Salivary Metabolite Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage
Abstract
1. Introduction
2. Material and Methods
2.1. Subject
2.2. Exercise Protocol
2.3. Sample Collection and Preservation
2.4. Analysis of Blood Biochemical Markers and Salivary Metabolites
2.5. Bioinformatics Analysis
- (i)
- The overall changes in salivary metabolites after exercise were examined by using principal component analysis (PCA) and hierarchical clustering heatmaps. PCA was used to identify patterns of variation among samples. Hierarchical clustering showed the relationships between different samples and metabolites based on the similarity in metabolite profiles.
- (ii)
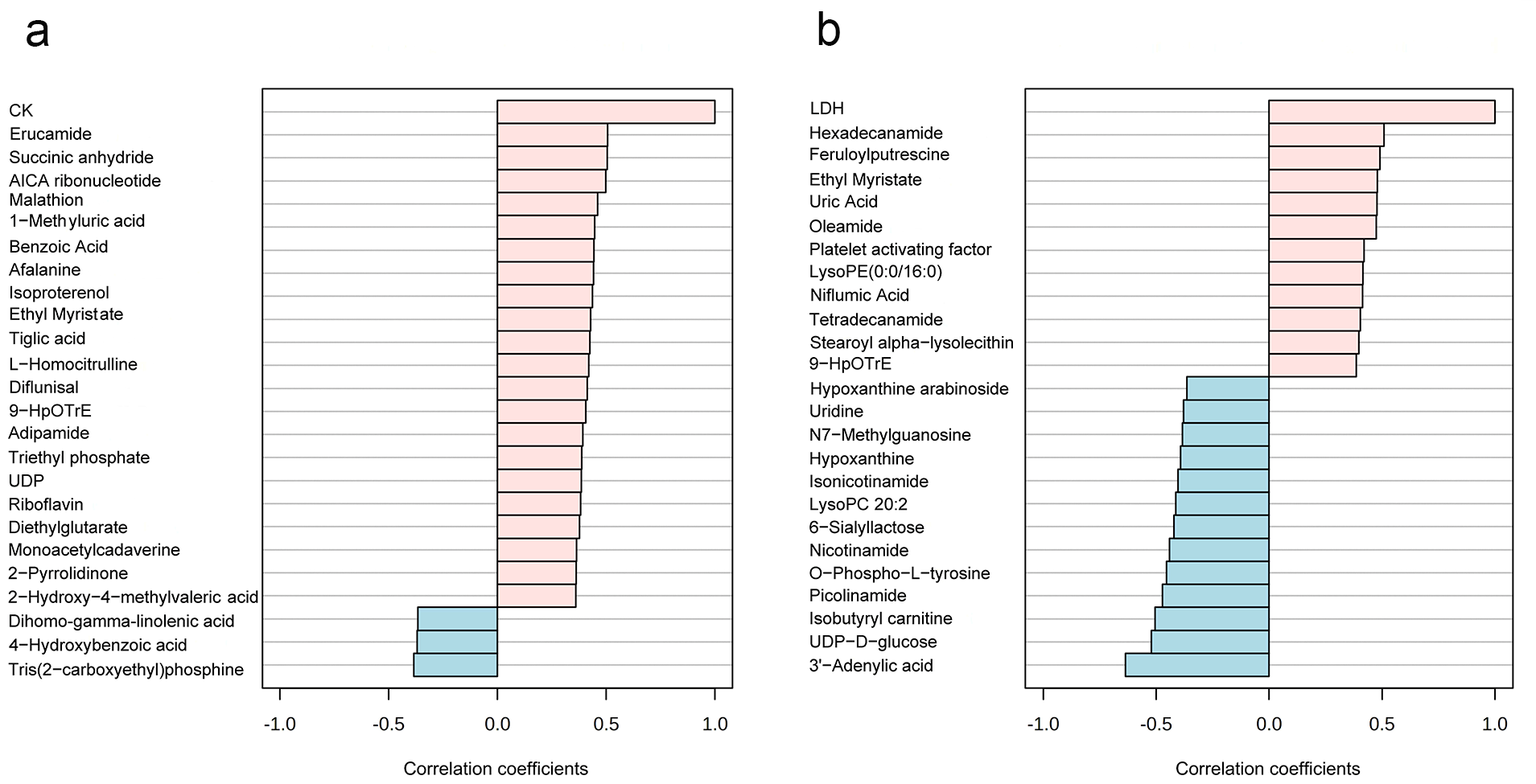
- The salivary metabolites strongly correlated with CK and LDH were identified by using the pattern search module, and they are two important blood biochemical indicators of EIMD. The high correlation between salivary metabolites and CK/LDH was significant for understanding the physiological response to exercise, developing non-invasive diagnostic methods for EIMD, and exploring the body’s metabolic adaptation mechanisms.
