Differential Expression of Lipid Metabolism Genes, CROT and ABCG1, in Obese Patients with Comorbid Depressive Disorder and Risk of MASLD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Hepatopathological Diagnosis
2.3. Anthropometric Evaluation and Biochemical Analysis
2.4. RNA Isolation and Analysis of the Gene Expression in Liver and Serum Samples
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Liver Histology Assessment According to Depression Diagnosis
3.3. Association Between Depressive Disorder and Comorbid Conditions
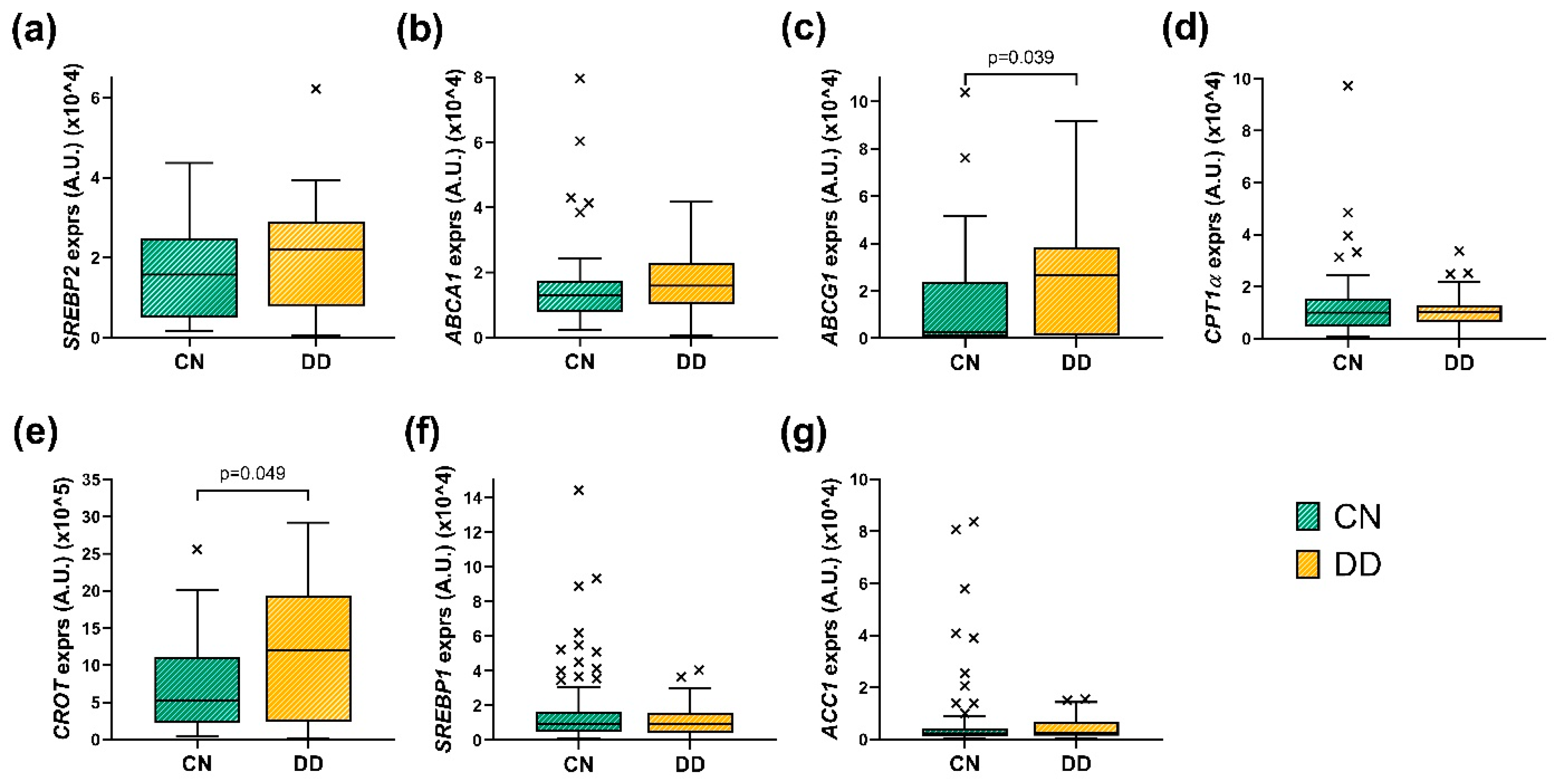
3.4. Evaluation of the Hepatic Gene Expression Between Cohorts
3.4.1. Evaluation of the Hepatic Gene Expression Between CN and DD Cohorts
3.4.2. Evaluation of the Hepatic Gene Expression in Respect to Liver Histology
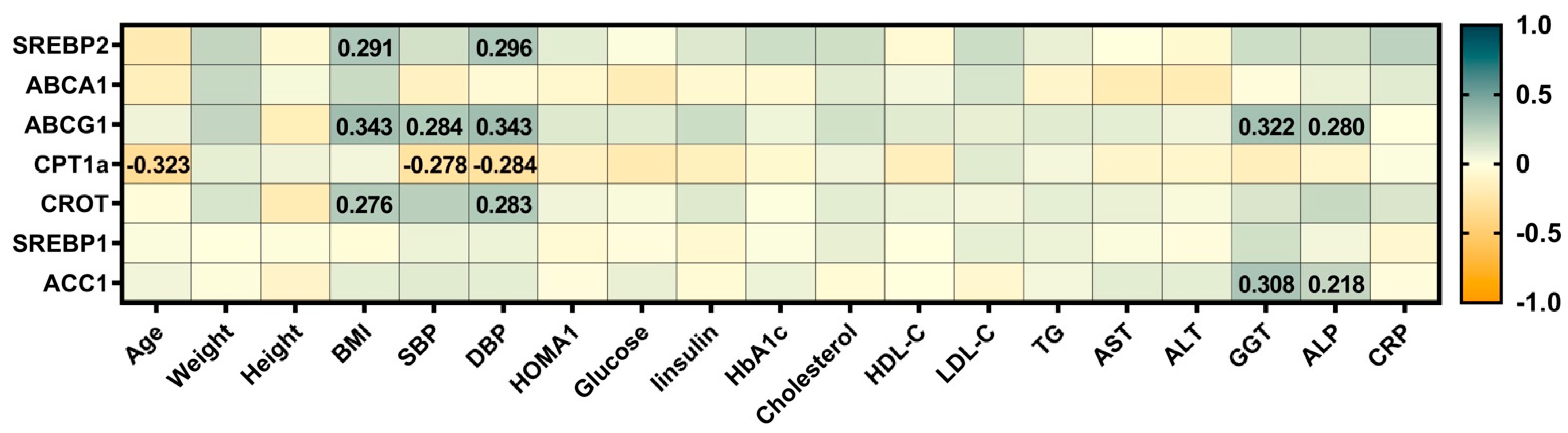
3.5. Correlations Between the Expression of Hepatic Genes Involved in the Lipid Metabolism with Clinical and Biochemical Measures of the Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. Metabolic-Dysfunction-Associated Steatotic Liver Disease—Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int. J. Mol. Sci. 2023, 24, 15473. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Lamers, F.; Peyrot, W.J.; Baune, B.T.; Breen, G.; Dehghan, A.; Forstner, A.J.; Grabe, H.J.; Homuth, G.; Kan, C.; et al. Genetic Association of Major Depression with Atypical Features and Obesity-Related Immunometabolic Dysregulations. JAMA Psychiatry 2017, 74, 1214–1225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, X.; Wang, Y.; Zhao, F.; Cui, R.; Xie, W.; Liu, Q.; Yang, W. Shared biological mechanisms of depression and obesity: Focus on adipokines and lipokines. Aging 2023, 15, 5917–5950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panganiban, J.; Kehar, M.; Ibrahim, S.H.; Hartmann, P.; Sood, S.; Hassan, S.; Ramirez, C.M.; Kohli, R.; Censani, M.; Mauney, E.; et al. Metabolic dysfunction-associated steatotic liver disease (MASLD) in children with obesity: An Obesity Medicine Association (OMA) and expert joint perspective 2025. Obes. Pillars 2025, 14, 100164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, J.; Lim, L.K.E.; Ng, C.H.; Tan, D.J.H.; Lim, W.H.; Ho, C.S.; Tan, E.X.X.; Sanyal, A.J.; Muthiah, M.D. Is Fatty Liver Associated with Depression? A Meta-Analysis and Systematic Review on the Prevalence, Risk Factors, and Outcomes of Depression and Non-alcoholic Fatty Liver Disease. Front. Med. 2021, 8, 691696. [Google Scholar] [CrossRef]
