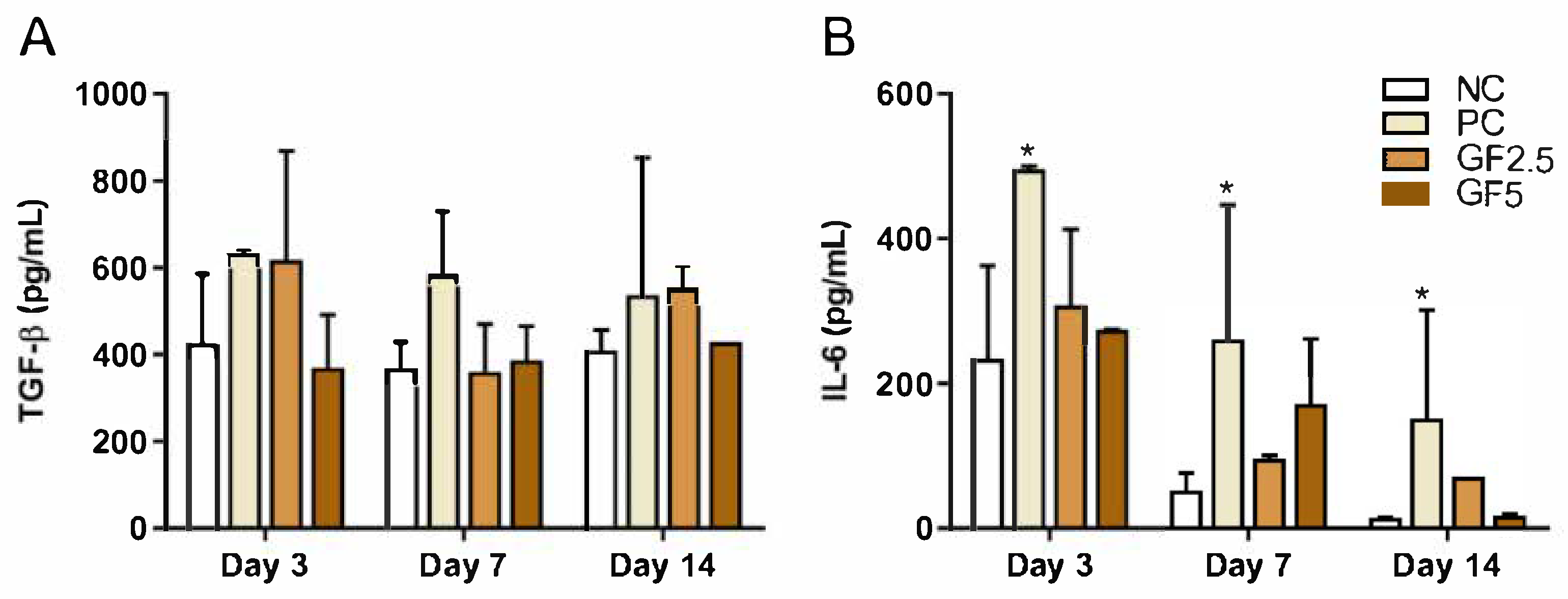
3.3. M. fasciculata Geopropolis Cream Does Not Alter Serum Cytokine Production in Mice
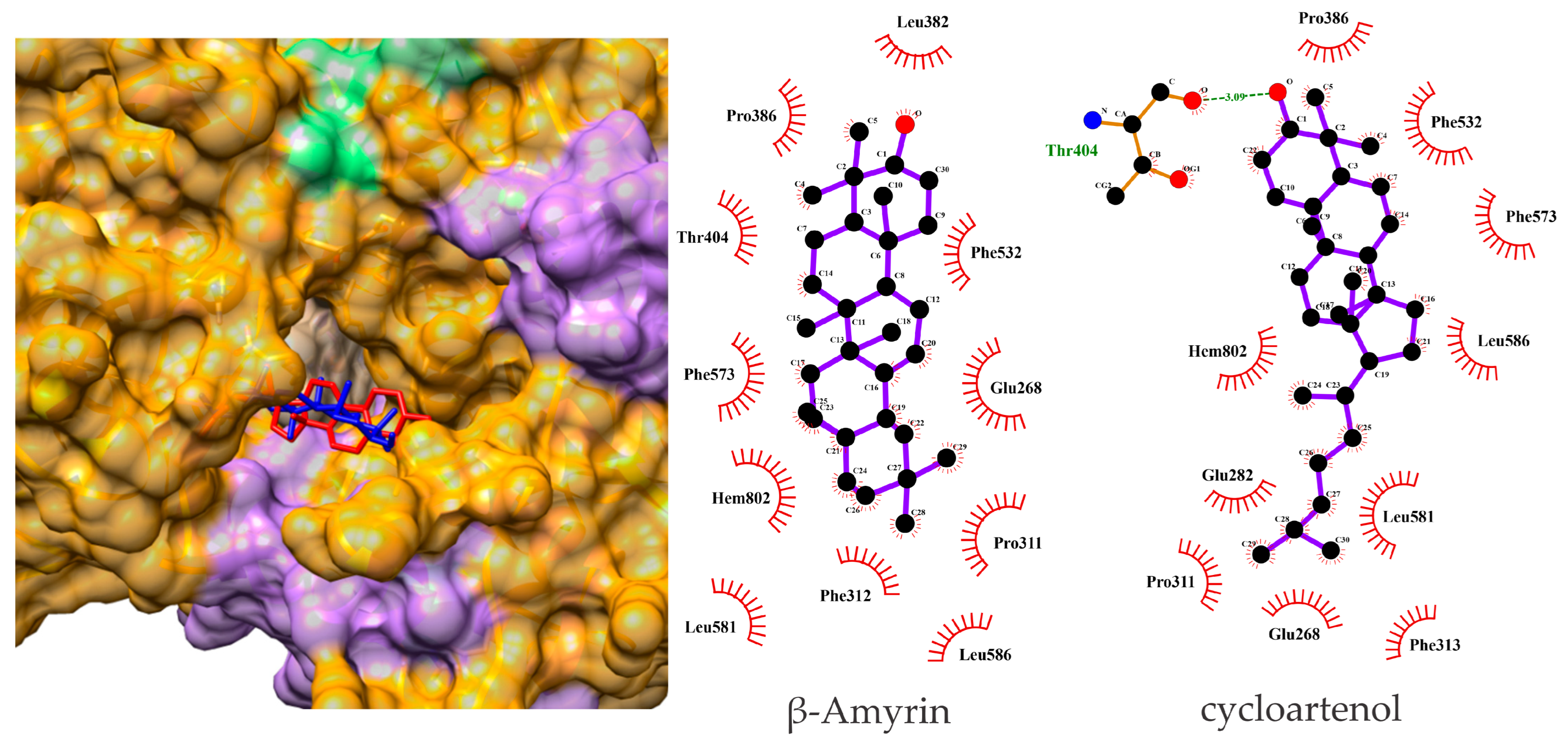
3.4. β-Amyrin and Cycloartenol Bind Effectively to hMPO in Docking Analysis
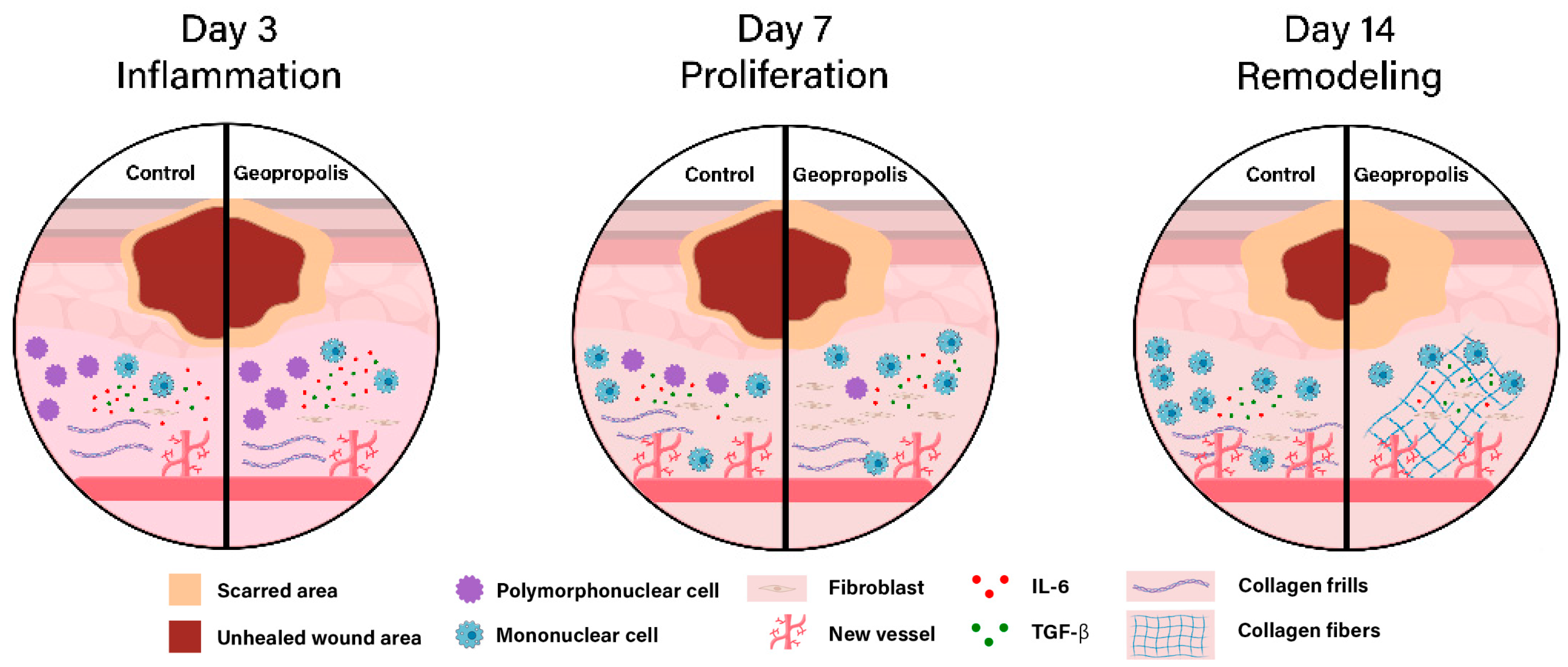
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Primous, N.R.; Elvin, P.T.; Carter, K.V.; Andrade, H.L.; La Fontaine, J.; Shibuya, N.; Biguetti, C.C. Bioengineered Skin for Diabetic Foot Ulcers: A Scoping Review. J. Clin. Med. 2024, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Malgrange, D. Physiopathologie Du Pied Diabétique. Rev. Med. Interne 2008, 29, S231–S237. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.; Attinger, C.; Abbas, Z.; Bal, A.; Rojas, N.; Xu, Z.R. Cost of Treating Diabetic Foot Ulcers in Five Different Countries. Diabetes/Metab. Res. Rev. 2012, 28, 107–111. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Dutra, R.P.; de Sousa, M.M., Jr.; Mignoni, M.S.P.M.; de Oliveira, K.G.M.; Pereira, E.B.; Figueredo, A.S.; da Costa, A.A.C.; Dias, T.G.; Vasconcelos, C.C.; Silva, L.A.; et al. Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis. Metabolites 2023, 13, 1027. [Google Scholar] [CrossRef]
