Abstract
Background/Objectives: In 4-octylphenol (4-OP), a toxic environmental pollutant with endocrine disruptive effect, the use of 4-OP causes pollution in the freshwater environment and poses risks to aquatic organisms. Common carps (Cyprinus carpio L.) live in freshwater and are experimental animals for studying the toxic effects of environmental pollutants on fish. Its heart is susceptible to toxicants. However, whether 4-OP has a toxic effect on common carp heart remains unknown. Methods: Here, we conducted a common carp 4-OP exposure experiment (carp treated with 17 μg/L 4-OP for 45 days), aiming to investigate whether 4-OP has a toxic effect on common carp hearts. We observed the microstructure and ultrastructure of carp heart and detected autophagy genes, mitochondrial fission genes, mitochondrial fusion genes, glycolytic enzymes, AMPK, ATPase, and oxidative stress factors, to investigate the molecular mechanism of 4-OP induced damage in common carp hearts. Results: Our results showed that 4-OP exposure caused mitochondrial damage, autophagy, and damage in common carp hearts. 4-OP exposure increased the levels of miR-144, and eight autophagy factors (Beclin1, RB1CC1, ULK1, LC3-I, LC3-II, ATG5, ATG12, and ATG13), and decreased the levels of four autophagy factors (PI3K, AKT, mTOR, and SQSTM1). Furthermore, 4-OP exposure induced the imbalance between mitochondrial fission and fusion and mitochondrial dynamics imbalance, as demonstrated by the increase in three mitochondrial fission factors (Mff, Drp1, and Fis1) and the decrease in three mitochondrial fusion factors (Mfn1, Mfn2, and Opa1). Moreover, excess 4-OP treatment caused energy metabolism disorder, as demonstrated by the reduction in four ATPase (Na+K+-ATPase, Ca2+Mg2+-ATPase, Ca2+-ATPase, and Mg2+-ATPase), elevation in four glycolysis genes (HK1, HK2, LDHA, and PGK1), reduction in glycolysis gen (PGAM2), and the elevation in energy-sensing AMPK. Finally, 4-OP treatment induced the imbalance between antioxidant and oxidant and oxidative stress, as demonstrated by the increase in oxidant H2O2, and the decreases in five antioxidant factors (CAT, SOD, T-AOC, Nrf2, and HO-1). Conclusions: miR-144 mediated autophagy by targeting PI3K, mTOR, and SQSTM1, and the miR-144/PI3K-AKT-mTOR/ULK1 pathway was involved in 4-OP-induced autophagy. Mff-Drp1 axis took part in 4-OP-caused mitochondrial dynamics imbalance, and mitochondrial dynamics imbalance mediated autophagy via Mfn2-SQSTM1, Mfn2/Beclin1, and Mff-LC3-II axes. Energy metabolism disorder mediated mitochondrial dynamics imbalance through the AMPK-Mff-Drp1 pathway. Oxidative stress mediated energy metabolism disorder via the H2O2-AMPK axis. Taken together, oxidative stress triggered energy metabolism disorder, induced mitochondrial dynamics imbalance, and caused autophagy via the H2O2-AMPK-Mff-LC3-II pathway. Our study provided references for the toxic effects of endocrine disruptor on common carp hearts, and provided a basis for assessing environmental pollutant-induced damage in common carp heart. We only studied the toxic effects of 4-OP on common carp, and the toxic effects of 4-OP on other fish species need to be further studied.
1. Introduction
4-Octylphenol (4-OP), a toxic endocrine disruptor with estrogenic activity, is used in the production of chemical industrial products, such as surfactants, adhesives, emulsifiers, rubber-processing chemicals, and fuel stabilizers, which resulted in 4-OP entering the freshwater environment. 4-OP was found in the raw wastewater (untreated influent wastewater) of five wastewater treatment plants in Saskatchewan, Canada; the concentration of 4-OP was 288.7–322.9 ng/L in one plant and 24.2–141.6 ng/L in another [1]. Surface water was collected from 14 sampling sites in the Three Gorges Reservoir Region of China, and 4-OP was detected in 10 sampling points, with concentrations ranging from 11 to 120 ng/L [2]. In Brazil, 4-OP was detected at concentrations of 80–124 ng/L in tap water and at concentrations of 5–17 μg/L in the Iraí River [3]. It is worrying that 4-OP in the environment entered and accumulated in aquatic organisms and humans, which may pose a potential threat to aquatic organisms and human health. In Taizhou, China, a 4-OP concentration of 9.50–10.87 ng/g was found in Coastal Mud Shrimp [4]. Among 96 fish samples from Terengganu River in Malaysia, 4-OP was found in 22 Arius sp. samples with concentrations of 10.52–26.48 ng/g [5]. In 59 human milk samples in Taiwan, China, 4-OP was found in 18 samples, and the 4-OP detection rate and 4-OP concentrations were 30.5% and 1.16–2.97 μg/kg, respectively [6]. Thus, we wanted to investigate the toxic mechanisms of freshwater fish in the 4-OP exposure condition.
Three studies reported that 4-OP had toxic effects on freshwater fish. 4-OP exposure reduced the survival rate of juvenile goldfish (Carassius auratus) [7], caused damage in primary hepatocytes derived from pearl mullet (Alburnus tarichi) [8], and in common carp gill [9]. However, whether 4-OP has negative effects on fish hearts remains unknown. In humans, exposure to environmental endocrine disruptors has been linked to an increased risk of cardiovascular disease [10]. Two research studies showed that the isomers of 4-OP had toxic effects on fish hearts. Exposure to the linear isomer of 4-OP, 4-nonylphenol (4-NP), resulted in a decreased heart rate in medaka (Oryzias latipes) [11]. The branched isomer of 4-OP, 4-tert-octylphenol (4-t-OP), was shown to reduce heart rate and induce cardiac damage and cardiovascular abnormalities in zebrafish (Danio rerio) [12]. Hence, we hypothesized that 4-OP exposure can cause cardiac damage in fish. Common carp is an omnivorous fish and a commonly eaten freshwater fish in daily life. Common carp is also a commonly used experimental animal in fish toxicology research. Therefore, we wanted to use common carp as a research object to investigate whether 4-OP exposure can cause cardiac injury in common carp.
The heart requires a continuous energy supply, and ATP serves as the primary energy source. ATP is hydrolyzed by ATPase to release energy, maintaining normal cardiac function. Research showed that toxic endocrine disruptors altered ATPase and disrupted energy metabolism in fish. Tao et al. (2020) demonstrated that monobutyl phthalate changed ATPase (Na+-K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase) and induced energy metabolism disturbance in zebrafish gills [13]. In common carp, cadmium (Cd) exposure resulted in altered activities of Na+-K+-ATPase, Ca2+-ATPase, Ca2+Mg2+-ATPase, and Mg2+-ATPase, and damaged heart [14]. However, whether 4-OP can cause ATPase activity changes in common carp remains unknown. Notably, AMPK, an energy sensor, plays an important role in regulating the heart’s energy metabolism. Mukherjee et al. (2022) reported that toxic endocrine disruptor nonylphenol exposure caused injury and altered AMPK in zebrafish livers [15]. However, it is unknown whether 4-OP can affect AMPK in common carp. Glycolysis is one of the basic pathways of energy metabolism and can provide energy to the heart. Research studies found that toxic endocrine disruptors caused poisoning in animals, and glycolysis factors were altered. In Cd-treated largemouth bass (Micropterus salmoides), the testis was injured, and lactate dehydrogenase A (LDHA) changed [16]. Bisphenol AF exposure caused changes in glycolysis enzymes hexokinase 1 (HK1) and hexokinase 2 (HK2), and inhibited glycolysis in zebrafish (Danio rerio) muscle [17]. Lipid deposition and phosphoglycerate kinase 1 (PGK1) change were observed in BPA-treated Rare Minnow Gobiocypris rarus livers [18]. In Cd-treated mice, phosphoglycerate mutase 2 (PGAM2) was altered and glycolysis was interrupted [19]. However, whether 4-OP can affect glycolysis in common carp remains unknown. Thereby, we hypothesized that 4-OP can induce energy metabolism disorder in terms of ATPase activity, AMPK, and glycolysis in common carp hearts.
Mitochondria are the most abundant organelle in cardiomyocytes, and most of the energy required by the heart is produced by mitochondria. The balance of mitochondrial dynamics is crucial for maintaining normal mitochondria and cardiac function. Research reported that toxic endocrine disruptors altered mitochondrial fission and mitochondrial fusion indicators and caused mitochondria damage in fish. In mono (2-ethylhexyl) phthalate (MEHP)-treated ctenopharyngodon idellus kidney (CIK) cells, mitochondrial fission factors (multitranche financing facility (Mff), dynamin-related protein 1 (Drp1)), mitochondrial fusion factors (mitofusin-1 (Mfn1), mitofusin-2 (Mfn2)), and optic atrophy 1 (OPA1) changed, and mitochondria were damaged [20]. After zebrafish (Danio rerio) were exposed to dibutyl phthalate, mitochondrial fission factor (Fis1) and fusion factors (Mfn1, Mfn2, and OPA1) were altered, as mitochondria damage was induced [21]. Liu et al. (2023) detected mitochondria fission factors (Drp1 and Mff) and mitochondrial fusion factors (Mfn1, Mfn2, and OPA1) in common carp hearts, and found mitochondria damage after Cd treatment [14]. However, whether 4-OP exposure can alter mitochondrial fission factors and mitochondrial fusion factors, and damage mitochondria in common carp heart, remains unclear. Therefore, we wanted to measure mitochondrial fission factors (Mff, Drp1, and Fis) and mitochondrial fusion factors (Mfn1, Mfn2, and OPA1).
Interestingly, oxidative stress occurred and energy homeostasis was dysregulated in zebrafish liver following nonylphenol exposure [15]. After endocrine disruptor 4-tert-butylphenol (4-tBP) exposure, oxidative stress occurred and mitochondria were damaged in common carp livers [22]. Thereby, we also wanted to explore if 4-OP exposure can induce oxidative stress in common carp. Several studies discovered that endocrine disruptors affected the oxidative indicator (hydrogen peroxide (H2O2)) and anti-oxidative indicators (catalase (CAT), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1(HO-1)), and induced oxidative stress in fish. 4-tBP treatment caused H2O2, CAT, SOD, and T-AOC changes and induced oxidative stress in common carp head kidneys [23]. Desai et al. (2023) demonstrated that Nrf2 altered and oxidative stress occurred in the brain of zebrafish treated with 4-nonylphenol [24]. In 1,2,3,7,8-polychlorinated dibenzo-p-dioxin-treated primary Atlantic cod hepatic cells, HO-1 changed and oxidative stress occurred [25]. It was reported that 4-OP exposure changed SOD and induced oxidative stress in primary cultured hepatocytes derived from pearl mullet (Alburnus tarichi) [8]. However, whether 4-OP exposure can induce oxidative stress in common carp is still unclear. Thus, we wanted to investigate whether 4-OP exposure can induce oxidative stress in common carp hearts by measuring oxidation (H2O2) and anti-oxidation (CAT, SOD, T-AOC, Nrf2, and HO-1) factors.
In common carp livers exposed to endocrine disruptor 2,4-di-tert-butylphenol, Xie et al. (2024) measured oxidative stress factors (CAT and SOD) and autophagy factors (Beclin1, microtubule-associated protein 1A/1B-light chain 3-I (LC3-I), microtubule-associated protein 1A/1B-light chain 3-II (LC3-II), autophagy-related 5 (ATG5), mechanistic target of rapamycin (mTOR), and sequestosome 1 (SQSTM1)), and found that oxidative stress and autophagy occurred [26]. Several studies found that autophagy was involved in the toxic mechanism of endocrine disruptors in human cell lines and fish. Mahemuti et al. (2018) showed that autophagy factors (RB1 inducible coiled-coil 1 (RB1CC1) and sequestosome 1 (SQSTM1)) altered, autophagy occurred, and cells were damaged in BPA-treated human fetal lung fibroblasts [27]. In MEHP-treated CIK cells, autophagy factors (Beclin1, autophagy activating kinase 1 (ULK1), LC3-II, autophagy-related 12 (Atg12), and autophagy-related 13 (Atg13)) altered, autophagy occurred, and cells were damaged [20]. In synthetic phenolic antioxidants-treated grass carp, autophagy factors (Beclin1, LC3-I, protein kinase B (AKT), phosphatidylinositol 3-kinase (PI3K), and mTOR) changed, and autophagy and damage occurred in livers [28]. After Cd treatment, autophagy factors (Beclin1, ULK1, LC3-I, LC3-II, autophagy-related 5 (ATG5), ATG12, mTOR, and SQSTM1) changed, inducing autophagy and damage in common carp hearts [14]. However, no studies have reported whether excessive 4-OP can trigger cardiac autophagy in fish. Therefore, we assumed that 4-OP can affect autophagy indicators and induce autophagy in fish. MicroRNAs (miRNAs), a class of endogenous, small, and non-coding single-stranded RNAs [29], can negatively regulate gene expression by targeting mRNA. miRNAs can be involved in the molecular mechanism of autophagy induced by endocrine disruptors via targeting autophagy genes in common carp [14,30]. A miRNA can participate in the physiological functions of an organism by targeting multiple genes. It is noted that our previous transcriptome sequencing analysis indicated that miR-144 was differentially upregulated in 4-OP-teated common carp (unpublished data, as shown in Table S1). We further predicted that three autophagy genes (PI3K, mTOR, and SQSTM1) were target genes of miR-144 using a bioinformatics website (http://www.bioinformatics.com.cn/, accessed on 10 January 2025). Accordingly, we wanted to study whether miR-144 was involved in the molecular mechanism of 4-OP-caused autophagy in common carp hearts via targeting PI3K, MTOR, and SQSTM1.
In summary, we wanted to investigate the effect of 4-OP exposure on common carp, to explore the complex mechanisms of 4-OP-induced cardiotoxicity from the perspectives of ATPases, AMPK, glycolysis enzymes, mitochondrial dynamics, OS, autophagy, and miR-144. Our study will offer novel foundation knowledge on assessing the risks of 4-OP exposure and investigating endocrine disruptor-induced toxicity in common carp.
2. Materials and Methods
2.1. Animal Ethics and Animal Sources
All procedures in this study were approved by the Animal Care and Use Committee of Northeast Agricultural University (No. NEAUEC20240229). Eighty-one healthy common carp (weight 78.57 ± 5.99 g and body length 14.24 ± 0.66 cm) were purchased from the Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences (Harbin, China).