- Nemiary, D.; Shim, R.; Mattox, G.; Holden, K. The Relationship Between Obesity and Depression Among Adolescents. Psychiatr. Ann. 2012, 42, 305–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youssef, N.A.; Abdelmalek, M.F.; Binks, M.; Guy, C.D.; Omenetti, A.; Smith, A.D.; Diehl, A.M.E.; Suzuki, A. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013, 33, 1062–1070. [Google Scholar] [CrossRef]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.-J. Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction. Cell. Mol. Life Sci. 2024, 81, 34. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, H. Gamma-Aminobutyric Acid Signaling in Damage Response, Metabolism, and Disease. Int. J. Mol. Sci. 2023, 24, 4584. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Han, Z.; Wu, Z.; Xia, Y.; Yang, G.; Yin, Y.; Ren, W. GABA regulates IL-1β production in macrophages. Cell Rep. 2022, 41, 111770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Sun, S.P.; Zhu, H.S.; Jiao, X.Q.; Zhong, K.; Guo, Y.J.; Zha, G.-M.; Han, L.-Q.; Yang, G.-Y.; Li, H.-P. GABA regulates the proliferation and apoptosis of MAC-T cells through the LPS-induced TLR4 signaling pathway. Res. Vet. Sci. 2018, 118, 395–402. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Duan, F.; Lu, B. Depression and NAFLD risk: A meta-analysis and Mendelian randomization study. J. Affect. Disord. 2024, 352, 379–385. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Ibrahim, R.; Theberge, K.; May, M.; Houseknecht, K.L. Non-alcoholic fatty liver disease (NAFLD) and mental illness: Mechanisms linking mood, metabolism and medicines. Front. Neurosci. 2022, 16, 1042442. [Google Scholar] [CrossRef]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef]
- Goedeke, L.; Vales-Lara, F.M.; Fenstermaker, M.; Cirera-Salinas, D.; Chamorro-Jorganes, A.; Ramírez, C.M.; Mattison, J.A.; de Cabo, R.; Suarez, Y.; Fernández-Hernando, C. A Regulatory Role for MicroRNA 33* in Controlling Lipid Metabolism Gene Expression. Mol. Cell. Biol. 2013, 33, 2339–2352. [Google Scholar] [CrossRef]
- Auguet, T.; Aragonès, G.; Berlanga, A.; Guiu-Jurado, E.; Martí, A.; Martínez, S.; Sabench, F.; Hernández, M.; Aguilar, C.; Sirvent, J.J.; et al. miR33a/miR33b* and miR122 as Possible Contributors to Hepatic Lipid Metabolism in Obese Women with Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 1620. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Martinez, S.; Porras, J.A.; Aragonès, G.; Sabench, F.; Hernandez, M.; Aguilar, C.; Sirvent, J.J.; et al. Altered Fatty Acid Metabolism-Related Gene Expression in Liver from Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 22173–22187. [Google Scholar] [CrossRef]
- Kathiresan, S.; Melander, O.; Anevski, D.; Guiducci, C.; Burtt, N.P.; Roos, C.; Hirschhorn, J.N.; Orho-Melander, M. Polymorphisms Associated with Cholesterol and Risk of Cardiovascular Events. N. Engl. J. Med. 2008, 358, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H. Osthol attenuates hepatic steatosis via decreased triglyceride synthesis not by insulin resistance. World J. Gastroenterol. 2014, 20, 11753. [Google Scholar] [CrossRef]