- Nogueira, D.S. Overview of Stingless Bees in Brazil (Hymenoptera: Apidae: Meliponini). EntomoBrasilis 2023, 16, e1041. [Google Scholar] [CrossRef]
- Pedro, S.R.M. The Stingless Bee Fauna in Brazil (Hymenoptera: Apidae). Sociobiology 2014, 61, 348–354. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent Advances in Chemistry and Plant Origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Dutra, R.P.; De Barros Abreu, B.V.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Batista, M.C.A.; Abreu, B.V.d.B.; Dutra, R.P.; Cunha, M.S.; Amaral, F.M.M.d.; Torres, L.M.B.; Ribeiro, M.N.d.S. Composição Química e Atividade Antioxidante Da Geoprópolis de Melipona fasciculata (Meliponinae) Produzida Em Áreas de Campos Alagados e de Cerrado No Estado Do Maranhão, Nordeste Do Brasil. Acta Amaz. 2016, 46, 315–322. [Google Scholar] [CrossRef]
- Liberio, S.A.; Pereira, A.L.A.; Dutra, R.P.; Reis, A.S.; Araújo, M.J.A.M.; Mattar, N.S.; Silva, L.A.; Ribeiro, M.N.S.; Nascimento, F.R.F.; Guerra, R.N.M.; et al. Antimicrobial Activity against Oral Pathogens and Immunomodulatory Effects and Toxicity of Geopropolis Produced by the Stingless Bee Melipona fasciculata Smith. BMC Complement. Altern. Med. 2011, 11, 108. [Google Scholar] [CrossRef]