2.2. Animal Rearing and Experimental Design
2.2.1. Animal Rearing
The carp were randomly assigned to 9 tanks (9 per tank), each containing 90 L of dechlorinated tap water. Following a 15-day acclimation period, a 4-OP exposure experiment was conducted. The fish were reared under the following conditions: water temperature at 24 ± 1 °C, pH of 7.2 ± 0.2, dissolved oxygen concentration of 7.2 ± 0.4 mg/L, and a photoperiod of 12 h light/12 h dark. Oxygen was delivered by an oxygen pump. One-third of the water and sponge filters were replaced daily. The carp were fed a commercial diet (Shuyang Wanbing Pet Supplies Co., Ltd., Xingtai, China) twice daily at 8:00 and 17:00.
2.2.2. Experimental Design
The 9 tanks (9 fish per tank) were randomly assigned to 3 groups (3 tanks per group): the control group (untreated dechlorinated tap water), the solvent control group (anhydrous ethanol-supplemented dechlorinated tap water, 33.3 μL/L anhydrous ethanol), and the 4-OP group (4-OP stock solution-supplemented dechlorinated tap water,17 μg/L 4-OP and 33.3 μL/L anhydrous ethanol). The 4-OP concentration (17 μg/L) was set based on the maximum environmental concentration reported in the freshwater environment by Wacheski et al. (2021) [3]. A 4-OP stock solution (510 mg/L) was prepared by dissolving 15.3 mg of 4-OP in 30 mL of anhydrous ethanol. The treatments were as follows: 3 mL of anhydrous ethanol was added to 90 L of water in each tank of the solvent control group, while 3 mL of the 4-OP stock solution was added to 90 L of water in each tank of the 4-OP group. Following the 45-day 4-OP exposure experiment, the carps were euthanized with anesthetic tricaine methane-sulfonate (MS-222, HY-W011777, MedChemExpress, Monmouth Junction, NJ, USA). The anesthetic solution was prepared by dissolving MS-222 in distilled water and buffering to pH 7.0 with sodium bicarbonate. After the fish were euthanized, the heart tissues were taken out immediately.
The heart tissues obtained in the solvent control group were fixed in 4% paraformaldehyde for microstructural observation. The heart tissues obtained in the control and 4-OP groups were divided into four parts for subsequent experiments: the first part was fixed in 4% paraformaldehyde for microstructural observation; the second part was fixed in 2.5% glutaraldehyde solution and was stored at 4 °C for transmission electron microscopy (TEM); the third part was used for tissue homogenate for assay kits; the last part was immediately frozen in liquid nitrogen and stored at −80 °C for quantitative real-time PCR (qRT-PCR) and western blot.
2.3. Observation of the Heart Microstructure with Hematoxylin and Eosin Staining
Heart tissues fixed in 4% paraformaldehyde were taken out and rinsed under running water to remove residual fixative [31]. The samples were dehydrated with gradient ethanol (30%, 50%, 70%, 80%, 90%, 95%, and 100%), embedded in paraffin, and sliced into 2–4 μm using microtome (Jinhua Wireless Power Plant, Jinhua, China). The slices obtained were deparaffinized and stained with hematoxylin and eosin (H&E), followed by dehydration and mounting. Finally, the stained slices were scanned using a digital slice scanning system (Shandong Winmedic Technology Co., Ltd., Jinan, China), and microstructural images were visualized and captured using the software ZYFViewer (version 1.0; Shandong Winmedic Technology Co., Ltd., Jinan, China).
2.4. Observation of the Heart Ultrastructure with Transmission Electron Microscopy
Heart tissues fixed in 2.5% glutaraldehyde were taken out and rinsed three times (15 min each) with 0.1 M phosphate buffer (pH 7.2). The tissues were fixed again with 1% osmium acid at room temperature for 2 h, followed by three time rinses (15 min each) with 0.1 M phosphate buffer (pH 7.2). The tissues were dehydrated in a graded ethanol series (50%, 70%, 90%, and 100%, 20 min each) and were incubated twice in acetone (10 min each). Subsequently, the samples were gradually permeated with a gradient mixture of pure acetone and embedded solution (1:1 for 40 min; 1:2 for 1.2 h; and 1:3 for 12 h). The tissues were embedded and sliced into 60–80 nm slices using an ultra-thin slicer (LR604, RMC company, San Diego, CA, USA). The slices were double-stained with uranyl acetate and lead citrate. Finally, the heart ultrastructure was observed using a transmission electron microscope (gem-1200es, Tokyo Electronic and Optical Laboratory, Tokyo, Japan).
2.5. Determination of Oxidative Stress Markers and ATPase Activities with Assay Kits
Heart tissue (0.1 g) was mixed with physiological saline (0.9 mL) in 1.5 mL tubes and homogenized at 4 °C using a frozen grinder (Hangzhou Suizhen Biotechnology Co., Ltd., Hangzhou, China) to prepare a 10% tissue homogenate. The grinding program was set as follows: 1 min of grinding at 6.5 m/s; 1 min of standing; followed by another 1 min of grinding at 6.5 m/s. The homogenate was then centrifuged at 620 RCF at 4 °C for 10 min. using a refrigerated centrifuge (Mikro 200R, Hettich, Kirchlengern, Germany). The supernatant was collected for the determination of four ATPase activities with ATPase assay kit (A016-2, Na+K+-ATPase, Ca2+Mg2+-ATPase, Ca2+-ATPase, and Mg2+-ATPase), of H2O2 content with H2O2 assay kit (A064-1), of CAT activity with CAT assay kit (A007-1), of SOD activity with SOD assay kit (A001-1), and of T-AOC content with T-AOC assay kit (A001-1). The five assay kits were purchased from Nanjing Jiancheng Biotechnology Research Institute (Nanjing, China). All assays were performed in triplicate (n = 3).
For the ATPase assay, ATPase catalyzes the hydrolysis of ATP to produce inorganic phosphate, and absorbance was measured at 660 nm using a microplate reader (Molecular Devices Co., Ltd., Shanghai, China). For the H2O2 assay, H2O2 reacts with molybdic acid to form a compound, and absorbance was measured at 405 nm. For the CAT assay, CAT decomposes H2O2, and the reaction is halted by ammonium molybdate being added to form a yellowish compound, and the absorbance was measured at 405 nm. For the SOD assay, SOD inhibits superoxide anions, leading to the formation of nitrite, and the absorbance was measured at 550 nm. For the T-AOC assay kit, the antioxidant reduces Fe3+ to Fe2+, Fe2+ and phenanthroline derivatives form a stable compound, and the absorbance was measured at 520 nm.
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis of Gene Expression
All primers in our experiment were synthesized by Sangon Bioengineering Co., Ltd. (Shanghai, China). The genes detected included 2 internal reference genes U6 (for miR-144) and β-Actin (for mRNA), along with 12 autophagy factors (PI3K, AKT, mTOR, SQSTM1, Beclin1, LC3-I, LC3-II, ULK1, ATG5, ATG12, ATG13, and RB1CC1), 6 mitochondrial dynamics factors (Mff, Drp1, Fis1, Mfn1, Mfn2, and Opa1), AMPK, 5 glycolysis factors (HK1, HK2, LDHA, PGK1, and PGAM2), and 2 oxidative stress factors (Nrf2 and HO-1). The primer sequence information is shown in Table 1.

Table 1.
Primer sequences for qRT-PCR.
Heart tissues stored at −80 °C were used for total RNA extraction. The steps for extracting total RNA were as follows: 0.1 g of the tissue was placed into 1.5 mL EP tubes with 1 mL of Trizol reagent (Takara, Kyoto, Japan) and was fully ground at 4 °C using a frozen grinder. The grinding program was set as follows: 1 min of grinding at 6.5 m/s; 1 min of standing; followed by another 1 min of grinding at 6.5 m/s. 400 μL of supernatant was collected and 400 μL of chloroform was added, and the mixture was shaken vigorously and kept at room temperature for 10 min. The mixture was then centrifuged at 14,480 RCF at 4 °C for 15 min using a refrigerated centrifuge (Mikro 200R, Hettich, Kirchlengern, Germany). 400 μL of supernatant was collected and 400 μL of isopropyl alcohol was added. The mixture was inverted gently and kept at room temperature for 10 min, followed by centrifugation at 14,480 RCF at 4 °C for 15 min. The supernatant was discarded, and 1 mL of cold 75% DEPC ethanol was added to precipitate. The mixture was shaken gently and was centrifuged at 10,060 RCF at 4 °C for 10 min. The supernatants were discarded, and the precipitate (the total RNA) was collected. RNA purity was assessed using microspectrophotometer (Hangzhou Allsheng Instrument Co., Ltd., Hangzhou, China). Our obtained ratio of OD260/OD280 was between 1.8 and 2.1, meaning that our RNA purity was reliable.
Total RNA was reverse-transcribed into cDNA with miRcute Plus miRNA First-Strand cDNA kit (B532451, Tiangen Biotechnology Co., Ltd., Beijing, China) and mRNA reverse transcription kit (BL696A, Biosharp, Beijing, China). For miRNA, a 20 μL reaction mixture was prepared containing 10 μL of 2 × miRNA RT Reaction Buffer, 2 μL of miRNA RT Enzyme Mix, and 8 μL of dissolved RNA. For mRNA, a 20 μL reaction mixture was prepared containing 4 μL of 5 × Reaction Mix, 3 μL of Supreme Enzyme Mix, and 13 μL of dissolved RNA. Reverse transcription was performed using a gradient PCR apparatus (Hangzhou, China). The reverse transcription reaction program for miRNA was set as follows: 42 °C for 60 min and 95 °C for 3 min. The reverse transcription reaction program for mRNA was set as follows: 25 °C for 10 min, 55 °C for 15 min, and 85 °C for 5 min. Subsequently, qRT-PCR reaction mixtures were prepared. For miRNA, a 10 μL qRT-PCR reaction mixture was prepared containing 5 μL of 2 × miRcute microRNA Premix (Tiangen Biotechnology Co., Ltd., Beijing, China), 0.2 μL of Forward Primer, 0.2 μL of Reverse Primer, 1 μL of cDNA, and 3.6 μL of RNase-free ddwater. For mRNA, a 10 μL qRT-PCR reaction mixture was prepared containing 5 μL of FastStart Universal SYBR Green Master Kit (Roche Diagnostic GmbH, Mannheim, Germany), 0.3 μL of forward primer, 0.3 μL of reverse primer, 1 μL of cDNA, and 3.4 μL of RNase-free water [32]. The reactions were performed using QuantStudio®3 real-time PCR instrument (Roche, Basel, Switzerland). Relative mRNA expression was calculated using the 2 −ΔΔCT method [15,31]. The experiments were performed in triplicate (n = 3).
2.7. Western Blot Analysis of Protein Expression
A 1 mL lysis was prepared by mixing 990 μL of western and IP lysis buffer (Beyotime Biotechnology, Nantong, China) with 10 μL of protease inhibitor phenylmethylsulfonyl fluoride (PMSF; Beyotime Biotechnology Nantong, China). Heart tissues stored at −80 °C were used for total protein extraction. The steps for extracting total protein were as follows: 0.1 g of tissue was paced into 1.5 mL EP tubes with 1 mL prepared lysis and was fully ground at 4 °C using a frozen grinder. The grinding program was set as follows: 1 min of grinding at 6.5 m/s; 1 min of standing; followed by another 1 min of grinding at 6.5 m/s. The lysate obtained was centrifuged at 14,480 RCF at 4 °C for 10 min. Protein concentration in the supernatant was determined with a BCA protein concentration assay kit (Beyotime Biotechnology, Nantong, China) in order to calculate the protein loading volume for SDS-PAGE. 600 μL of the supernatant was transferred in new 1.5 mL EP tubes and mixed with 150 μL of SDS-PAGE sample loading buffer (Beyotime Biotechnology, Nantong, China). The mixture was boiled at 98 °C for 10 min to denature proteins and the total protein was obtained.
The preparation involved 4% stacking gels and 12% resolving gels. The protein loading volume was calculated based on the measured protein concentration to perform gel electrophoresis. The conditions for electrophoresis were 80 V for 30 min, followed by 120 V for 1 h and 40 min. After effective separation by molecular weight, proteins on the gels were electroblotted onto PVDF membranes at a constant current (200 mA) using Tris-glycine buffer supplemented with 20% methanol. The membrane was then blocked in 5% non-fat dry milk at 37 °C for 2 h and was subsequently incubated with three primary antibodies (anti-β-actin, anti-SQSTM1, and anti-Beclin1) at 4 °C overnight. Anti-β-actin (WL01372, the dilution ratio is 1:500) and anti-Beclin1 (WL02508, the dilution ratio is 1:500) were from Wanleibio (Shenyang, China). Anti-SQSTM1 (GTX100685, the dilution ratio is 1:500) was bought from GeneTex (San Antonio, TX, USA). The membranes were washed three times (15 min each) with TBST (Servicebio, Wuhan, China), followed by incubation with HRP-conjugated goat anti-rabbit IgG secondary antibody (sc-2004, the dilution ratio is 1:5000; Santa Cruz, CA, USA) at room temperature for 1 h. The membranes were washed three times (15 min each) in TBST. BeyoECL Star (Beyotime Biotechnology, Nantong, China) was dropped on the membranes [33], and protein bands were visualized and captured using Azure c300 chemiluminescence imaging system (Azure Biosystems, Dublin, CA, USA). The gel images were placed as Supplemental Material, as shown in Figure S1. The relative band intensities were quantified using ImageJ software (Version 1.53t) and β-actin was used as the internal reference protein. The experiments were performed in triplicate (n = 3).