- (iii)
- Subsequently, differential metabolites after exercise were analyzed by using a paired t-test. The statistical test helped to determine which metabolites had significant changes between the Pre-Ex and Post-Ex groups. The enriched metabolic pathways were revealed by using the Kyoto Encyclopedia of Genes and Genomes (KEGG) module. KEGG is a comprehensive database that integrates genomic, chemical, and systemic functional information, which helped us to understand the biological functions and pathways associated with the differential metabolites.
- (iv)
- A discriminant model for classification was established using partial least squares discriminant analysis (PLS-DA). The potential salivary biomarkers for EIMD prediction were screened by the operating characteristic curve (ROC). Based on the area under the curve (AUC) ranking, potential salivary biomarkers for EIMD prediction were screened. The prediction performances of multiple salivary metabolites were evaluated by using random forest, which contributed to determining the optimal number of metabolites for more accurate EIMD prediction.
3. Results
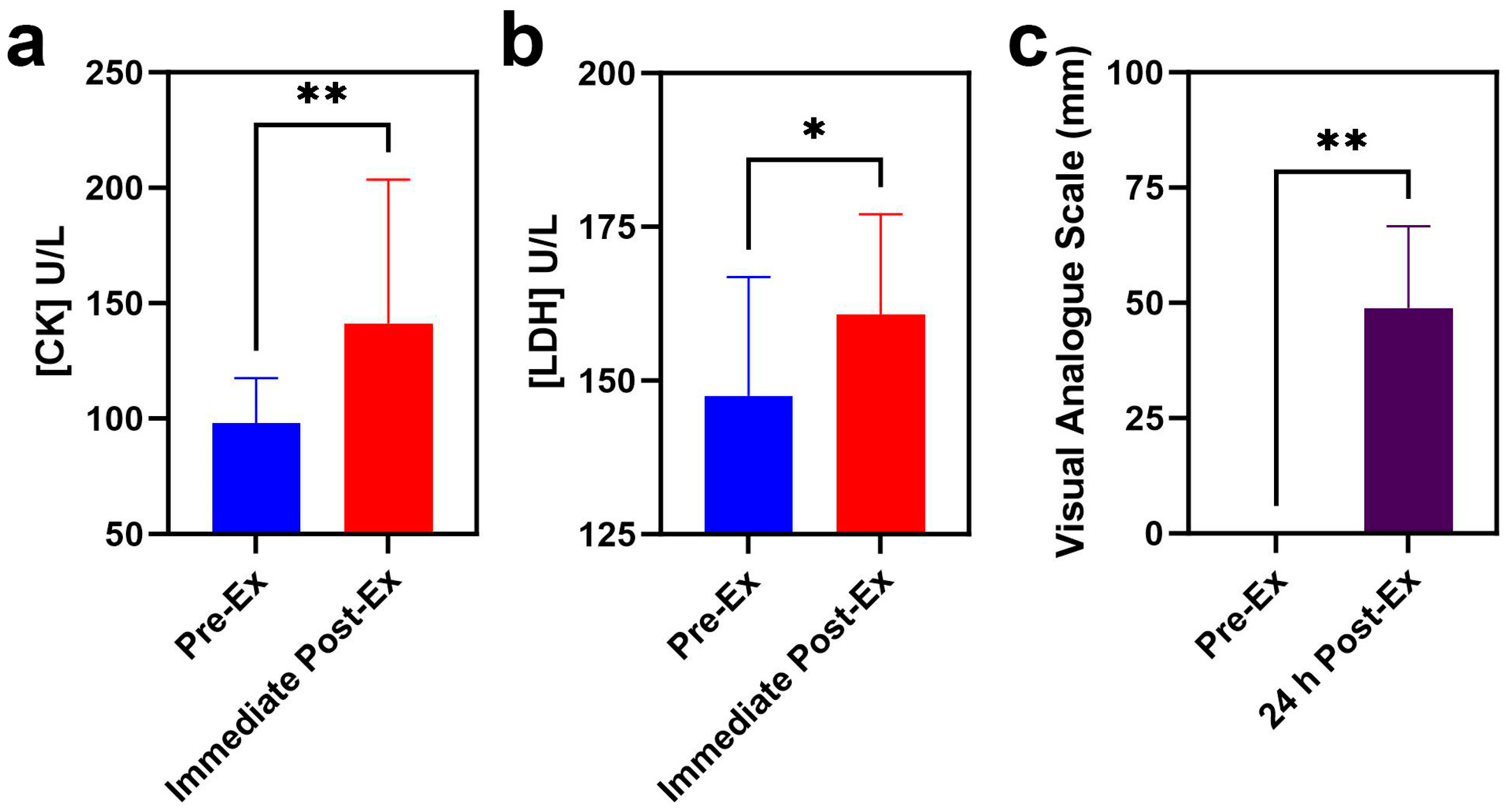
3.1. Blood Biochemical Indicators Analyses
3.2. Changes in Salivary Metabolites After High-Intensity Rowing Training
3.3. Potential Saliva Biomarker Screening for EIMD Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Saatmann, N.; Zaharia, O.P.; Loenneke, J.P.; Roden, M.; Pesta, D.H. Effects of Blood Flow Restriction Exercise and Possible Applications in Type 2 Diabetes. Trends Endocrinol. Metab. 2021, 32, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Hupin, D.; Roche, F.; Gremeaux, V.; Chatard, J.C.; Oriol, M.; Gaspoz, J.M.; Barthélémy, J.C.; Edouard, P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1262–1267. [Google Scholar] [CrossRef]
- Oja, P.; Memon, A.R.; Titze, S.; Jurakic, D.; Chen, S.T.; Shrestha, N.; Em, S.; Matolic, T.; Vasankari, T.; Heinonen, A.; et al. Health Benefits of Different Sports: A Systematic Review and Meta-Analysis of Longitudinal and Intervention Studies Including 2.6 Million Adult Participants. Sports Med. Open 2024, 10, 46. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Blain, G.M.; Rossman, M.J.; Park, S.Y.; Hart, C.R.; Trinity, J.D.; Gifford, J.R.; Sidhu, S.K.; Weavil, J.C.; Hureau, T.J.; et al. Acute high-intensity exercise impairs skeletal muscle respiratory capacity. Med. Sci. Sports Exerc. 2018, 50, 2409–2417. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef]
- Stozer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Cleak, M.J.; Eston, R.G. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br. J. Sports Med. 1992, 26, 267–272. [Google Scholar] [CrossRef]