- Barboza, J.R.; Pereira, F.A.N.; Fernandes, R.A.; Vasconcelos, C.C.; Cartágenes, M.d.S.d.S.; Oliveira Lopes, A.J.; Melo, A.C.d.; Guimarães, I.d.S.; Rocha, C.Q.d.; Ribeiro, M.N.d.S. Cytotoxicity and Pro-Apoptotic, Antioxidant and Anti-Inflammatory Activities of Geopropolis Produced by the Stingless Bee Melipona fasciculata Smith. Biology 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Fontoura, D.M.N.; Olinda, R.G.; Viana, G.A.; Kizzy, K.M.; Batista, J.S.; Serrano, R.M.O.T.; Silva, O.M.D.; Camara, C.A.; Silva, T.M.S. Wound Healing Activity and Chemical Composition of Geopropolis from Melipona subnitida. Rev. Bras. Farmacogn. 2020, 30, 367–373. [Google Scholar] [CrossRef]
- Martins, A.C.L.; Rêgo, M.M.C.; Carreira, L.M.M.; de Albuquerque, P.M.C. Espectro Polínico de Mel de Tiúba (Melipona fasciculata Smith, 1854, Hymenoptera, Apidae). Acta Amaz. 2011, 41, 183–190. [Google Scholar] [CrossRef]
- Perazzo, A.; Preziosi, V.; Guido, S. Phase Inversion Emulsification: Current Understanding and Applications. Adv. Colloid Interface Sci. 2015, 222, 581–599. [Google Scholar] [CrossRef]