2.8. Statistical Analysis
The obtained data from assay kits, qRT-PCR, and western blot in the control and 4-OP groups were statistically analyzed. IBM SPSS Statistics (version 25, Chicago, IL, USA) was used to analyze the obtained data. The Shapiro–Wilk test was used to assess the normality of the data. Levene’s test was used to verify homogeneity of variance. Subsequently, an independent samples t-test was used to analyze the significant differences. Figures were plotted using GraphPad Prism (version 10.0, GraphPad Software, Inc., La Jolla, CA, USA). Data were presented as mean ± standard deviation (SD). * Represented a significant difference (p < 0.05). ** Represented an extremely significant difference (p < 0.01)
3. Results
3.1. The Effect of 4-OP Exposure on Heart Tissue Microstructure
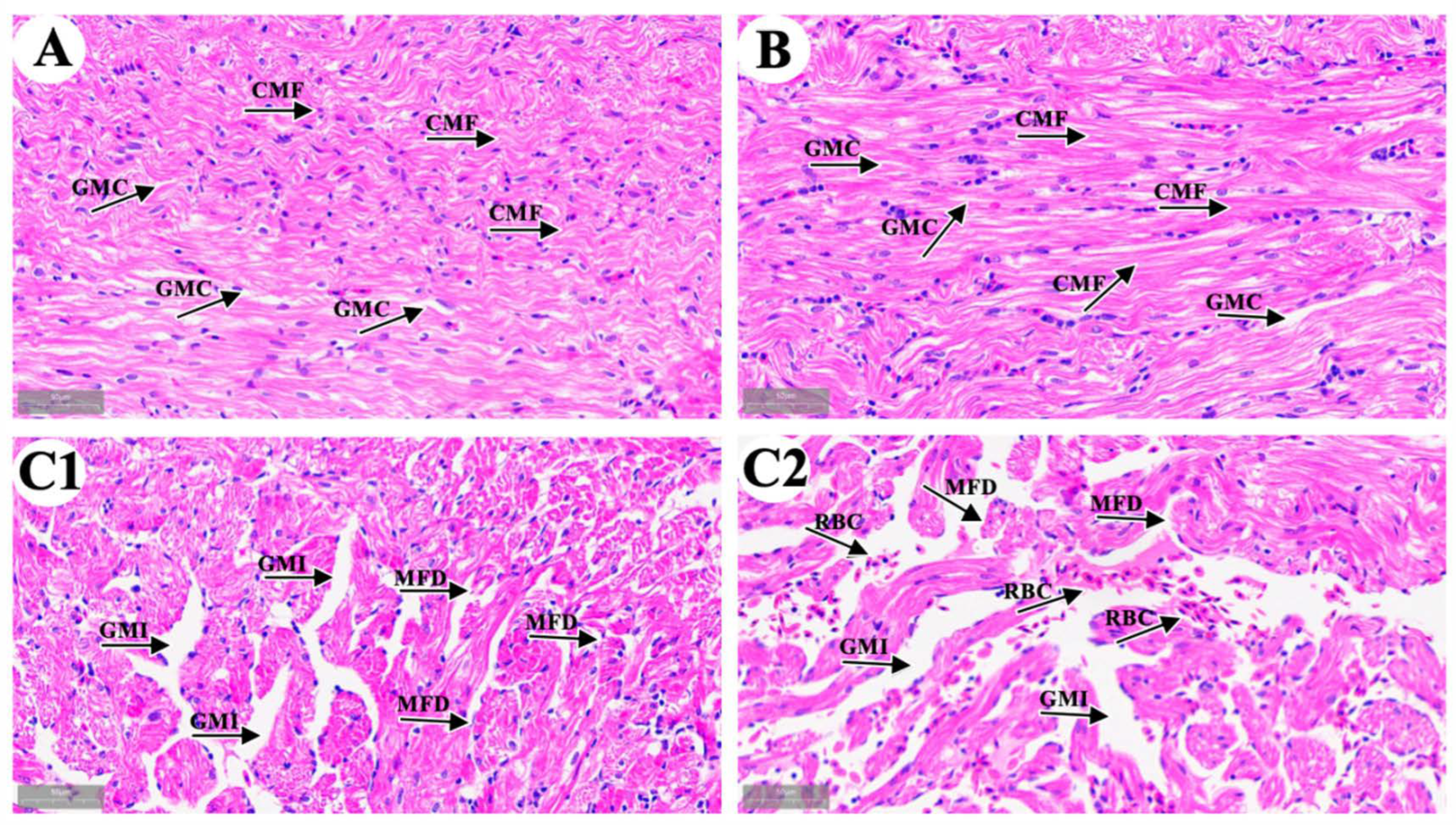
We observed cardiac microstructure in the control group (Figure 1A), the solvent control group (Figure 1B), and the 4-OP group (Figure 1(C1,C2)). In both the control and solvent groups, longitudinal sections of cardiac muscle fibers (CMF) were striped with an orderly arrangement, and the gaps between myocardial fibers (GMC) were uniform. The observation results in the solvent control group indicated that 33.3 μL/L anhydrous ethanol had no harmful effect on common carp hearts. In the 4-OP group, the cardiac muscle fibers were disrupted and disarranged (MFD), and gaps between myocardial fibers were increased (GMI). In addition, as shown in Figure 1(C2), the accumulation of red blood cell (RBC) was observed between myocardial fibers. The observation results in the 4-OP group indicated that exposure to 17 μg/L 4-OP induced cardiac damage in common carp.
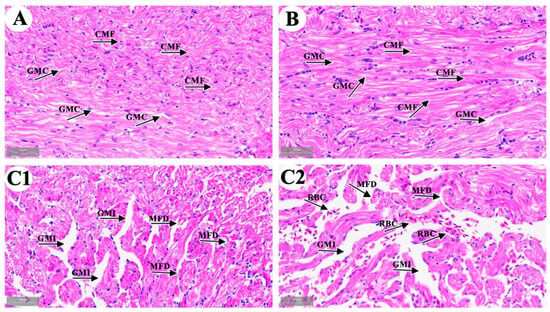
Figure 1.
Representative images of common carp heart microstructure. (A) control group; (B) solvent control group; (C1,C2) 4-OP group. (A,B,C1,C2) (×40). CMF: cardiac muscle fibers; GMC: gaps between myocardial fibers; MFD: myocardial fibers disrupted and disarranged; GMI: gaps between myocardial fibers increased; RBC: red blood cells.
3.2. The Effect of 4-OP Exposure on Heart Tissue Ultrastructure
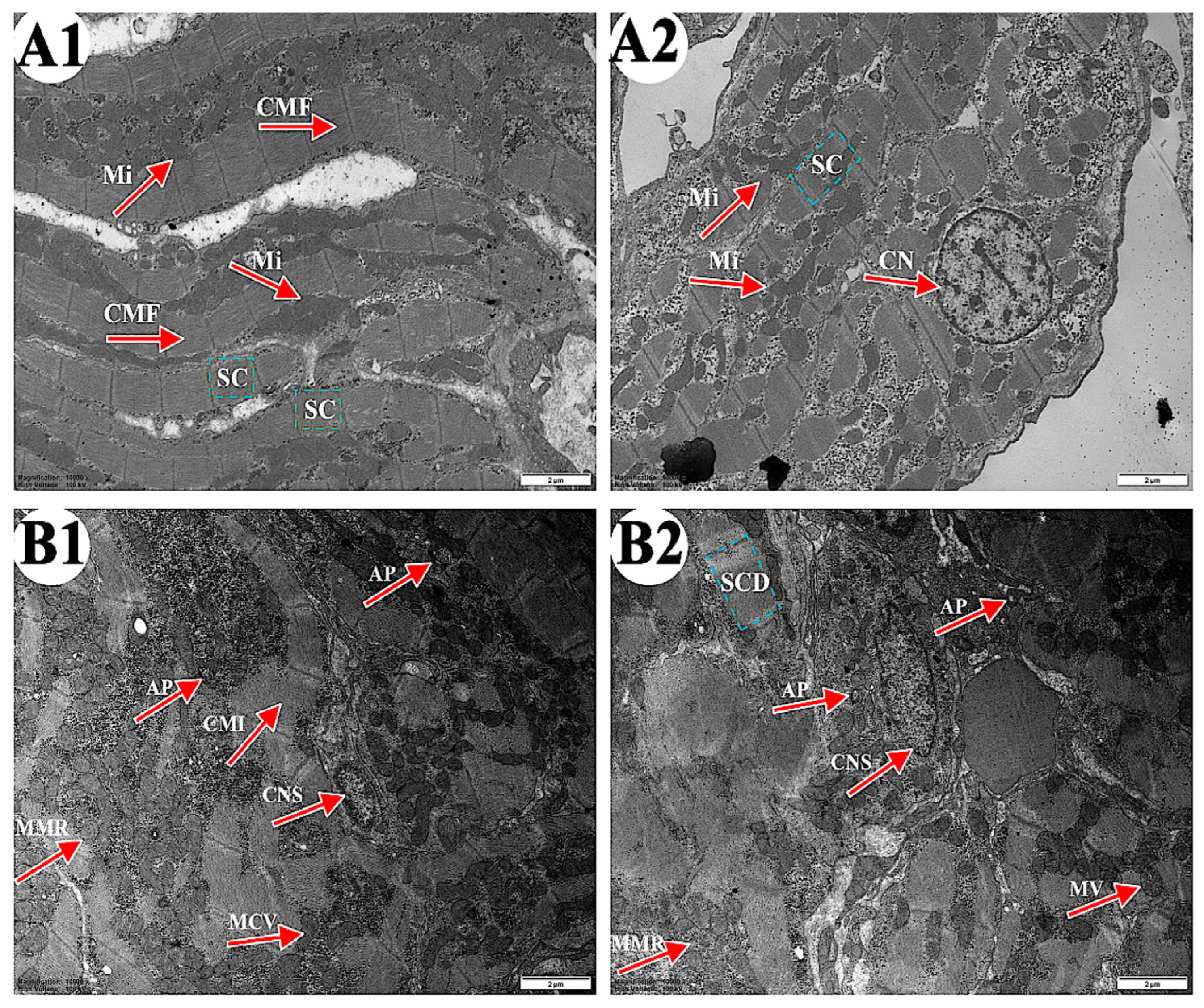
As shown in Figure 2, the ultrastructure of carp heart in the control and the 4-OP groups were observed. In the control group (Figure 2(A1,A2)), cardiac muscle fibers (CMF) were arranged in an orderly manner and cardiac sarcomere (CS) displayed a well-organized arrangement. Mitochondria (Mi) were arranged along cardiac muscle fibers and the nucleus of cardiomyocyte nucleus (CN) were rounded. In the 4-OP group (Figure 2(B1,B2)), cardiac muscle fiber appeared irregular (CMI) in arrangement, and cardiac sarcomere was disorganized (CSD). Moreover, autophagosomes (AP), mitochondrial membrane rupture (MMR), mitochondrial cristae vague (MCV), mitochondrial vacuole (MV), and cell nuclei shrink (CNS) were observed.
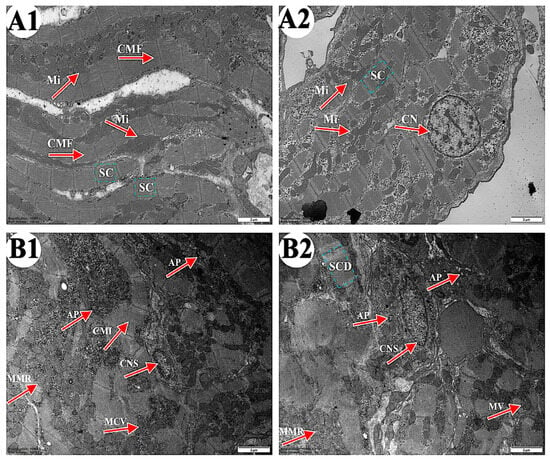
Figure 2.
Representative images of common carp heart ultrastructure. (A1,A2) control groups (×10,000). (B1,B2) 4-OP groups (×10,000). Magnification: ×15,000. CMF: cardiac muscle fiber; CS: cardiac sarcomere; Mi: mitochondria; CN: cardiomyocyte nucleus; CMI: cardiac muscle fiber irregular; AP: autophagosome; CSD: cardiac sarcomere disorganized; MMR: mitochondrial membrane rupture; MCV: mitochondrial cristae vague; MV: mitochondrial vacuole; CNS: cell nuclei shrink.
3.3. The Effect of 4-OP Exposure on Autophagy Factors
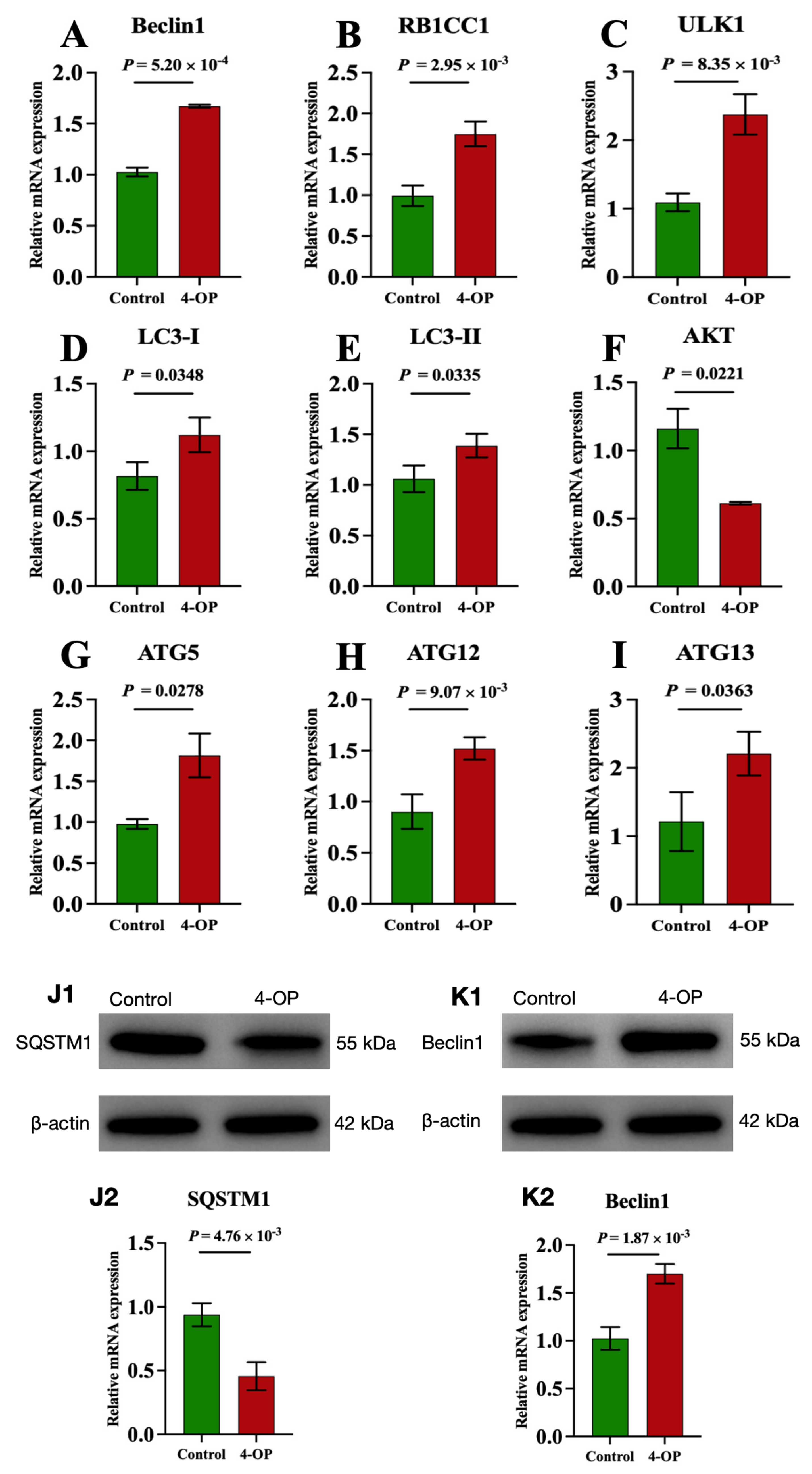
We investigated the effect of 4-OP on autophagy by measuring mRNA levels of nine autophagy genes (AKT, Beclin1, LC3-I, LC3-II, ULK1, ATG5, ATG12, ATG13, and RB1CC1) and two protein levels of autophagy factors (SQSTM1 and Beclin1), as shown in Figure 3A–K. At the mRNA level, our results revealed that compared with the control group, the mRNA levels of Beclin1 (Figure 3A), RB1CC1 (Figure 3B), ULK1 (Figure 3C), and ATG12 (Figure 3H) in the 4-OP group were extremely significantly (p < 0.01) increased, mRNA levels of LC3-I (Figure 3D), LC3-II (Figure 3E), ATG5 (Figure 3G), and ATG13 (Figure 3I) were significantly (p < 0.05) increased, while the mRNA level of AKT (Figure 3F) was significantly (p < 0.05) decreased. At the protein level, we found an extremely significant (p < 0.01) decrease in SQSTM1 (Figure 3(J1,J2)) and an extremely significant (p < 0.01) increase in Beclin1 (Figure 3(K1,K2)) following 4-OP treatment.