- Petejova, N.; Martinek, A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: A critical review. Crit. Care 2014, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, C.B.; Clarkson, P.M. Exercise-induced muscle damage and adaptation. Sports Med. 1989, 7, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Eshaghi, H. Effect of omega-3 fatty acids supplementation on indirect blood markers of exercise-induced muscle damage: Systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2021, 9, 6429–6442. [Google Scholar] [CrossRef]
- Tota, Ł.; Piotrowska, A.; Pałka, T.; Morawska, M.; Mikuľáková, W.; Mucha, D.; Żmuda-Pałka, M.; Pilch, W. Muscle and intestinal damage in triathletes. PLoS ONE 2019, 14, e0210651. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Ford, L.; Wittmann, B.; Conner, J.; Wulff, J.; Mitchell, M.; Evans, A.M.; Toal, D.R. Global biochemical analysis of plasma, serum and whole blood collected using various anticoagulant additives. PLoS ONE 2021, 16, e0249797. [Google Scholar] [CrossRef]
- Lindsay, A.; Costello, J.T. Realising the Potential of Urine and Saliva as Diagnostic Tools in Sport and Exercise Medicine. Sports Med. 2017, 47, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, Z.; Kang, Y.; Sha, X.; Zhang, Y. Preliminary study on the evaluation of skeletal muscle damage in patients with diabetes mellitus using ultrasonic shear wave elastography. Altern. Ther. Health Med. 2024, 30, 314–317. [Google Scholar]
- Fulford, J.; Eston, R.G.; Rowlands, A.V.; Davies, R.C. Assessment of magnetic resonance techniques to measure muscle damage 24 h after eccentric exercise. Scand. J. Med. Sci. Sports 2015, 25, E28–E39. [Google Scholar] [CrossRef]
- Silver, F.H.; Kelkar, N.; Deshmukh, T. Use of vibrational optical coherence tomography to measure viscoelastic properties of muscle and tendon: A new method to follow musculoskeletal injury and pathology In vivo. J. Mech. Behav. Biomed. Mater. 2021, 119, 104479. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.V.A.; Alvarez, P.D.; Sena, A.F.D.; Serpa, T.K.; de Assis, M.G.; Pimenta, E.M.; Costa, H.A.; de Oliveira, M.N.S.; Cabido, C.E.T.; Veneroso, C.E. Relationship between infrared thermography and muscle damage markers in physically active men after plyometric exercise. J. Therm. Biol. 2022, 104, 103187. [Google Scholar]
- Ahmadi, S.; Sinclair, P.J.; Foroughi, N.; Davis, G.M. Electromyographic activity of the biceps brachii after exercise-induced muscle damage. J. Sports Sci. Med. 2007, 6, 461–470. [Google Scholar] [PubMed]
- Qu, C.; Wu, Z.; Xu, M.; Qin, F.; Dong, Y.; Wang, Z.; Zhao, J. Cryotherapy models and timing-sequence recovery of exercise-induced muscle damage in middle- and long-distance runners. J. Athl. Train. 2020, 55, 329–335. [Google Scholar] [CrossRef]