- Khosravi-Maharlooei, M.; Madley, R.; Borsotti, C.; Ferreira, L.M.R.; Sharp, R.C.; Brehm, M.A.; Greiner, D.L.; Parent, A.V.; Anderson, M.S.; Sykes, M.; et al. Modeling Human T1D-Associated Autoimmune Processes. Mol. Metab. 2022, 56, 101417. [Google Scholar] [CrossRef]
- Anderson, M.S.; Bluestone, J.A. The NOD Mouse: A Model of Immune Dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Fan, W.; Huang, R.; Wang, H.; Liu, G. Preclinical study of diabetic foot ulcers: From pathogenesis to vivo/vitro models and clinical therapeutic transformation. Int. Wound J. 2023, 20, 4394–4409. [Google Scholar] [CrossRef]
- Frade, M.A.C.; Assis, R.V.C.d.; Coutinho Netto, J.; Andrade, T.A.M.d.; Foss, N.T. The vegetal biomembrane in the healing of chronic venous ulcers. An. Bras. Dermatol. 2012, 87, 45–51. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.; Reddy, V.K.; Veigas, J.M.; Murthy, U.D. Study on wound healing activity of Punica granatum peel. J. Med. Food 2004, 7, 256–259. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 5; Semichem Inc.: Shawnee, KS, USA, 2009.
- Frisch, M. Gaussian 09, Revision d. 01; Gaussian. Inc.: Wallingford, CT, USA, 2009.