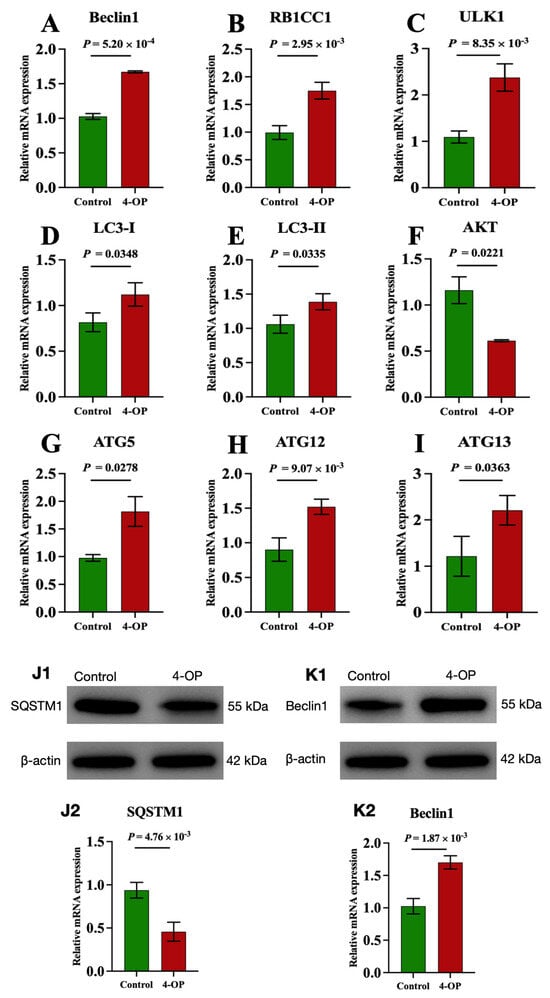
Figure 3.
mRNA and protein expressions of autophagy factors in common carp hearts. (A) mRNA expression of Beclin1; (B) mRNA expression of RB1CC1; (C) mRNA expression of ULK1; (D) mRNA expression of LC3−I; (E) mRNA expression of LC3−II; (F) mRNA expression of AKT; (G) mRNA expression of ATG5; (H) mRNA expression of ATG12; (I) mRNA expression of ATG13. (J1,K1) Representative images of the protein SQSTM1 and Beclin1, respectively; (J2,K2) Quantization results of protein expression of SQSTM1 and Beclin1, respectively. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
3.4. The Effect of 4-OP Exposure on miR-144 and Its Three Target Genes
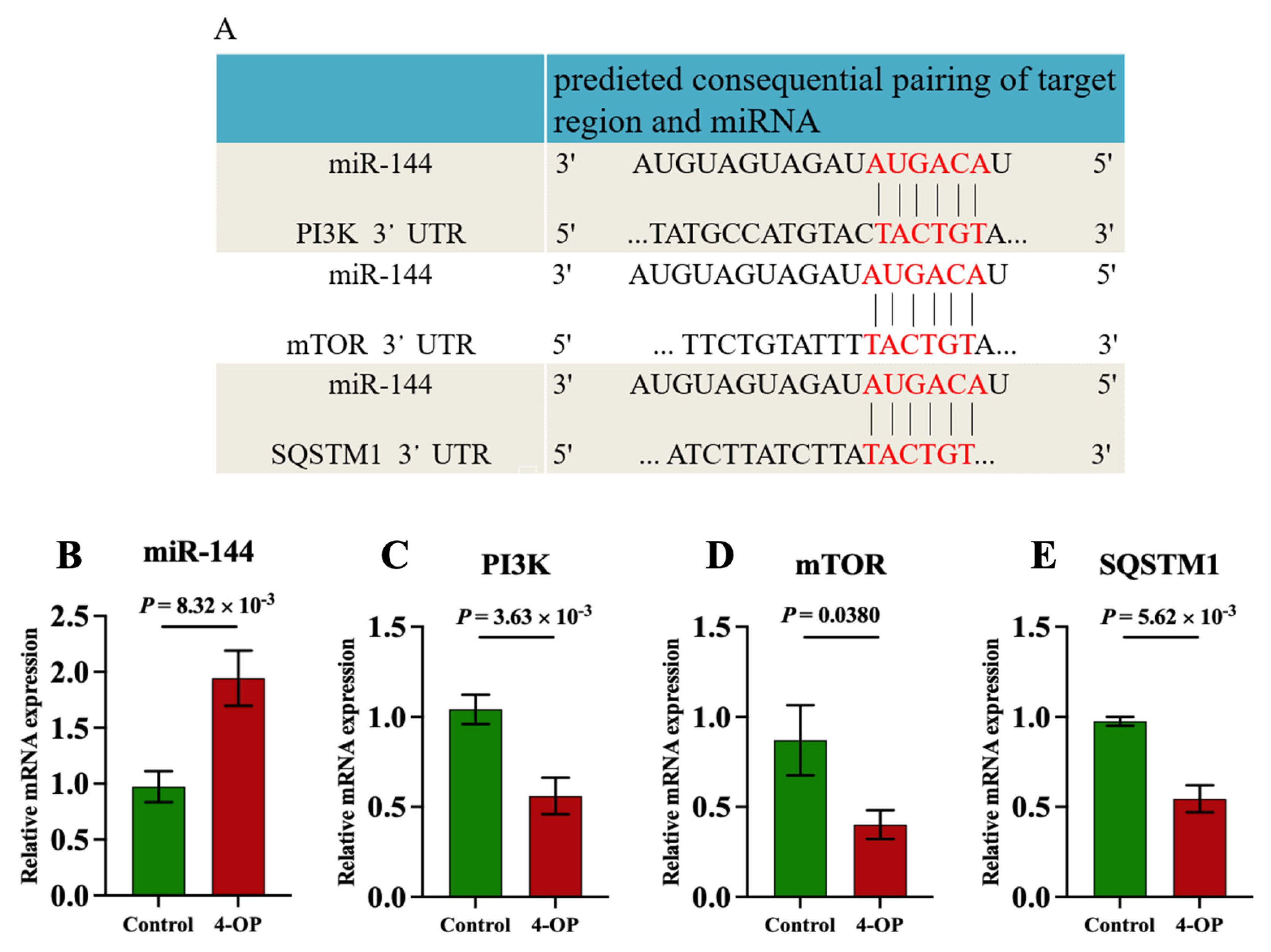
Our previous transcriptome sequencing analysis revealed that miR-144 was differentially upregulated, and PI3K, mTOR, and SQSTM1 were differentially downregulated genes following 4-OP exposure. Furthermore, a bioinformatics website (http://www.bioinformatics.com.cn/, accessed on 10 January 2025) was used to predict the potential target genes of miR-144. As shown in Figure 4A, the same six binding sites were identified between miR-144 and the 3′ untranslated regions (3′UTR) of PI3K, mTOR, and SQSTM1, indicating that PI3K, mTOR, and SQSTM1 were target genes of miR-144. Moreover, we further validated that PI3K, mTOR, and SQSTM1 were target genes of miR-144 at transcription levels (Figure 4B–E). The results showed that compared with the control group, the mRNA level of miR-144 (Figure 4B) was extremely significantly (p < 0.01) upregulated in the 4-OP group. Compared with the control group, the mRNA level of mTOR (Figure 4D) in the 4-OP group was significantly (p < 0.05) downregulated, and the mRNA levels of PI3K (Figure 4C) and SQSTM1 (Figure 4E) in the 4-OP group were extremely significantly (p < 0.01) downregulated.
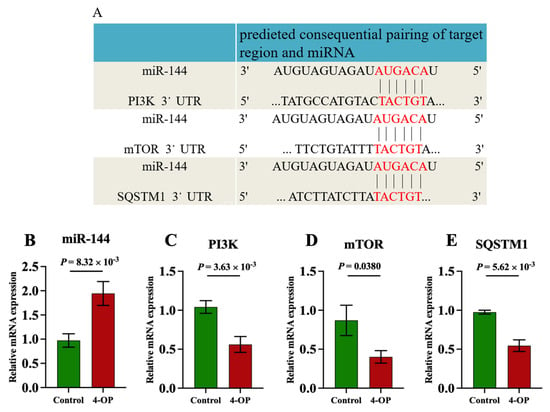
Figure 4.
Effects of 4−OP exposure on miR-144 and its three target gene expressions. (A) The potential binding site of miR−144 on 3′UTR of PI3K, mTOR, and SQSTM1. (B) mRNA level of miR−144. (C) mRNA level of PI3K. (D) mRNA level of mTOR. (E) mRNA level of SQSTM1. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
3.5. The Effect of 4-OP Exposure on Mitochondrial Fission and Mitochondrial Fusion Factors
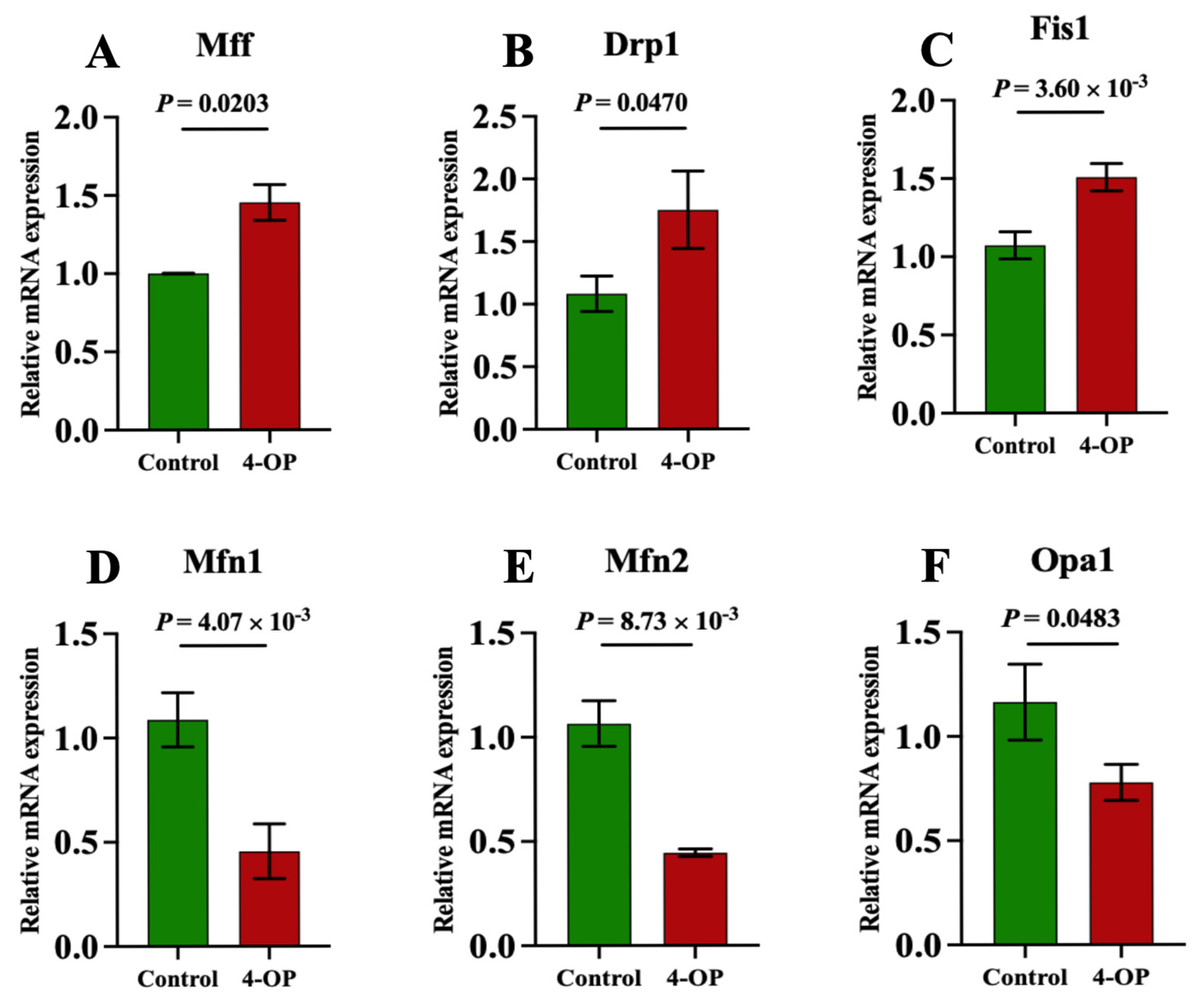
We performed qRT-PCR to assess the expression of genes involved in mitochondrial fission and fusion (Figure 5A–F). Compared with the control group, the mRNA expression of Mff (Figure 5A) and Drp1 (Figure 5B) was significantly (p < 0.05) elevated, and the mRNA expressions of Fis1 (Figure 5C) were extremely significantly (p < 0.01) elevated following 4-OP treatment. The mRNA expressions of Mfn1 (Figure 5D) and Mfn2 (Figure 5E) were extremely significantly (p < 0.01) reduced, and Opa1 (Figure 5F) was significantly (p < 0.05) reduced in the 4-OP group compared with the control group.