- Miyagawa, S.; Horie, T.; Nishino, T.; Koyama, S.; Watanabe, T.; Baba, O.; Yamasaki, T.; Sowa, N.; Otani, C.; Matsushita, K.; et al. Inhibition of microRNA-33b in humanized mice ameliorates nonalcoholic steatohepatitis. Life Sci. Alliance 2023, 6, e202301902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qiao, Y.; Wang, M.; Liang, X.; Zhang, M.; Li, C.; Cairang, J.; Wang, J.; Bi, H.; Gao, T. The influence of genetic and acquired factors on the vulnerability to develop depression: A review. Biosci. Rep. 2023, 43, BSR20222644. [Google Scholar] [CrossRef] [PubMed]
- Chourpiliadis, C.; Zeng, Y.; Lovik, A.; Wei, D.; Valdimarsdóttir, U.; Song, H.; Hammar, N.; Fang, F. Metabolic Profile and Long-Term Risk of Depression, Anxiety, and Stress-Related Disorders. JAMA Netw. Open 2024, 7, e244525. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, G.; Lang, X.; Fu, Z.; Zhang, P.; Zheng, Y.; Zhang, X. Prevalence and clinical correlates of abnormal lipid metabolism in first-episode and drug-naïve patients with major depressive disorder with abnormal glucose metabolism. Sci. Rep. 2023, 13, 8078. [Google Scholar] [CrossRef]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef]
- Jin, M.; Ji, L.; Ran, M.; Bi, Y.; Zhang, H.; Tao, Y.; Xu, H.; Zou, S.; Zhang, H.; Yu, T.; et al. Interactions between ABC gene polymorphisms and processing speed in predicting depression severity. BMC Psychiatry 2025, 25, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Rao, Q.; Lin, K.; He, Y.; Cai, J.; Yang, M.; Xu, Y.; Hou, L.; Lin, Y.; Liu, H. CYP2C19-rs4986893 confers risk to major depressive disorder and bipolar disorder in the Han Chinese population whereas ABCB1-rs1045642 acts as a protective factor. BMC Psychiatry 2023, 23, 69. [Google Scholar] [CrossRef]
- Kleiner, D.E. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Leutner, M.; Dervic, E.; Bellach, L.; Klimek, P.; Thurner, S.; Kautzky, A. Obesity as pleiotropic risk state for metabolic and mental health throughout life. Transl. Psychiatry 2023, 13, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, I.Y.; Chang, Y.; Sung, E.; Kang, J.H.; Wild, S.H.; Byrne, C.D.; Shin, H.; Ryu, S. Depression and increased risk of non-alcoholic fatty liver disease in individuals with obesity. Epidemiol. Psychiatr. Sci. 2021, 30, e23. [Google Scholar] [CrossRef] [PubMed]
- Radford-Smith, D.E.; Patel, P.J.; Irvine, K.M.; Russell, A.; Siskind, D.; Anthony, D.C.; Powell, E.E.; Probert, F. Depressive symptoms in non-alcoholic fatty liver disease are identified by perturbed lipid and lipoprotein metabolism. PLoS ONE 2022, 17, e0261555. [Google Scholar] [CrossRef]