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Lopes, A.J.O.; Calado, G.P.; Fróes, Y.N.; Araújo, S.A.d.; França, L.M.; Paes, A.M.d.A.; Morais, S.V.d.; Rocha, C.Q.d.; Vasconcelos, C.C. Plant metabolites as SARS-CoV-2 inhibitors candidates: In silico and in vitro studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Rarey, M. PoseView--molecular interaction patterns at a glance. J. Cheminform. 2010, 2 (Suppl. 1), P50. [Google Scholar] [CrossRef]
- Sehn, E.; Hernandes, L.; Franco, S.L.; Gonçalves, C.C.M.; Baesso, M.L. Dynamics of Reepithelialization and Penetration Rate of a Bee Propolis Formulation during Cutaneous Wounds Healing. Anal. Chim. Acta 2009, 635, 115–120. [Google Scholar] [CrossRef]
- McLennan, S.V.; Bonner, J.; Milne, S.; Lo, L.; Charlton, A.; Kurup, S.; Jia, J.; Yue, D.K.; Twigg, S.M. The Anti-Inflammatory Agent Propolis Improves Wound Healing in a Rodent Model of Experimental Diabetes. Wound Repair Regen. 2008, 16, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.j.d.; Vianna, L.d.A.C.; Gamba, M.A. Avaliação Da Eficácia Da Pomada de Própolis Em Portadores de Feridas Crônicas. Acta Paul. Enferm. 2007, 20, 199–204. [Google Scholar] [CrossRef]
- Araujo, M.; Bufalo, M.; Conti, B.; Fernandes, A., Jr.; Trusheva, B.; Bankova, V.; Sforcin, J. The Chemical Composition and Pharmacological Activities of Geopropolis Produced by Melipona fasciculata Smith in Northeast Brazil. J. Mol. Pathophysiol. 2015, 4, 12–20. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.P.G.; Conte, F.L.; Cardoso, E.d.O.; Conti, B.J.; Santiago, K.B.; Golim, M.d.A.; Cruz, M.T.; Sforcin, J.M. Immunomodulatory/Inflammatory Effects of Geopropolis Produced by Melipona Fasciculata Smith in Combination with Doxorubicin on THP-1 Cells. J. Pharm. Pharmacol. 2016, 68, 1551–1558. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Aragão, G.F.; Cunha Pinheiro, M.C.; Nogueira Bandeira, P.; Gomes Lemos, T.L.; de Barros Viana, G.S. Analgesic and Anti-Inflammatory Activities of the Isomeric Mixture of Alpha- and Beta-Amyrin from Protium heptaphyllum (Aubl.) March. J. Herb. Pharmacother. 2007, 7, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Mathew, L.E.; Vijayalakshmi, N.R.; Helen, A. Anti-Inflammatory Potential of beta-Amyrin, a Triterpenoid Isolated from Costus Igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-Inflammatory Effect of α, beta-Amyrin, a Pentacyclic Triterpene from Protium heptaphyllum in Rat Model of Acute Periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical Role of Tumor Necrosis Factor-α in the Early Process of Wound Healing in Skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the Application of Medicinal Plants and Natural Products in Wound Healing: A Mechanistic Review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Ahumada, C.; Sáenz, T.; García, D.; De La Puerta, R.; Fernandez, A.; Martinez, E. The Effects of a Triterpene Fraction Isolated from Crataegus Monogyna Jacq. on Different Acute Inflammation Models in Rats and Mice. Leucocyte Migration and Phospholipase A2 Inhibition. J. Pharm. Pharmacol. 1997, 49, 329–331. [Google Scholar] [CrossRef]
- Islam, M.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef]
- Miller, A.; Majauskaite, L.; Engel, K.-H. Enzyme-catalyzed hydrolysis of γ-oryzanol. Eur. Food Res. Technol. 2004, 218, 349–354. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, S.; Li, S.; Gong, S.; Zhang, Q. Neutrophil extracellular traps in wound healing. Trends Pharmacol. Sci. 2024, 45, 1033–1045. [Google Scholar] [CrossRef]