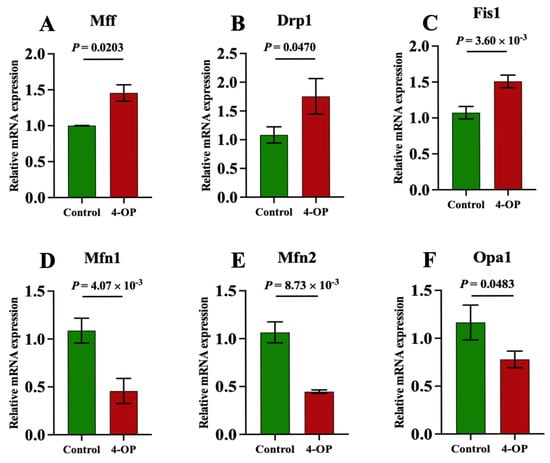
Figure 5.
mRNA expression of mitochondrial fission and mitochondrial fusion genes in common carp. (A) mRNA expression of Mff; (B) mRNA expression of Drp1; (C) mRNA expression of Fis1; (D) mRNA expression of Mfn1; (E) mRNA expression of Mfn2; (F) mRNA expression of Opa1. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
3.6. The Effect of 4-OP Exposure on Energy Metabolism Factors
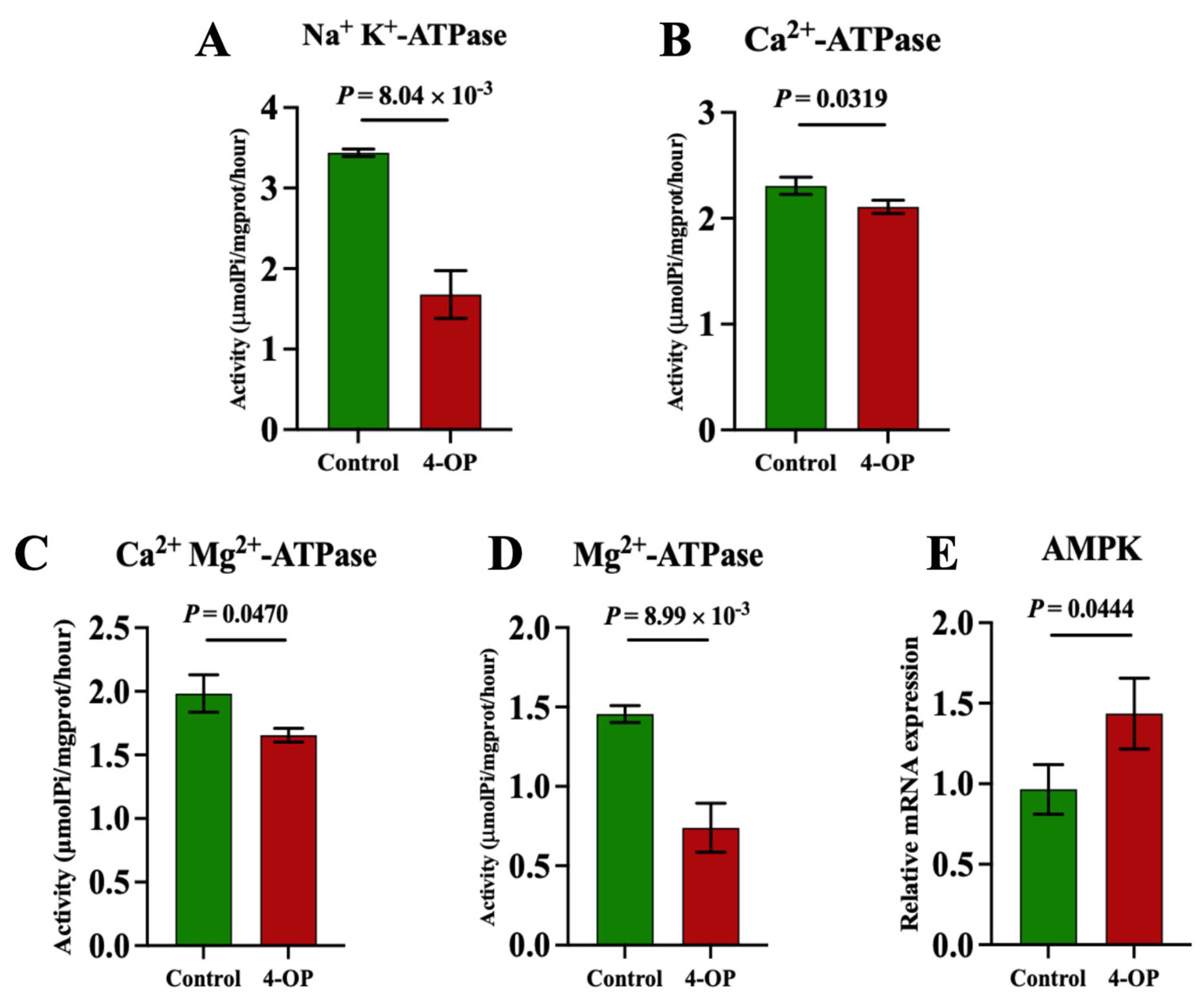
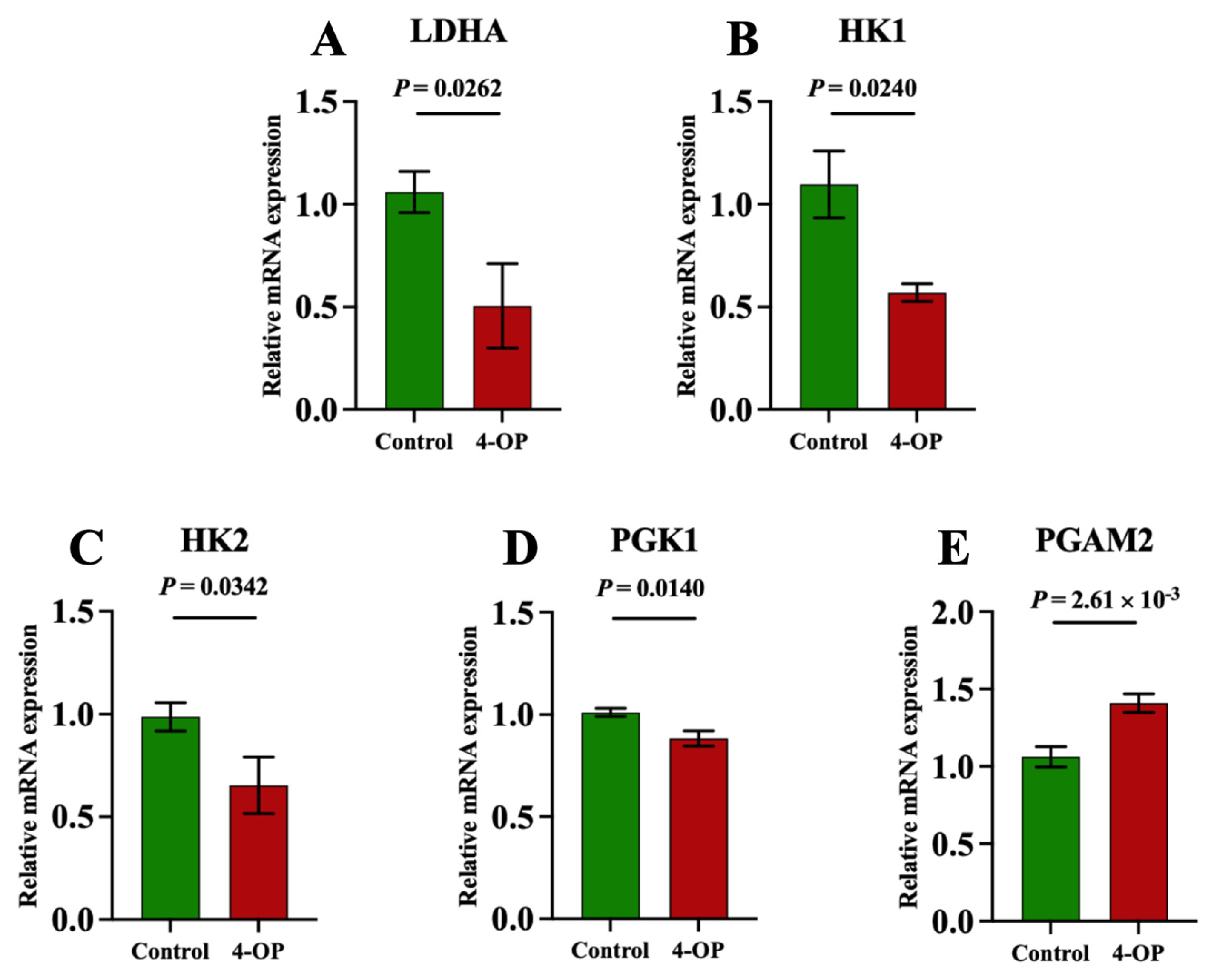
To investigate whether 4-OP exposure can affect energy metabolism in the hearts of common carp, we assessed four ATPase (Figure 6A–D), AMPK (Figure 6E), and five glycolysis genes (Figure 7A–E). Among the four ATPases, the activities of Na+K+-ATPase and Mg2+-ATPase in the 4-OP group were extremely significant (p < 0.01) and lower than those in the control group, and the activities of Ca2+Mg2+-ATPase and Ca2+-ATPase in the 4-OP group were significantly (p < 0.05) lower than those in the control group. The mRNA expression of AMPK in the 4-OP group was significantly (p < 0.05) higher than that in the control group. For 5 glycolysis genes, the mRNA expressions of LDHA (Figure 7A), HK1 (Figure 7B), HK2 (Figure 7C), and PGK1 (Figure 7D) were significantly (p < 0.05) lower than those in the control group, the while mRNA level of PGAM2 (Figure 7E) was extremely significantly (p < 0.01) higher than that in the control group.
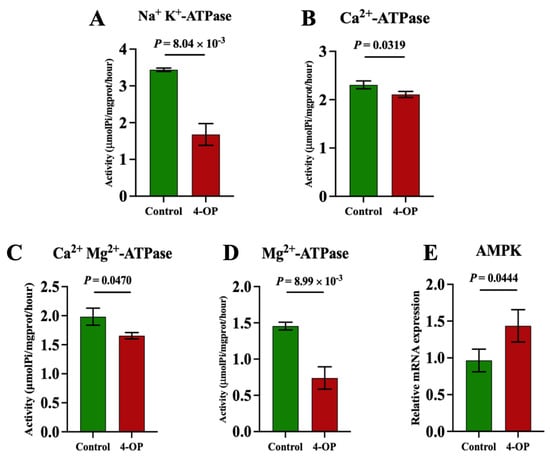
Figure 6.
Activities of ATPase and mRNA expression of AMPK in common carp hearts. (A) Activity of Na+K+−ATPase; (B) activity of Ca2+Mg2+−ATPase; (C) activity of Ca2+−ATPase; (D) activity of Mg2+−ATPase. (E) mRNA expression of AMPK. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
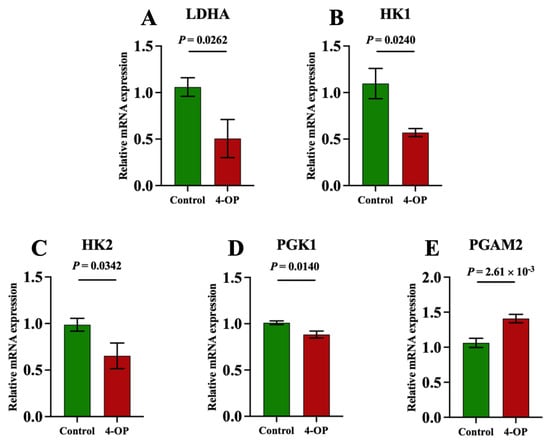
Figure 7.
mRNA expression of glycolysis factors in common carps. (A) mRNA expression of LDHA; (B) mRNA expression of HK1; (C) mRNA expression of HK2; (D) mRNA expression of PGK1; (E) PGAM2 mRNA expression. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
3.7. The Effect of 4-OP Exposure on Oxidative Stress Indexes
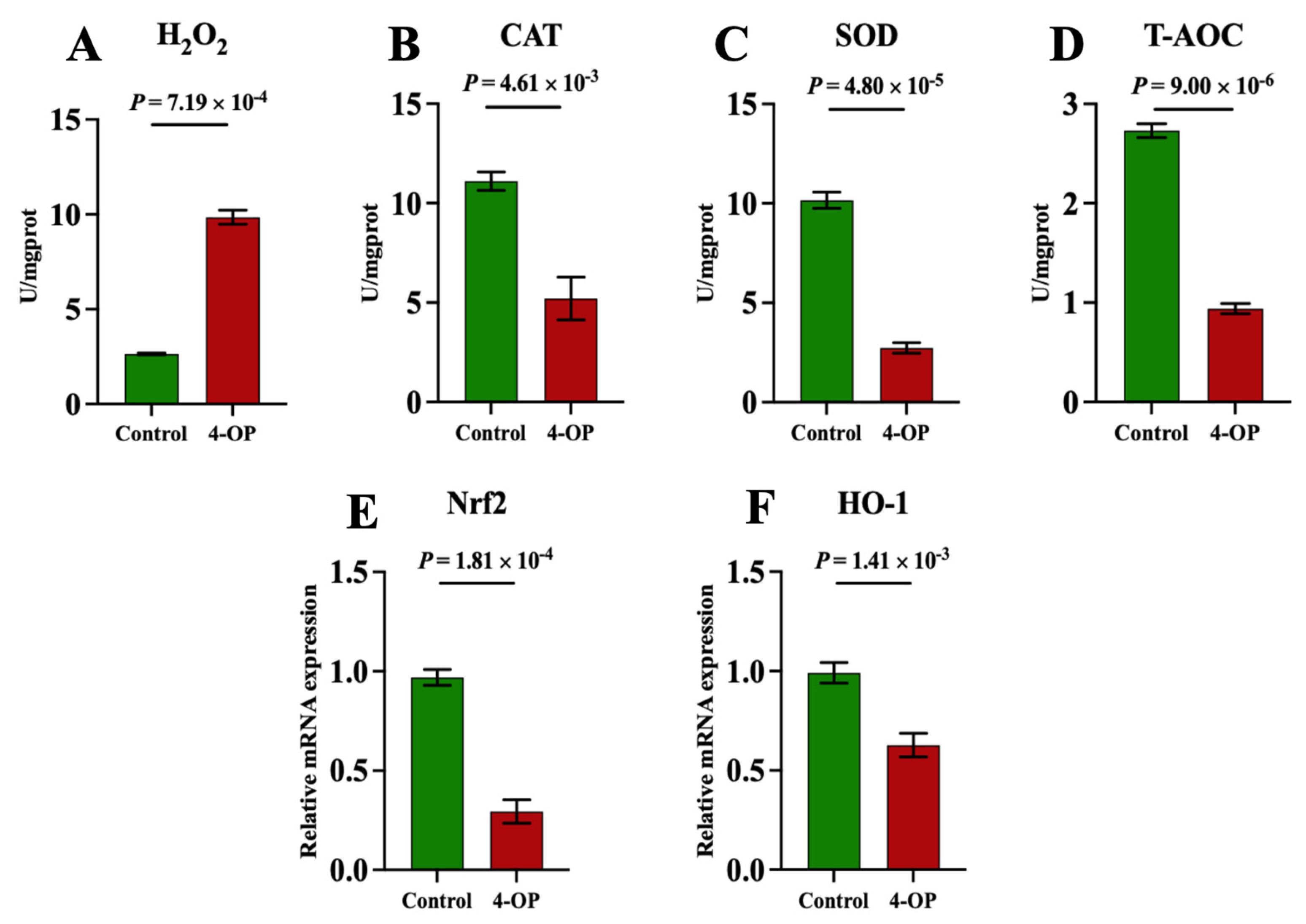
To investigate whether 4-OP can induce oxidative stress in common carp hearts, we measured the content of oxidant H2O2, and the activities of antioxidants CAT, SOD, and T-AOC, as well as the mRNA levels of the antioxidant factors Nrf2 and HO-1, as shown in Figure 8A–F. The H2O2 (Figure 8A) content was found to be extremely significantly (p < 0.01) upregulated in the 4-OP group compared to the control group. CAT (Figure 8B), SOD (Figure 8C), and T-AOC (Figure 8D) activities, as well as the mRNA levels of Nrf2 (Figure 8E) and HO-1 (Figure 8F), were found to be extremely significantly (p < 0.01) downregulated in the 4-OP group compared to the control group.