- Labenz, C.; Huber, Y.; Michel, M.; Nagel, M.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Nonalcoholic Fatty Liver Disease Increases the Risk of Anxiety and Depression. Hepatol. Commun. 2020, 4, 1293–1301. [Google Scholar] [CrossRef]
- Dong, T.; Li, J.; Liu, Y.; Zhou, S.; Wei, X.; Hua, H.; Tang, K.; Zhang, X.; Wang, Y.; Wu, Z.; et al. Roles of immune dysregulation in MASLD. Biomed. Pharmacother. 2024, 170, 116069. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, T.; Ma, L.; Zhang, Z.; Xu, M.; Zhang, T.; Xu, H.; Tang, X.; Yang, S.; Yu, S.; et al. Clinical Features and Treatment Outcomes of Chronic Nonbacterial Osteomyelitis in Children: A Multicenter Study in China. Med. J. Peking Union Med. Coll. Hosp. 2023, 14, 278–284. [Google Scholar] [CrossRef]
- Vieta, E.; Alonso, J.; Pérez-Sola, V.; Roca, M.; Hernando, T.; Sicras-Mainar, A.; Sicras-Navarro, A.; Herrera, B.; Gabilondo, A. Epidemiology and costs of depressive disorder in Spain: The EPICO study. Eur. Neuropsychopharmacol. 2021, 50, 93–103. [Google Scholar] [CrossRef]
- Frank, P.; Jokela, M.; Batty, G.D.; Lassale, C.; Steptoe, A.; Kivimäki, M. Overweight, obesity, and individual symptoms of depression: A multicohort study with replication in UK Biobank. Brain Behav. Immun. 2022, 105, 192–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wang, Y.; Yang, X.; Li, Y.; Gui, J.; Mei, Y.; Liu, H.; Guo, L.; Li, J.; Lei, Y.; et al. Obesity and lipid indices as predictors of depressive symptoms in middle-aged and elderly Chinese: Insights from a nationwide cohort study. BMC Psychiatry 2024, 24, 351. [Google Scholar] [CrossRef]
- Tomeno, W.; Kawashima, K.; Yoneda, M.; Saito, S.; Ogawa, Y.; Honda, Y.; Kessoku, T.; Imajo, K.; Mawatari, H.; Fujita, K.; et al. Non-alcoholic fatty liver disease comorbid with major depressive disorder: The pathological features and poor therapeutic efficacy. J. Gastroenterol. Hepatol. 2015, 30, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kronsten, V.T.; Tranah, T.H.; Pariante, C.; Shawcross, D.L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J. Hepatol. 2022, 76, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.B.; Whang, W.; Shimbo, D.; Shah, A.; Shapiro, P.A.; Davidson, K.W. Exposure to tricyclic antidepressants is associated with an increased risk of incident CHD events in a population-based study. Int. J. Cardiol. 2010, 145, 124–125. [Google Scholar] [CrossRef]
- Khalil, S.M.; MacKenzie, K.R.; Maletic-Savatic, M.; Li, F. Metabolic bioactivation of antidepressants: Advance and underlying hepatotoxicity. Drug Metab. Rev. 2024, 56, 97–126. [Google Scholar] [CrossRef]