- Hasmann, A.; Wehrschütz-Sigl, E.; Marold, A.; Wiesbauer, H.; Schoeftner, R.; Gewessler, U.; Kandelbauer, A.; Schiffer, D.; Schneider, K.; Binder, B. Analysis of myeloperoxidase activity in wound fluids as a marker of infection. Ann. Clin. Biochem. 2013, 50, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.W.; Finkielsztein, A.; Mascarenhas, L.A.; Mehl, L.C.; Butin-Israeli, V.; Sumagin, R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J. Immunol. 2017, 198, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Komesu, M.C.; Tanga, M.B.; Buttros, K.R.; Nakao, C. Effects of Acute Diabetes on Rat Cutaneous Wound Healing. Pathophysiology 2004, 11, 63–67. [Google Scholar] [CrossRef]
- Tanaka, M.; Misawa, E.; Yamauchi, K.; Abe, F.; Ishizaki, C. Effects of Plant Sterols Derived from Aloe vera Gel on Human Dermal Fibroblasts in Vitro and on Skin Condition in Japanese Women. Clin. Cosmet. Investig. Dermatol. 2015, 8, 95–104. [Google Scholar] [CrossRef]
- Siqueira, J.S.; Mescouto, C.S.T.; Lemos, M.d.S.; Junior, J.B.P.; Venturieri, G.C.; Filho, H.A.D.; Dantas, K.d.G.F. Determination of Inorganic Elements in Geopropolis Samples by Inductively Coupled Plasma–Optical Emission Spectrometry. J. Apic. Res. 2022, 61, 400–407. [Google Scholar] [CrossRef]
- Vaidyanathan, L. Growth Factors in Wound Healing: A Review. Biomed. Pharmacol. J. 2021, 14, 1469–1480. [Google Scholar] [CrossRef]
- Ansorge, S.; Reinhold, D.; Lendeckel, U. Propolis and Some of Its Constituents Down-Regulate DNA Synthesis and Inflammatory Cytokine Production but Induce TGF-1 Production of Human Immune Cells. Zeitschrift Naturforschung C 2003, 58, 580–589. [Google Scholar] [CrossRef]
| Parameters Analyzed | Day 3 | Day 7 | Day 14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | GF2.5 | GF5 | NC | PC | GF2.5 | GF5 | NC | PC | GF2.5 | GF5 | |
| Inflammatory Infiltrate | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ | +++ | + | ++ |
| Edema | ++ | ++ | ++ | - | + | + | + | + | + | + | - | + |
| Angiogenesis | + | + | + | + | ++ | ++ | + | + | ++ | ++ | + | ++ |
| Fibroblasts | - | + | ++ | ++ | + | ++ | +++ | +++ | + | + | ++ | ++ |
| Collagen Fibers | + | + | + | + | + | + | + | + | + | + | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.S.; de Souza, G.C.; Fontoura, G.M.G.; Silva, L.A.d.C.; Lopes, A.J.O.; Dutra, R.P.; Silva, L.A.; Guerra, R.N.M.; Ribeiro, M.N.S.; Nascimento, F.R.F. Geopropolis from Melipona fasciculata Smith Accelerates Wound Healing in Diabetic Mice. Metabolites 2025, 15, 413. https://doi.org/10.3390/metabo15060413
Reis AS, de Souza GC, Fontoura GMG, Silva LAdC, Lopes AJO, Dutra RP, Silva LA, Guerra RNM, Ribeiro MNS, Nascimento FRF. Geopropolis from Melipona fasciculata Smith Accelerates Wound Healing in Diabetic Mice. Metabolites. 2025; 15(6):413. https://doi.org/10.3390/metabo15060413
Chicago/Turabian StyleReis, Aramys Silva, Gabriel Carvalho de Souza, Guilherme Martins Gomes Fontoura, Luecya Alves de Carvalho Silva, Alberto Jorge Oliveira Lopes, Richard Pereira Dutra, Lucilene Amorim Silva, Rosane Nassar Meireles Guerra, Maria Nilce Sousa Ribeiro, and Flávia Raquel Fernandes Nascimento. 2025. "Geopropolis from Melipona fasciculata Smith Accelerates Wound Healing in Diabetic Mice" Metabolites 15, no. 6: 413. https://doi.org/10.3390/metabo15060413
APA StyleReis, A. S., de Souza, G. C., Fontoura, G. M. G., Silva, L. A. d. C., Lopes, A. J. O., Dutra, R. P., Silva, L. A., Guerra, R. N. M., Ribeiro, M. N. S., & Nascimento, F. R. F. (2025). Geopropolis from Melipona fasciculata Smith Accelerates Wound Healing in Diabetic Mice. Metabolites, 15(6), 413. https://doi.org/10.3390/metabo15060413