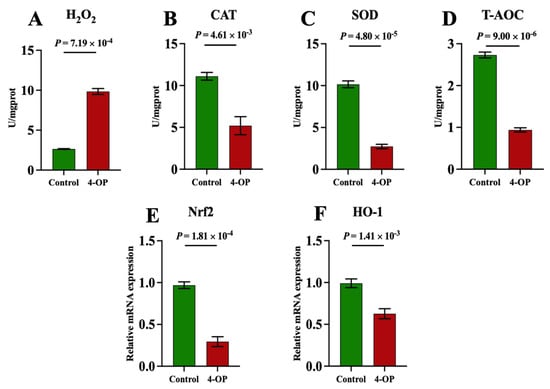
Figure 8.
Oxidative stress indexes in common carp hearts. (A) H2O2. (B) CAT. (C) SOD. (D) T−AOC. (E) mRNA expression of Nrf2. (F) mRNA expression of HO−1. Each value is represented by mean ± SD. The number of experimental replications was three (n = 3).
4. Discussion
4.1. 4-OP Treatment Induced Heart Damage in Common Carp
Two studies reported that 4-OP caused damage in fish. 4-OP exposure impaired primary hepatic cells derived from pearl danios [8], and caused gill damage in common carp [9]. However, no studies have reported whether 4-OP can damage the heart in fish. Microstructure and ultrastructure observation are effective methods to study animal damage caused by environmental stress [15,28,34]. Thus, we observed the microstructure and ultrastructure of carp hearts after 4-OP exposure to investigate whether 4-OP exposure can damage carp hearts. Both our microstructural observation and ultrastructural observation revealed that 4-OP exposure damaged carp hearts. After 4-OP exposure, microstructural observation showed disrupted and disarranged cardiac muscle fibers and increased gaps between myocardial fibers. Ultrastructural observation showed ruptured mitochondrial membrane, vague mitochondrial cristae, shrunken cell nuclei, and autophagosomes. The observation of autophagosomes further revealed that 4-OP exposure induced autophagy and mitochondrial damage in carp hearts. Our findings are supported by two studies showing that harmful endocrine disruptors damaged heart tissue in common carp. A study by Wu et al. (2022) found that exposure to polystyrene nanoplastics increased the gap between cardiomyocytes and broke muscle fibers in common carp hearts [35]. Liu et al. (2023) observed ruptured cardiac muscle fibers, disordered arrangement of cardiac muscle fibers, ruptured mitochondrial cristae, and autophagosomes, and found autophagic damage in common carp hearts following cd treatment [14].
4.2. 4-OP Exposure Induced Autophagy
Recent studies have found that autophagy plays a crucial role in the mechanism underlying fish damage caused by toxic environmental pollutants [20,26,28]. PI3K, AKT, mTOR, SQSTM1, Beclin1, LC3-I, LC3-II, ULK1, ATG5, ATG12, ATG13, and RB1CC1 are autophagy factors. PI3K, AKT, and mTOR are negative regulators of autophagy. Beclin1, ATG5, ATG12, LC3I, and LC3II contribute to autophagosome assembly, with LC3-II recruited to the autophagosomal membrane to facilitate autophagosome formation and initiate autophagy. ULK1 participates in autophagy initiation by forming a complex with ATG13 and RB1CC1 [36]. SQSTM1 (also known as p62), an autophagy substrate protein, acts as a negative regulator of autophagy. In our study, we found that exposure to 4-OP increased the levels of ULK1, Beclin1, ATG5, ATG12, ATG13, RB1CC1, LC3-I, and LC3-II and decreased the levels of PI3K, AKT, mTOR, and SQSTM1, which indicated that 4-OP treatment induced autophagy in common carp hearts. Other experiments are consistent with our findings. Liu et al. (2022) reported that cobalt chloride upregulated the levels of ATG5, RB1CC1, LC3I, and LC3-II, and induced autophagy in mouse kidneys [37]. In MEHP-treated CIK cells, Beclin1, ULK1, LC3-II, Atg12, and Atg13 increased, and autophagy occurred [20]. Exposure to synthetic phenolic antioxidants upregulated the levels of Beclin1, LC3-I, and ATG13, and downregulated the levels of PI3K, AKT, and mTOR, and induced autophagy in grass carp livers [28]. After 2,4-di-tert-butylphenol exposure, mTOR and SQSTM1 decreased, Beclin1, LC3-I, LC3-II, and ATG5 increased, and autophagy occurred in common carp livers [26]. Moreover, it was discovered that LY294002 (PI3K inhibitor) treatment inhibited the level of PI3K, as well as the levels of AKT and mTOR in human breast cancer cells [38]. Zhao et al. (2021) demonstrated that MK2206 (AKT inhibitor) treatment inhibited the levels of Akt and mTOR and aggravated stigmasterol-induced autophagy in gastric cancer cells [39]. mTOR agonist MHY1485 alleviated hypoxia-induced mTOR reduction, ULK1 elevation, and autophagy in the pulmonary artery cells of rats [40]. Therefore, our results indicated that the PI3K-AKT-mTOR/ULK1 pathway took part in 4-OP-induced autophagic damage in common carp hearts. For the first time, we found that the PI3K-AKT-mTOR/ULK1 pathway was involved in the molecular mechanism of autophagy caused by estrogen, and further research is needed.
4.3. miR-144 Mediated 4-OP-Induced Autophagy
miRNAs are key regulators of various biological processes, such as autophagy. Three studies reported that miRNAs mediated autophagy caused by toxic endocrine disruptors. miR-30a was shown to mediate estrogen diethylstilbestrol exposure-caused autophagy via targeting Beclin1 in mouse T cell lymphoma cell line (EL4) [41]. miR-155 mediated autophagy by targeting PI3K in polychlorinated biphenyls (PCBs)-exposed rat chondrocytes [42]. Zhou et al. (2023) found that miR-144 mediated autophagy in rat ovarian cells treated with 4-vinylcyclohexene diepoxide by targeting PTEN [43]. However, whether miRNAs were involved in 4-OP exposure-induced autophagy in common carp hearts was still unknown. miRNAs regulate biological processes mainly through binding the 3′UTR of target mRNAs. Thereby, a bioinformatics website was used in our study and we found six binding sites between miR-144 and the 3′UTR of PI3K, mTOR, and SQSTM1 genes, predicting that PI3K, mTOR, and SQSTM1 are target genes of miR-144. Furthermore, we measured miR-144, PI3K, mTOR, and SQSTM1 at the transcriptional level and found that 4-OP treatment increased the level of miR-144 and decreased the levels of PI3K, mTOR, and SQSTM1 in common carp hearts, further demonstrating that PI3K, mTOR, and SQSTM1 were target genes of miR-144. The findings indicated that miR-144 mediated 4-OP-induced autophagy in common carp hearts via targeting PI3K, mTOR, and SQSTM1. Two studies support the targeted relationship of our study. Ren et al. (2018) confirmed that mTOR was a direct target of miR-144 through a luciferase reporter assay and overexpression experiment; miR-144 overexpression increased the level of miR-144 and reduced the level of mTOR in osteosarcoma cells [44]. Similarly, Wang et al. (2019) reported that miR-144 overexpression upregulated the level of miR-144 and downregulated the level of PI3K in SW1990 cells [45]. Notably, based on our above finding regarding the PI3K-AKT-mTOR/ULK1 pathway, our results indicated that the miR-144/PI3K-AKT-mTOR/ULK1 pathway participated in 4-OP-induced autophagy in common carp hearts. To our knowledge, for the first time, we found that miR-144 targeted three genes PI3K, mTOR, and SQSTM1 to mediate autophagy in common carp hearts following 4-OP exposure and the miRNA-144/PI3K-AKT-mTOR/ULK1 pathway took part in the molecular mechanism underlying toxic environmental pollutant-caused autophagic damage in animals, which needs to be explored in the future.
4.4. 4-OP Exposure Caused Energy Metabolism Disorder
The heart maintains blood circulation mainly through energy generated by ATP hydrolysis, a process catalyzed by ATPase that converts ATP into ADP to release energy. Four ATPases—Na+K+-ATPase, Ca2+Mg2+-ATPase, Ca2+-ATPase, and Mg2+-ATPase—play important roles in the process. Three reports found that environmental pollutants caused decreases in the four ATPases and energy metabolism disorder in fish [13,14,46]. In monobutyl phthalate-treated zebrafish, Na+-K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase decreased and energy metabolism was disturbed in gills [13]. Cui et al. (2023) found that chlorpyrifos treatment decreased the activities of four ATPases (Na+K+-ATPase, Mg2+-ATPase, Ca2+-ATPase, and Ca2+Mg2+-ATPase) and disrupted energy metabolism in common carp livers [46]. After Cd treatment in common carp, Na+K+-ATPase, Ca2+Mg2+-ATPase, Ca2+-ATPase, and Mg2+-ATPase decreased and energy metabolism impairment occurred in hearts [14]. Thus, we hypothesized that 4-OP can reduce the four ATPases in common carp hearts. We measured the activities of four ATPases and found that 4-OP exposure reduced the activities of the four ATPases, indicating that 4-OP caused energy metabolism disorder in common carp hearts.
Glycolysis is one of the major energy metabolism pathways, generating ATP through the breakdown of glucose. HK1, HK2, PGAM2, LDHA, and PGK1 are glycolysis enzymes. HK1 is a first-rate-limiting enzyme in glycolysis. The knockdown of HK1 induced energy metabolism disorders and accelerated the invasion of cervical cancer HeLa cells [47]. HK2 is a key enzyme in glucose metabolism. HK2 depletion suppressed glycolysis and led to energy impairment in mice glioma cells [48] (Hu et al., 2022). Mikawa et al. (2021) reported that the overexpression of PGAM2 inhibited glycolysis and reduced glucose metabolism in mouse cells [49]. It has been found that knockdown LDHA impaired glucose metabolism in Hep3B cells [50]. PGK1 silencing inhibited glycolysis in mouse glioma cells [51]. Therefore, we detected the five glycolysis indicators and further investigated whether toxic substance 4-OP can induce energy metabolism disorders from the perspective of glycolysis. In our experiment, HK1, HK2, LDHA, and PGK1 mRNA expression decreased, while PGAM2 mRNA expression increased after 4-OP treatment, indicating that 4-OP induced glycolysis disorders in common carp hearts. Similar studies supported our findings. Bisphenol AF exposure decreased the levels of HK1 and HK2, and inhibited glycolysis in zebrafish (Danio rerio) muscle [17]. In Cd-treated largemouth bass (Micropterus salmoides), LDHA decreased [16]. In addition, two studies have found that endocrine disruptors increased AMPK and induced energy metabolism disorder in fish. In nonylphenol-treated zebrafish, AMPK increased and energy metabolism disorder occurred [15]. Cui et al. (2023) found that chlorpyrifos exposure increased AMPK and induced energy metabolism disorder in common carp livers [46]. However, it was unknown if AMPK can take part in the molecular mechanism of 4-OP treatment-caused energy metabolism disorder in common carp. In our experiment, we found that AMPK increased after 4-OP treatment, confirming that 4-OP disrupted energy metabolism in common carp hearts. Interestingly, it was found that treatment of duck embryonic cells with compound C (an AMPK inhibitor) reduced AMPK, increased mTOR, and inhibited autophagy [52]. AMPK activator O304 activated AMPK, reduced the level of mTOR, and caused autophagy in mouse kidney cells [53] (Zhu et al., 2022). Therefore, our findings also suggested that energy metabolism disorder mediated autophagy via AMPK/mTOR axis in the context of 4-OP poisoning in common carp hearts, which requires further investigation.
4.5. 4-OP Treatment Induced Mitochondrial Damage
Mitochondria are essential organelles that serve as the primary energy producers in cells, supplying a large amount of energy to sustain continuous systolic and diastolic functions of the heart. The functional state of mitochondria directly determines cardiac energy supply efficiency. Therefore, we wanted to investigate whether 4-OP exposure can damage mitochondria in common carp hearts. Our morphological observation showed that 4-OP caused cardiac mitochondrial damage in carp, as evidenced by mitochondrial vacuole and mitochondrial crest fracture. Several studies reported that six factors (Mff, Drp1, Fis1, Mfn1, Mfn2, and Opa1) were involved in the mechanism of mitochondrial damage. Mfn1 and Mfn2 knockout in mice cardiomyocytes and Opa1 knockout in mice embryonic fibroblasts resulted in mitochondrial damage [54,55,56]. The overexpression of Mff induced mitochondrial fragmentation in HeLa cells [57]. Treatment with Mdivi-1 (a Drp1 inhibitor) alleviated mitochondrial fragmentation in mouse mammary epithelial cells [58]. Fis1 knockout mitigated palmitic acid-induced mitochondrial damage in rat primary cardiomyocytes [59]. Mitochondrial dynamics are regulated by two opposing processes: fission and fusion. Mff, Drp1, and Fis1 are mitochondrial fission proteins, while Mfn1, Mfn2, and Opa1 are mitochondrial fusion proteins. In our experiment, we measured the six factors and found that exposure to 4-OP increased the levels of three mitochondrial fission factors (Mff, Drp1, and Fis1) and decreased the levels of three mitochondrial fusion factors (Mfn1, Mfn2, and Opa1). The results indicated that 4-OP exposure disrupted the balance between mitochondrial fission and fusion, suggesting that 4-OP induced mitochondrial dynamics imbalance and damage in common carp hearts. Previous studies demonstrated that toxic endocrine disruptors disrupted mitochondrial dynamics through similar mechanisms. Fan et al. (2024) reported that dibutyl phthalate exposure in zebrafish increased the level of Fis1 and decreased the levels of Mfn1, Mfn2, and Opa1, and damaged mitochondria [21]. Liu et al. (2023) found that Cd treatment upregulated Mff and Drp1 while it downregulated the levels of Mfn1, Mfn2, and OPa1, and caused the imbalance between mitochondrial fission and fusion mitochondrial dynamics imbalance and damage in common carp hearts [14]. Notably, Peng et al. (2022) demonstrated that Mff knockdown decreased the level of Drp1 in the 12-SV40 human corneal epithelial cell line [60]. Therefore, our results suggested that the Mff-Drp1 axis was involved in 4-OP-caused mitochondrial dynamics imbalance. Interestingly, Yuan et al. (2023) found that the transfection of siRNA-Mfn2 into SH-SY5Y cells reduced the protein expression of SQSTM1 and increased the protein expression of Beclin1 [61]. The knockdown of Mff depressed the level of LC3-II in the 12-SV40 human corneal epithelial cell line [60]. Therefore, our data suggested that mitochondrial dynamics imbalance mediated autophagy through the Mfn2-SQSTM1, Mfn2/Beclin1, and Mff-LC3-II axes in the hearts of 4-OP exposed common carp. In addition, treatment with AICAR (AMPK activator) increased the levels of AMPK and Mff in bovine luteal cells [62]. Therefore, our results suggested that energy metabolism disruption mediated mitochondrial damage via the AMPK-Mff-Drp1 pathway in common carp hearts. Energy metabolism disorder took part in mitochondrial fission and fusion imbalance-mediated autophagy through the AMPK-Mff-LC3-II pathway. For the first time, we found that the AMPK-Mff-LC3-II pathway partook in the molecular mechanism of animal poisoning, and our new finding needs to be further investigated.