- Mullish, B.H.; Kabir, M.S.; Thursz, M.R.; Dhar, A. Review article: Depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment. Pharmacol. Ther. 2014, 40, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Nebhinani, N.; Sharma, P.; Pareek, V.; Suthar, N.; Jakhotia, S.; Gehlot, M.; Purohit, P. Association of Inflammatory and Liver Markers with Cardiometabolic Risk Factors in Patients with Depression. Ind. J. Clin. Biochem. 2019, 34, 219–224. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.H. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. J. Affect. Disord. 2021, 278, 259–263. [Google Scholar] [CrossRef]
- Sanford, J.D.; Franklin, D.; Grois, G.A.; Jin, A.; Zhang, Y. Carnitine o-octanoyltransferase is a p53 target that promotes oxidative metabolism and cell survival following nutrient starvation. J. Biol. Chem. 2023, 299, 104908. [Google Scholar] [CrossRef]
- Demircan, A.; Coskunpinar, E.; Kanca, D.; Ozkara, G.; Yanar, F.; Kurnaz-Gomleksiz, O.; Burga, Z.; Ozturk, O.; Yilmaz Aydogan, H. Role of SNPs of CPTIA and CROT genes in the carnitine-shuttle in coronary artery disease: A case-control study. Turk. J. Biochem. 2019, 44, 822–830. [Google Scholar] [CrossRef]
- Okui, T.; Kuraoka, S.; Iwashita, M.; Itagawa, R.; Kasai, T.; Aikawa, M.; Singh, S.A.; Aikawa, E. Carnitine O-octanoyltransferase (CROT) deficiency in mice leads to an increase of omega-3 fatty acids. Front. Mol. Biosci. 2024, 11, 1374316. [Google Scholar] [CrossRef]
- Hardy, L.; Frisdal, E.; Le Goff, W. Critical Role of the Human ATP-Binding Cassette G1 Transporter in Cardiometabolic Diseases. Int. J. Mol. Sci. 2017, 18, 1892. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.L.; Parkinson, P.F.; Racke, M.M.; Hirsch-Reinshagen, V.; Fan, J.; Wong, C.; Stukas, S.; Theroux, L.; Chan, J.Y.; Donkin, J.; et al. ABCG1 influences the brain cholesterol biosynthetic pathway but does not affect amyloid precursor protein or apolipoprotein E metabolism in vivo. J. Lipid Res. 2008, 49, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Brückl, T.M.; Uhr, M. ABCB1 Genotyping in the Treatment of Depression. Pharmacogenomics 2016, 17, 2039–2069. [Google Scholar] [CrossRef]
- Barbhuiya, P.A.; Yoshitomi, R.; Pathak, M.P. Understanding the Link Between Sterol Regulatory Element Binding Protein (SREBPs) and Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD). Curr. Obes. Rep. 2025, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Raeder, M.B.; Fernø, J.; Glambek, M.; Stansberg, C.; Steen, V.M. Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci. Lett. 2006, 395, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.M.; Niskanen, L.; Tolmunen, T.; Hintikka, J.; Viinamäki, H.; Heiskanen, T.; Honkalampi, K.; Kokkonen, M.; Koivumaa-Honkanen, H. Low serum HDL-cholesterol levels are associated with long symptom duration in patients with major depressive disorder. Psychiatry Clin. Neurosci. 2010, 64, 279–283. [Google Scholar] [CrossRef]