- Aminian-Far, A.; Hadian, M.R.; Olyaei, G.; Talebian, S.; Bakhtiary, A.H. Whole-body vibration and the prevention and treatment of delayed-onset muscle soreness. J. Athl. Train. 2011, 46, 43–49. [Google Scholar] [CrossRef]
- Al Rawi, N.; Elmabrouk, N.; Abu Kou, R.; Mkadmi, S.; Rizvi, Z.; Hamdoon, Z. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 2021, 125, 105018. [Google Scholar] [CrossRef]
- Ohshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, H.; Huang, R. A pilot study of the metabolomic profiles of saliva from female orthodontic patients with external apical root resorption. Clin. Chim. Acta 2018, 478, 188–193. [Google Scholar] [CrossRef]
- AlQallaf, H.; Hamada, Y.; Blanchard, S.; Shin, D.; Gregory, R.; Srinivasan, M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS ONE 2018, 13, e0200231. [Google Scholar] [CrossRef]
- Figura, M.; Friedman, A. In search of Parkinson’s disease biomarkers-is the answer in our mouths? A systematic review of the literature on salivary biomarkers of Parkinson’s disease. Neurol. Neurochir. Pol. 2020, 54, 14–20. [Google Scholar] [CrossRef]
- Chang, W.; Lin, C. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Eur. J. Oncol. Nurs. 2017, 29, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Alaee, M.; Amri, J.; Baghinia, M.R.; Rafiee, M. Serum and saliva concentrations of biochemical parameters in men with prostate cancer and benign prostate hyperplasia. Lab. Med. 2020, 51, 243–251. [Google Scholar] [CrossRef]
- Shah, V.S.; Pareikh, D.; Manjunatha, B.S. Salivary alpha-amylase-biomarker for monitoring type II diabetes. J. Oral Maxillofac. Pathol. 2021, 25, 441–445. [Google Scholar] [CrossRef]
- Shu, J.; Yu, H.; Du, H.; Zhang, J.; Zhang, K.; Li, X.; Xie, H.; Li, Z. Identification of N- and O-linked glycans recognized by AAL in saliva of patients with atrophic gastritis and gastric cancer. Cancer Biomark. 2018, 22, 669–681. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ren, J.; Xu, Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2018, 275, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Lien, H.; Liang, K.; Liu, S.; Lin, W.; Wu, S. In response to saliva pepsin detection and proton pump inhibitor response in suspected laryngopharyngeal reflux. Laryngoscope 2019, 129, E120. [Google Scholar] [CrossRef]
- Hayat, J.O.; Gabieta-Somnez, S.; Yazaki, E.; Kang, J.Y.; Woodcock, A.; Dettmar, P.; Mabary, J.; Knowles, C.H.; Sifrim, D. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut 2015, 64, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lang, X.; Wu, D.; He, Y.; Lan, C.; Xiao, X.; Wang, B.; Zou, D.; Wu, J.; Zhao, Y.; et al. Salivary pepsin as an intrinsic marker for diagnosis of sub-types of gastroesophageal reflux disease and gastroesophageal reflux disease-related disorders. J. Neurogastroenterol. Motil. 2020, 26, 74–84. [Google Scholar] [CrossRef]