4.6. Exposure to 4-OP Caused Oxidative Stress
Two studies found that toxic environmental pollutants induced oxidative stress, autophagy, energy metabolism disorder, and mitochondrial dynamics imbalance in fish. Oxytetracycline exposure induced oxidative stress, mitochondrial dynamics imbalance, and autophagy in largemouth bass (Micropterus salmoides) livers [63]. Cd exposure induced oxidative stress, autophagy, energy metabolism disorder, and mitochondrial dynamics imbalance in common carp hearts [14]. However, whether 4-OP can induce oxidative stress in common carp hearts was still unknown. Therefore, we wanted to explore whether 4-OP exposure can induce oxidative stress in common carp hearts. Oxidative stress arises from an imbalance between cellular antioxidants and oxidants. H2O2 is an oxidant factor, and CAT, SOD, T-AOC, Nrf2, and HO-1 are antioxidant factors. H2O2 is an aerobic metabolite and induces oxidative stress. CAT, an antioxidant enzyme, degrades H2O2 into H2O and oxygen. Antioxidant agent SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide (H2O2) and ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2) for scavenging free radicals in the body. T-AOC reflects the total antioxidant capacity of the body. Nrf2 and HO-1 play a role in activating the antioxidant response to defend against oxidative stress. Therefore, we detected oxidant H2O2 and five antioxidant factors (CAT, SOD, T-AOC, Nrf2, and HO-1), and found that 4-OP exposure increased H2O2 while reducing the five antioxidant factors. The results suggested that 4-OP led to oxidative stress by disrupting the balance between antioxidants and oxidants in common carp hearts. Several studies support our results. 4-tBP exposure upregulated H2O2, downregulated CAT, SOD, and T-AOC, and induced oxidative stress in common carp head kidneys [23]. An increase in H2O2 and decreases in CAT and SOD were found in Cd-induced oxidative stress in common carp hearts [14]. Desai et al. (2023) demonstrated that Nrf2 decreased and oxidative stress occurred in the brain of zebrafish treated with 4-nonylphenol [24]. In 1,2,3,7,8-polychlorinated dibenzo-p-dioxin-treated primary Atlantic cod hepatic cells, the level of HO-1 decreased and induced oxidative stress [25]. Additionally, Xu et al. (2022) reported that H2O2 treatment disturbed mitochondrial dynamics and caused mitochondrial damage by increasing Drp1 and decreasing Mfn2 in rat retinal neuronal cell lines [64]. Therefore, our results indicated that oxidative stress mediated mitochondrial dynamics imbalance via the H2O2-Drp1 and H2O2/Mfn2 axes following 4-OP exposure, and that oxidative stress triggered 4-OP-induced autophagy mediated by mitochondrial dynamics imbalance. In addition, exposure to H2O2 upregulated the AMPK in MLO-Y4 cells [65] (Wei et al., 2023). Thereby, our findings indicated that oxidative stress mediated energy metabolism disorder via the H2O2-AMPK axis in common carp hearts. Therefore, our findings indicated that oxidative stress triggered energy metabolism disorder, induced mitochondrial dynamics imbalance, and caused autophagy via the H2O2-AMPK-Mff pathway.
5. Conclusions
In conclusion, our results demonstrated that the heart was a target organ of 4-OP and that 4-OP exposure led to mitochondrial damage, autophagy, and damage in common carp hearts. 4-OP exposure increased the levels of miR-144, Beclin1, RB1CC1, ULK1, LC3-I, LC3-II, ATG5, ATG12, and ATG13, and decreased the levels of PI3K, AKT, mTOR, and SQSTM1, and induced autophagy. miR-144 mediated autophagy via targeting PI3K, mTOR, and SQSTM1, and the miR-144/PI3K-AKT-mTOR/ULK1 pathway may took part in 4-OP induced autophagy. In addition, 4-OP exposure upregulated mitochondrial fission genes (Mff, Drp1, and Fis1) and downregulated mitochondrial fusion genes (Mfn1, Mfn2, and Opa1), and resulted in an imbalance between mitochondrial fission and mitochondrial fusion and mitochondrial dynamics imbalance, as well as mitochondrial damage. The Mff-Drp1 axis was involved in 4-OP-caused mitochondrial dynamics imbalance. Mitochondrial dynamics imbalance mediated autophagy via the Mfn2-SQSTM1, Mfn2/Beclin1, and Mff-LC3-II axes. Interestingly, 4-OP induced energy metabolism disorder, as evidenced by the decrease in four ATPases (Na+K+-ATPase, Ca2+Mg2+-ATPase, Ca2+-ATPase, and Mg2+-ATPase), the increases in four glycolytic enzymes (HK1, HK2, LDHA, and PGK1), the decrease in glycolytic enzyme PGAM2, and the increase in energy sensor AMPK. Energy metabolism disorder mediated 4-OP-caused mitochondrial dynamics imbalance via the AMPK-Mff-Drp1 pathway, and energy metabolism disorder triggered mitochondrial dynamics imbalance-mediated autophagy induced by 4-OP via the AMPK-Mff-LC3-II pathway. We also found that exposure to 4-OP increased H2O2, decreased CAT, SOD, T-AOC, Nrf2, and HO-1, and resulted in oxidative stress. Oxidative stress mediated energy metabolism disorder via the H2O2-AMPK axis. Therefore, our results suggested that oxidative stress triggered energy metabolism disorder, induced mitochondrial dynamics imbalance, and caused autophagy via the H2O2-AMPK-Mff-LC3-II pathway. Our study provided new insights into the molecular mechanism of 4-OP-induced toxicity in common carp and provided potential targets and new information on endocrine disruptors-induced cardiotoxicity in fish. However, whether 4-OP is toxic to other fish species remains unclear, which is a limitation of our study and requires further investigation in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo15060391/s1, Figure S1: The gel images of three biological replicates for β-actin, SQSTM1, and Beclin1; Table S1: Transcriptome sequencing result of common carp under 4-OP exposure.
Author Contributions
Writing—original draft preparation, methodology, and supervision: M.Q.; Writing—original draft preparation, conceptualization, and methodology: C.J.; conceptualization and methodology: J.L.; formal analysis and investigation: Q.Z.; formal analysis and validation: Y.L. (Yuhao Liu); investigation and validation: Z.H.; investigation and methodology: Y.L. (Yuhang Liu); visualization and data curation: X.L.; writing—review and editing, resources and project administration: X.T.; writing—review and editing and funding acquisition: W.S.; project administration and funding acquisition: Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Plan Project of Jilin Province (20240302074GX), the Academic Backbone fund of Northeast Agricultural University (20XG29), and the Postdoctoral scientific research developmental fund of Heilongjiang Province (LBH-Z22066).
Institutional Review Board Statement
All procedures in this study were approved by the Animal Care and Use Committee of Northeast Agricultural University (No. NEAUEC20240229; approval date: 11 March 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
You Tang is an employee of Jilin Dianchuang Information Technology. The paper reflects the views of the scientists, and not the company.
Abbreviations
| Abbreviations | Original words |
| 4-OP | 4-Octylphenol |
| HK1 | hexokinase 1 |
| HK2 | hexokinase 2 |
| LDHA | lactate dehydrogenase A |
| PGK1 | phosphoglycerate kinase 1 |
| PGAM2 | phosphoglycerate mutase 2 |
| AMPK | AMP-activated protein kinases |
| PI3K | phosphatidylinositol 3-kinase |
| AKT | Akt kinase |
| mTOR | mechanistic target of rapamycin kinase |
| ULK1 | unc-51 like autophagy activating kinase 1 |
| ATG13 | autophagy related 13 |
| RB1CC1/FIP200 | RB1 inducible coiled-coil 1 |
| P62/SQSTM1 | sequestosome 1 |
| Beclin1 | beclin 1 |
| ATG5 | autophagy related 5 |
| ATG12 | autophagy related 12 |
| LC3-I | Microtubule-associated protein 1A/1B-light chain 3-I |
| LC3-II | Microtubule-associated protein 1A/1B-light chain 3-II |
| Mfn1 | mitofusin 1 |
| Mfn2 | mitofusin 2 |
| Opa1 | OPA1 mitochondrial dynamin like GTPase |
| Drp1 | Dynamin-related protein 1 |
| Fis1 | fission, mitochondrial 1 |
| Mff | mitochondrial fission factor |
References
- Aborkhees, G.; Raina-Fulton, R.; Thirunavokkarasu, O. Determination of Endocrine Disrupting Chemicals in Water and Wastewater Samples by Liquid Chromatography-Negative Ion Electrospray Ionization-Tandem Mass Spectrometry. Molecules 2020, 25, 3906. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ndungu, A.W.; Wang, J. Monitoring of Endocrine-Disrupting Compounds in Surface Water and Sediments of the Three Gorges Reservoir Region, China. Arch. Environ. Contam. Toxicol. 2016, 71, 509–517. [Google Scholar] [CrossRef]
- Wacheski, T.; Hara, E.; Soares, B.; da Silva, B.; Abate, G.; Grassi, M. o-DGTDevices for the determination of emerging contaminants in aqueous matrices. J. Braz. Chem. Soc. 2021, 23, 72–82. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, J.; Hu, X.; Yin, D. Characteristics of the alkylphenol and bisphenol A distributions in marine organisms and implications for human health: A case study of the East China Sea. Sci. Total Environ. 2016, 539, 460–469. [Google Scholar] [CrossRef]
- Nurulnadia, M.Y.; Zahid, B.M.; Yusof, K.M.K.K. Accumulation of octylphenol and nonylphenol in the fishes of Terengganu, Malaysia. Reg. Stud. Mar. Sci. 2025, 83, 104061. [Google Scholar] [CrossRef]
- Chen, G.W.; Ding, W.H.; Ku, H.Y.; Chao, H.R.; Chen, H.Y.; Huang, M.C.; Wang, S.L. Alkylphenols in human milk and their relations to dietary habits in central Taiwan. Food Chem. Toxicol. 2010, 48, 1939–1944. [Google Scholar] [CrossRef]
- Isidori, M.; Cangiano, M.; Palermo, F.A.; Parrella, A. E-screen and vitellogenin assay for the detection of the estrogenic activity of alkylphenols and trace elements. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 51–56. [Google Scholar] [CrossRef]
- Kaptaner, B. Cytotoxic effects of 4-octylphenol on fish hepatocytes. Cytotechnology 2016, 68, 1577–1583, Erratum in Cytotechnology 2016, 68, 175. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Liu, Y.; Teng, X.; Luan, P.; Teng, X.; Yin, X. Immunosuppression participated in complement activation-mediated inflammatory injury caused by 4-octylphenol via TLR7/IκBα/NF-κB pathway in common carp (Cyprinus common carpio) gills. Aquat. Toxicol. 2022, 249, 106211. [Google Scholar] [CrossRef]
- Fu, X.; Xu, J.; Zhang, R.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef]
- Yokota, H.; Seki, M.; Maeda, M.; Oshima, Y.; Tadokoro, H.; Honjo, T.; Kobayashi, K. Life-cycle toxicity of 4-nonylphenol to medaka (Oryzias latipes). Environ. Toxicol. Chem. 2001, 20, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Yen, C.H.; Hsieh, C.Y.; Ou, T.Y.; Risjani, Y.; Cheah, W.K.; Hu, S.Y. Toxicity effects of the environmental hormone 4-tert-octylphenol in zebrafish (Danio rerio). Int. J. Mar. Sci. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, Y.; Jiao, Y.; Wu, S.; Zhu, G.; Akindolie, M.S.; Zhu, T.; Qu, J.; Wang, L.; Zhang, Y. Monobutyl phthalate (MBP) induces energy metabolism disturbances in the gills of adult zebrafish (Danio rerio). Environ. Pollut. 2020, 266 Pt 1, 115288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, X.; Hao, Z.; Yu, M.; Tang, Y.; Teng, X.; Sun, W.; Kang, L. Cadmium exposure caused cardiotoxicity in common carps (Cyprinus carpio L.): miR-9-5p, oxidative stress, energetic impairment, mitochondrial division/fusion imbalance, inflammation, and autophagy. Fish Shellfish Immunol. 2023, 138, 108853. [Google Scholar] [CrossRef]
- Mukherjee, U.; Samanta, A.; Biswas, S.; Ghosh, S.; Das, S.; Banerjee, S.; Maitra, S. Chronic exposure to nonylphenol induces oxidative stress and liver damage in male zebrafish (Danio rerio): Mechanistic insight into cellular energy sensors, lipid accumulation and immune modulation. Chem. Biol. Interact. 2022, 351, 109762. [Google Scholar] [CrossRef]
- Mehinto, A.C.; Prucha, M.S.; Colli-Dula, R.C.; Kroll, K.J.; Lavelle, C.M.; Barber, D.S.; Vulpe, C.D.; Denslow, N.D. Gene networks and toxicity pathways induced by acute cadmium exposure in adult largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2014, 152, 186–194. [Google Scholar] [CrossRef]