- Han, A.L. Association between lipid ratio and depression: A cross-sectional study. Sci. Rep. 2022, 12, 6190. [Google Scholar] [CrossRef]

| Variable | Total Cohort (n = 152) | CN Group (n = 107) | DD Group (n = 45) | p Value |
|---|---|---|---|---|
| Age (years) | 49.0 (39.8–56.1) | 48.6 (39.3–55.1) | 50.4 (42.9–58.9) | 0.035 * |
| Gender (n, % females) | 137 (90.1%) | 92 (86.0%) | 45 (100%) | 0.008 * |
| Weight (kg) | 119.0 (108.0–131.8) | 120.0 (107.7–132.0) | 117.0 (109.0–128.5) | 0.884 |
| Height (m) | 1.6 (1.6–1.7) | 1.6 (1.6–1.7) | 1.6 (1.6–1.6) | 0.054 |
| BMI (kg/m2) | 46.2 (43.1–49.8) | 45.9 (42.7–48.9) | 47.0 (43.9–51.6) | 0.064 |
| SBP (mmHg) | 133.0 (120.0–145.0) | 133.0 (120.0–142.5) | 137.0 (120.0–150.0) | 0.455 |
| DBP (mmHg) | 75.0 (65.0–85.0) | 71.0 (65.0–85.0) | 80.0 (63.0–89.0) | 0.319 |
| HOMA-1R | 4.8 (2.5–8.2) | 5.0 (2.6–8.2) | 4.0 (1.6–7.5) | 0.737 |
| Glucose (mg/dL) | 107.0 (89.0–133.0) | 105.0 (89.8–133.5) | 112.0 (87.5–134.0) | 0.955 |
| Insulin (mUI/L) | 16.0 (8.6–24.8) | 16.0 (8.5–24.9) | 15.3 (8.8–24.2) | 0.798 |
| HbA1c (%) | 5.5 (5.0–6.1) | 5.5 (5.0–6.1) | 5.6 (5.1–6.7) | 0.251 |
| Cholesterol (mg/dL) | 168.6 (148.0–195.3) | 169.5 (149.5–192.6) | 166.9 (147.0–200.9) | 0.823 |
| HDL-C (mg/dL) | 38.0 (33.0–44.0) | 38.0 (33.0–43.8) | 38.3 (31.3–47.0) | 0.509 |
| LDL-C (mg/dL) | 98.0 (80.0–122.6) | 97.0 (82.5–123.6) | 100.1 (78.0–118.8) | 0.920 |
| TG (mg/dL) | 151.0 (117.0–205.8) | 153.0 (116.5–206.8) | 147.5 (117.0–203.0) | 0.970 |
| AST (UI/L) | 34.0 (24.0–48.8) | 33.0 (24.6–46.0) | 34.0 (21.0–56.0) | 0.903 |
| ALT (UI/L) | 35.0 (24.8–53.0) | 35.0 (25.5–53.0) | 35.0 (24.0–54.5) | 0.914 |
| GGT (UI/L) | 24.0 (14.5–45.5) | 21.6 (14.0–42.3) | 33.0 (17.5–52.2) | 0.071 |
| ALP (Ul/L) | 69.0 (56.0–77.5) | 68.0 (53.5–78.0) | 69.0 (57.0–76.5) | 0.562 |
| CRP (mg/dL) | 10 (0.5–2.0) | 1.0 (0.5–2.0) | 1.0 (0.4–2.0) | 0.537 |
| Medication | CN Group (n = 107) | DD Group (n = 45) | Xi2 (df) | p Value |
|---|---|---|---|---|
| Antidepressants | 0 (0%) | 39 (86.7%) | 123.856 (1) | <0.001 * |
| Antihypertensive | 49 (45.8%) | 27 (60%) | 2.557 (1) | 0.110 |
| Lipid-lowering agents—Statins | 17 (15.9%) | 13 (28.9%) | 3.380 (1) | 0.066 |
| Lipid-lowering agents—Fibrates | 4 (3.7%) | 2 (4.4%) | 0.042 (1) | 0.838 |
| Diabetes treatment—Insulin | 5 (4.7%) | 3 (6.7%) | 0.253 (1) | 0.615 |
| Diabetes treatment—Oral | 25 (23.4%) | 14 (31.1%) | 0.997 (1) | 0.318 |
| Analgesics | 10 (9.4%) | 4 (8.9%) | 0.011 (1) | 0.916 |
| Analgesics—opioids | 3 (2.8%) | 1 (2.2%) | 0.045 (1) | 0.831 |
| Anticoagulants—oral | 1 (0.9%) | 1 (2.2%) | 0.395 (1) | 0.530 |
| Antiepileptics | 0 (0%) | 4 (8.9%) | 9.679 (1) | 0.002 * |
| Antihistamines | 2 (1.9%) | 0 (0%) | 0.860 (1) | 0.354 |