- Alzharani, M.A.; Alshuwaier, G.O.; Aljaloud, K.S.; Al-Tannak, N.F.; Watson, D.G. Metabolomics profiling of plasma, urine and saliva after short term training in young professional football players in Saudi Arabia. Sci. Rep. 2020, 10, 19759. [Google Scholar] [CrossRef]
- Ra, S.G.; Maeda, S.; Higashino, R.; Imai, T.; Miyakawa, S. Metabolomics of salivary fatigue markers in soccer players after consecutive games. Appl. Physiol. Nutr. Metab. 2014, 39, 1120–1126. [Google Scholar] [CrossRef]
- Pitti, E.; Petrella, G.; Di Marino, S.; Summa, V.; Perrone, M.; D’Ottavio, S.; Bernardini, A.; Cicero, D.O. Salivary metabolome and soccer match: Challenges for understanding exercise induced changes. Metabolites 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Casamichana, D.; Barba, E.; Martín-García, A.; Ulloa, I.; Nakamura, F.Y.; Castellano, J. Comparison of the external load of professional goalkeepers in different weekly training sessions. Biol. Sport 2024, 41, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Grimson, S.; Brickley, G.; Smeeton, N.J.; Brett, A.; Abbott, W. The relationship between subjective wellness and external training load in elite english premier league goalkeepers and a comparison with outfield soccer players. Int. J. Sport Physiol. Perform. 2023, 18, 262–267. [Google Scholar] [CrossRef]
- Pokora, I.; Kempa, K.; Chrapusta, S.J.; Langfort, J. Effects of downhill and uphill exercises of equivalent submaximal intensities on selected blood cytokine levels and blood creatine kinase activity. Biol. Sport 2014, 31, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Lee, M.C.; Chang, C.; Chen, W.; Huang, W. Beneficial effects of a negative ion patch on eccentric exercise-induced muscle damage, inflammation, and exercise performance in badminton athletes. Chin. J. Physiol. 2020, 63, 35–42. [Google Scholar] [CrossRef]
- Devantier-Thomas, B.; Deakin, G.B.; Crowther, F.; Schumann, M.; Doma, K. The repeated bout effect of traditional resistance training on cycling efficiency and performance. Eur. J. Appl. Physiol. 2024, 124, 2005–2017. [Google Scholar] [CrossRef]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Exercise-induced muscle damage and recovery in young and middle-aged males with different resistance training experience. Sports 2019, 7, 132. [Google Scholar] [CrossRef]
- Ye, X.; Beck, T.W.; Wages, N.P. Reduced susceptibility to eccentric exercise-induced muscle damage in resistance-trained men is not linked to resistance training-related neural adaptations. Biol. Sport 2015, 32, 199–205. [Google Scholar] [CrossRef]
- Yusof, A.M.; Harun, M.N.; Nasruddin, F.A.; Syahrom, A. Rowing biomechanics, physiology and hydrodynamic: A systematic review. Int. J. Sports Med. 2022, 43, 577–585. [Google Scholar] [CrossRef]