- Wei, P.; Jiang, G.; Wang, H.; Ru, S.; Zhao, F. Bisphenol AF exposure causes fasting hyperglycemia in zebrafish (Danio rerio) by interfering with glycometabolic networks. Aquat. Toxicol. 2021, 241, 106000. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, Z.; Wang, J.; Zhu, M.; Yang, H. Effects of Dietary Resveratrol, Bile Acids, Allicin, Betaine, and Inositol on Recovering the Lipid Metabolism Disorder in the Liver of Rare Minnow Gobiocypris rarus Caused by Bisphenol A. Aquac. Nutr. 2022, 2022, 6082343. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, W.; Zhang, S. Important roles of heme-regulated eIF-2α kinase in cadmium-induced glycolysis under acute exposure. Environ. Sci. Pollut. Res. Int. 2023, 30, 88444–88449. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Liu, Y.; Han, B.; Yu, Y.; Lin, H. MEHP induced mitochondrial damage by promoting ROS production in CIK cells, leading to apoptosis, autophagy, cell cycle arrest. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 288, 110064. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, D.; Hou, T.; Zhang, Q.; Tao, L.; Bian, C.; Wang, Z. Mitochondrial DNA Stress-Mediated Health Risk to Dibutyl Phthalate Contamination on Zebrafish (Danio rerio) at Early Life Stage. Environ. Sci. Technol. 2024, 58, 7731–7742. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, Q.; Yu, M.; Liu, Y.; Teng, X.; Gu, X. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022, 242, 113944. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lin, X.; Jiang, C.; Liu, Y.; Hao, Z.; Qiu, M.; Liu, X.; Chen, D.; Teng, X.; Tang, Y. Molecular mechanism of apoptosis induced by 4-tBP in common carp (Cyprinus carpio L.) head kidneys was explored from various angles: Hippo pathway, miR-203a, oxidative stress, ER stress, and mitochondrial pathway. Aquaculture 2024, 289, 740981. [Google Scholar] [CrossRef]
- Desai, J.K.; Trangadia, B.J.; Patel, U.D.; Patel, H.B.; Kalaria, V.A.; Kathiriya, J.B. Neurotoxicity of 4-nonylphenol in adult zebrafish: Evaluation of behaviour, oxidative stress parameters and histopathology of brain. Environ. Pollut. 2023, 334, 122206, Erratum in Environ. Pollut. 2024, 357, 124411. [Google Scholar] [CrossRef]
- Søfteland, L.; Holen, E.; Olsvik, P.A. Toxicological application of primary hepatocyte cell cultures of Atlantic cod (Gadus morhua)—effects of BNF, PCDD and Cd. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 401–411. [Google Scholar] [CrossRef]
- Xie, J.; Yin, Y.; Lin, B.; Li, X.; Li, Q.; Tang, X.; Pan, L.; Xiong, X. Autophagy and PPARs/NF-κB-associated inflammation are involved in hepatotoxicity induced by the synthetic phenolic antioxidant 2,4-di-tert-butylphenol in common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2024, 284, 116937. [Google Scholar] [CrossRef]
- Mahemuti, L.; Chen, Q.; Coughlan, M.C.; Qiao, C.; Chepelev, N.L.; Florian, M.; Dong, D.; Woodworth, R.G.; Yan, J.; Cao, X.L.; et al. Bisphenol A induces DSB-ATM-p53 signaling leading to cell cycle arrest, senescence, autophagy, stress response, and estrogen release in human fetal lung fibroblasts. Arch. Toxicol. 2018, 92, 1453–1469. [Google Scholar] [CrossRef]
- Li, S.; Xie, J.; Bai, Y.; Jiang, Z.; Li, K.; Wu, C. Synthetic phenolic antioxidants evoked hepatoxicity in grass carp (Ctenopharyngodon idella) through modulating the ROS-PI3K/mTOR/AKT pathway: Apoptosis-autophagy crosstalk. Fish Shellfish Immunol. 2023, 139, 108906. [Google Scholar] [CrossRef]
- Hao, Z.; Qiu, M.; Liu, Y.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Sun, W.; Teng, X.; Tang, Y. Co-exposure to ammonia and lipopolysaccharide-induced impaired energy metabolism via the miR-1599/HK2 axis and triggered autophagy, ER stress, and apoptosis in chicken cardiomyocytes. Poult. Sci. 2025, 104, 104965. [Google Scholar] [CrossRef]
- Cui, J.; Liang, Z.; Liu, Y.; Hao, Z.; Tang, Y.; Zhou, L.; Teng, X. Environmental endocrine disrupting chemical 4-tert-butylphenol induced calcium overload and subsequent autophagy impairment via miRNA-363/CACNA1D Axis in epithelioma papulosum cyprini cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109968. [Google Scholar] [CrossRef]
- Zhou, Q.; Hao, Z.; Qiu, M.; Liu, Y.; Chang, M.; Liu, X.; Wang, Y.; Tang, Y.; Sun, W.; Teng, X.; et al. Amino acid metabolism disorder and oxidative stress took part in EGCG alleviating Mn-caused ferroptosis via miR-9-5p/got1 axis. J. Hazard. Mater. 2025, 489, 137656. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Hao, Z.; Liu, Y.; Liu, Y.; Chang, M.; Lin, X.; Liu, X.; Dong, N.; Sun, W.; Teng, X. ROS acted as an initial role in selenium nanoparticles alleviating insecticide chlorpyrifos-induced oxidative stress, pyroptosis, and intestinal barrier dysfunction in porcine intestinal epithelial cells. Pestic. Biochem. Physiol. 2025, 211, 106418. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Liu, X.; Wang, Y.P.; Xu, X.; Zhu, L.; Zhang, W.; Ren, K. Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway. Metabolites 2025, 15, 329. [Google Scholar] [CrossRef]
- Che, X.; Shang, X.; WeiXu Xing, M.; Wei, H.; Li, W.; Li, Z.; Teng, X.; Geng, L. Selenium-enriched Lactiplantibacillus plantarum alleviates alkalinity stress-induced selective hepatic insulin resistance in common carp. Int. J. Biol. Macromol. 2025, 305, 141204. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in common carp by promoting ROS production. Fish Shellfish Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef]
- Thorne, R.F.; Yang, Y.; Wu, M.; Chen, S. TRIMming down autophagy in breast cancer. Autophagy 2022, 18, 2512–2513. [Google Scholar] [CrossRef]
- Liu, W.; Gan, Y.; Ding, Y.; Zhang, L.; Jiao, X.; Liu, L.; Cao, H.; Gu, Y.; Yan, L.; Wang, Y.; et al. Autophagy promotes GSDME-mediated pyroptosis via intrinsic and extrinsic apoptotic pathways in cobalt chloride-induced hypoxia reoxygenation-acute kidney injury. Ecotoxicol. Environ. Saf. 2022, 242, 113881. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Ozturk, M. Therapeutic potential of the PI3K inhibitor LY294002 and PARP inhibitor Talazoparib combination in BRCA-deficient triple negative breast cancer cells. Cell Signal. 2022, 91, 110229. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Gui, D.; Cui, Z.; Zhang, L.; Yu, C.; Yao, D.; Xu, M.; Chen, M.; Wu, P.; Li, G.; Wang, L.; et al. Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway. BMC Pulm. Med. 2017, 17, 191. [Google Scholar] [CrossRef]
- Singh, N.P.; Miranda, K.; Singh, U.P.; Nagarkatti, P.; Nagarkatti, M. Diethylstilbestrol (DES) induces autophagy in thymocytes by regulating Beclin-1 expression through epigenetic modulation. Toxicology 2018, 410, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Y.; Jin, X.; Liu, L.; Gu, H. PCB153 suppressed autophagy via PI3K/Akt/mTOR and RICTOR/Akt/mTOR signaling by the upregulation of microRNA-155 in rat primary chondrocytes. Toxicol. Appl. Pharmacol. 2022, 449, 116135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jin, X.; Wang, J.; Li, H.; Yang, L.; Wu, W.; Chen, W. 4-vinylcyclohexene diepoxide induces premature ovarian insufficiency in rats by triggering the autophagy of granule cells through regulating miR-144. J. Reprod. Immunol. 2023, 157, 103928. [Google Scholar] [CrossRef]
- Ren, Y.F.; Zhang, T.H.; Zhong, S.; Zhao, Y.T.; Lv, Y.N. miR-144 suppresses proliferation and induces apoptosis of osteosarcoma cells via direct regulation of mTOR expression. Oncol. Lett. 2018, 15, 1163–1169. [Google Scholar] [CrossRef]
- Wang, K.Q.; Xing, Y.L.; Qiao, X.; Li, S.Z.; Gong, D.W.; Yu, Z.W.; Wu, Y.Q. Effects of miR-144 on proliferation, migration, invasion and PI3K pathway of pancreatic cancer SW1990 cells. Cancer Res. Prev. Treat. 2019, 46, 879–883. [Google Scholar] [CrossRef]
- Cui, J.; Hao, Z.; Zhou, Q.; Qiu, M.; Liu, Y.; Liu, Y.; Teng, X.; Kang, L. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol. Environ. Saf. 2023, 258, 114983. [Google Scholar] [CrossRef]
- Tseng, P.L.; Chen, C.W.; Hu, K.H.; Cheng, H.C.; Lin, Y.H.; Tsai, W.H.; Cheng, T.J.; Wu, W.H.; Yeh, C.W.; Lin, C.C.; et al. The decrease of glycolytic enzyme hexokinase 1 accelerates tumor malignancy via deregulating energy metabolism but sensitizes cancer cells to 2-deoxyglucose inhibition. Oncotarget 2018, 9, 18949–18969. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Cao, K.; Wang, F.; Wu, W.; Mai, W.; Qiu, L.; Luo, Y.; Ge, W.P.; Sun, B.; Shi, L.; et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat. Metab. 2022, 4, 1756–1774. [Google Scholar] [CrossRef]
- Mikawa, T.; Shibata, E.; Shimada, M.; Ito, K.; Ito, T.; Kanda, H.; Takubo, K.; Shimada, A.; Lleonart, M.E.; Inagaki, N.; et al. Characterization of genetically modified mice for phosphoglycerate mutase, a vitally-essential enzyme in glycolysis. PLoS ONE 2021, 16, e0250856. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189.e15. [Google Scholar] [CrossRef]
- Cao, W.; Feng, Z.; Zhu, D.; Li, S.; Du, M.; Ye, S.; Qi, D.; Li, P.; Chen, Y.; Fang, Y. The Role of PGK1 in Promoting Ischemia/Reperfusion Injury-Induced Microglial M1 Polarization and Inflammation by Regulating Glycolysis. Neuromol. Med. 2023, 25, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, L.; Li, S.; Xu, L.; Wang, Y.; Chen, H. Impaired Cellular Energy Metabolism Contributes to Duck-Enteritis-Virus-Induced Autophagy via the AMPK-TSC2-MTOR Signaling Pathway. Front. Cell Infect. Microbiol. 2017, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shen, W.; Li, J.; Jia, N.; Xiong, Y.; Miao, J.; Xie, C.; Chen, Q.; Shen, K.; Meng, P.; et al. AMPK Activator O304 Protects Against Kidney Aging Through Promoting Energy Metabolism and Autophagy. Front. Pharmacol. 2022, 13, 836496. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Shimizu, I.; Wakasugi, T.; Yoshida, Y.; Ikegami, R.; Hayashi, Y.; Suda, M.; Katsuumi, G.; Nakao, M.; Ozawa, T.; et al. Cardiac mitofusin-1 is reduced in non-responding patients with idiopathic dilated cardiomyopathy. Sci. Rep. 2021, 11, 6722. [Google Scholar] [CrossRef]
- Fu, D.; Luo, J.; Wu, Y.; Zhang, L.; Li, L.; Chen, H.; Wen, T.; Fu, Y.; Xiong, W. Angiotensin II-induced calcium overload affects mitochondrial functions in cardiac hypertrophy by targeting the USP2/MFN2 axis. Mol. Cell Endocrinol. 2023, 571, 111938. [Google Scholar] [CrossRef]
- Sessions, D.T.; Kim, K.B.; Kashatus, J.A.; Churchill, N.; Park, K.S.; Mayo, M.W.; Sesaki, H.; Kashatus, D.F. Opa1 and Drp1 reciprocally regulate cristae morphology, ETC function, and NAD+ regeneration in KRas-mutant lung adenocarcinoma. Cell Rep. 2022, 41, 111818. [Google Scholar] [CrossRef]
- Ma, Y.; Du, M.; Yang, F.; Mai, Z.; Zhang, C.; Qu, W.; Wang, B.; Wang, X.; Chen, T. Quantifying the inhibitory effect of Bcl-xl on the action of Mff using live-cell fluorescence imaging. FEBS Open Bio 2019, 9, 2041–2051. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Wang, Z.; Lin, X.; Xu, Y.; Feng, S.; Miao, J. AMPK/Drp1 pathway mediates Streptococcus uberis-Induced mitochondrial dysfunction. Int. Immunopharmacol. 2022, 113 Pt A, 109413. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Q.; Xiao, Q.; Chen, Y.; Deng, Z.; Cen, C.; Lin, J. Lipotoxicity-induced upregulation of FIS1 exacerbates mitochondrial fragmentation and promotes NLRP3-dependent pyroptosis in diabetic cardiomyopathy. Free Radic. Biol. Med. 2025, 228, 183–196. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, D.; Xu, W.; Sun, Y.; Zha, Z.; Tan, X.; Yu, J.; Pan, C.; Zheng, Q.; Chen, W. AMPK/MFF Activation: Role in Mitochondrial Fission and Mitophagy in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2022, 63, 18. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Mo, Y.Z.; Li, D.L.; Xie, L.; Chen, M.H. Inhibition of ERK downregulates autophagy via mitigating mitochondrial fragmentation to protect SH-SY5Y cells from OGD/R injury. Cell Commun. Signal. 2023, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Przygrodzka, E.; Monaco, C.F.; Snider, A.P.; Keane, J.A.; Burns, P.D.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Prostaglandin F2α regulates mitochondrial dynamics and mitophagy in the bovine corpus luteum. Life Sci. Alliance 2023, 6, e202301968. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jin, M.; Huang, L.; Zhang, Y.; Zong, J.; Shan, H.; Kang, H.; Xu, M.; Liu, H.; Zhao, Y.; et al. Oxytetracycline-induced oxidative liver damage by disturbed mitochondrial dynamics and impaired enzyme antioxidants in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2023, 261, 106616. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, K.; Qu, X.H.; Wang, T.; Yang, P.; Yang, Y.; Jiang, L.P.; Wan, Y.Y.; Tou, F.F.; Chen, Z.P.; et al. Curcumin protects retinal neuronal cells against oxidative stress-induced damage by regulating mitochondrial dynamics. Exp. Eye Res. 2022, 224, 109239. [Google Scholar] [CrossRef]
- Wei, L.; Chai, S.; Yue, C.; Zhang, H.; Li, J.; Qin, N. Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis. Cell Death Discov. 2023, 9, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).