| Anti-inflammatories | 8 (7.5%) | 8 (17.8%) | 3.490 (1) | 0.062 |
| Benzodiazepines | 5 (4.7%) | 18 (40%) | 30.457 (1) | <0.001 * |
| Corticosteroids | 1 (0.9%) | 1 (2.2%) | 0.395 (1) | 0.530 |
| Morphine | 0 (0%) | 1 (2.2%) | 2.371 (1) | 0.124 |
| Hepatoprotectors | 1 (1%) | 0 (0%) | 0.422 (1) | 0.516 |
| Vitamins | 5 (4.9%) | 4 (8.9%) | 0.864 (1) | 0.353 |
| Hepatic Diagnosis | CN Group (n = 107) | DD Group (n = 45) | Xi2 (df) | p Value |
|---|---|---|---|---|
| NL | 18 (16.8%) | 10 (22.2%) | 3.429 (2) | 0.180 |
| SS | 31 (29%) | 18 (40%) | ||
| MASH | 58 (54.2%) | 17 (37.8%) | ||
| Liver histology | ||||
| Steatosis | 89 (83.2%) | 35 (77.8%) | 0.615 (1) | 0.433 |
| Lobular inflammation | 16 (55.2%) | 6 (40%) | 0.910 (1) | 0.340 |
| Ballooning | 16 (55.2%) | 6 (40%) | 0.910 (1) | 0.340 |
| Liver fibrosis | 12 (11.2%) | 4 (8.9%) | 0.182 (1) | 0.670 |
| Comorbid Condition | CN Group (n = 107) | DD Group (n = 45) | OR | p Value |
|---|---|---|---|---|
| T2DM | 32 (30.5%) | 15 (33.3%) | 0.943 | 0.883 |
| Metabolic Syndrome | 83 (77.6%) | 33 (73.3%) | 0.928 | 0.928 |
| High Blood Pressure | 49 (55.1%) | 23 (57.5%) | 1.189 | 0.672 |
| MASLD | 89 (83.2%) | 35 (77.8%) | 0.851 | 0.720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, J.M.; Rusu, E.C.; Camaron, J.; Clavero-Mestres, H.; Aguilar, C.; Riesco, D.; Xifré, B.; Chicote, J.U.; Martinez, S.; Vives, M.; et al. Differential Expression of Lipid Metabolism Genes, CROT and ABCG1, in Obese Patients with Comorbid Depressive Disorder and Risk of MASLD. Metabolites 2025, 15, 392. https://doi.org/10.3390/metabo15060392
Jurek JM, Rusu EC, Camaron J, Clavero-Mestres H, Aguilar C, Riesco D, Xifré B, Chicote JU, Martinez S, Vives M, et al. Differential Expression of Lipid Metabolism Genes, CROT and ABCG1, in Obese Patients with Comorbid Depressive Disorder and Risk of MASLD. Metabolites. 2025; 15(6):392. https://doi.org/10.3390/metabo15060392
Chicago/Turabian StyleJurek, Joanna Michalina, Elena Cristina Rusu, Javier Camaron, Helena Clavero-Mestres, Carmen Aguilar, David Riesco, Belen Xifré, Javier U. Chicote, Salomé Martinez, Marga Vives, and et al. 2025. "Differential Expression of Lipid Metabolism Genes, CROT and ABCG1, in Obese Patients with Comorbid Depressive Disorder and Risk of MASLD" Metabolites 15, no. 6: 392. https://doi.org/10.3390/metabo15060392
APA StyleJurek, J. M., Rusu, E. C., Camaron, J., Clavero-Mestres, H., Aguilar, C., Riesco, D., Xifré, B., Chicote, J. U., Martinez, S., Vives, M., Sabench, F., & Auguet, T. (2025). Differential Expression of Lipid Metabolism Genes, CROT and ABCG1, in Obese Patients with Comorbid Depressive Disorder and Risk of MASLD. Metabolites, 15(6), 392. https://doi.org/10.3390/metabo15060392