- Benitez-Sillero, J.D.; Perez-Navero, J.; Tasset, I.; Castillo, M.G.-D.; Gil-Campos, M.; Tunez, I. Influence of intense exercise on saliva glutathione in prepubescent and pubescent boys. Eur. J. Appl. Physiol. 2009, 106, 181–186. [Google Scholar] [CrossRef]
- França, T.C.L.; Muniz-Santos, R.; Caetano, L.C.; Souza, G.; Goulart, H.F.; Assis, M.; Bottino, A.; Bassini, A.; Santana, A.E.G.; Prado, E.S.; et al. A sportomics soccer investigation unveils an exercise-induced shift in tyrosine metabolism leading to hawkinsinuria. Front. Nutr. 2023, 10, 1169188. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Schön, M.; Naviaux, J.C.; Monk, J.M.; Alchus-Laiferová, N.; Wang, L.; Straka, I.; Matejicka, P.; Valkovic, P.; Ukropec, J.; et al. Cerebrospinal fluid and plasma metabolomics of acute endurance exercise. FASEB J. 2022, 36, e22408. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma Metabolome Profiling of Resistance Exercise and Endurance Exercise in Humans. Cell Rep. 2020, 33, 108554. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Uric and 1-methyluric acids: Metabolic wastes or antiradical protectors? J. Phys. Chem. B 2011, 115, 15430–15438. [Google Scholar] [CrossRef]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- Simpson, R.J.; Bosslau, T.K.; Weyh, C.; Niemiro, G.M.; Batatinha, H.; Smith, K.A.; Krüger, K. Exercise and adrenergic regulation of immunity. Brain Behav. Immun. 2021, 97, 303–318. [Google Scholar] [CrossRef]
- Nag, A.C.; Carey, T.R.; Cheng, M. DNA synthesis in rat heart cells after injury and the regeneration of myocardia. Tissue Cell 1983, 15, 597–613. [Google Scholar] [CrossRef]




| Physical Indicators | Range | Average Value ± Standard Error |
|---|---|---|
| Height/cm | 168–186 | 177.6 ± 4.6 |
| Weight/kg | 58–86 | 72.3 ± 7.0 |
| BMI/kg·m−2 | 20.3–27.1 | 22.9 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Ding, J.; Wang, B.; Li, Y.; Wang, L.; Bo, S.; Ren, Q.; Luo, A. Salivary Metabolite Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. Metabolites 2025, 15, 405. https://doi.org/10.3390/metabo15060405
Yi Y, Ding J, Wang B, Li Y, Wang L, Bo S, Ren Q, Luo A. Salivary Metabolite Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. Metabolites. 2025; 15(6):405. https://doi.org/10.3390/metabo15060405
Chicago/Turabian StyleYi, Yue, Junjie Ding, Baoguo Wang, Yuxian Li, Liming Wang, Shumin Bo, Qiongqiong Ren, and Aiqin Luo. 2025. "Salivary Metabolite Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage" Metabolites 15, no. 6: 405. https://doi.org/10.3390/metabo15060405
APA StyleYi, Y., Ding, J., Wang, B., Li, Y., Wang, L., Bo, S., Ren, Q., & Luo, A. (2025). Salivary Metabolite Variation After High-Intensity Rowing Training and Potential Biomarker Screening for Exercise-Induced Muscle Damage. Metabolites, 15(6), 405. https://doi.org/10.3390/metabo15060405







