Alteration of Metabolic Profile in Patients with Narcolepsy Type 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Participants
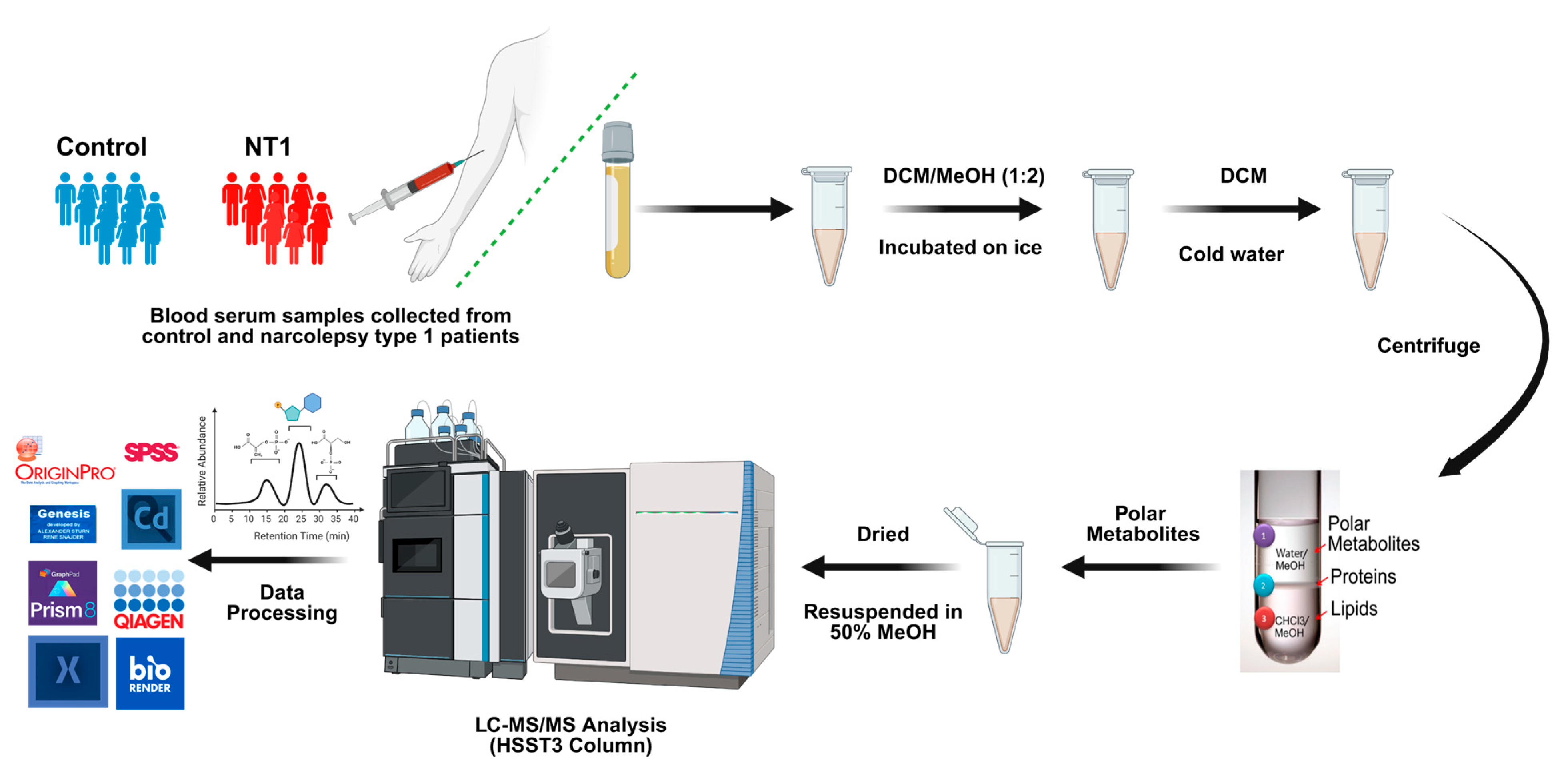
2.3. Metabolite Extraction
2.4. LC-MS/MS Parameters
2.5. Data Analysis
2.6. Ingenuity Pathway Analysis
2.7. Validation with LC-PRM-MS
3. Results
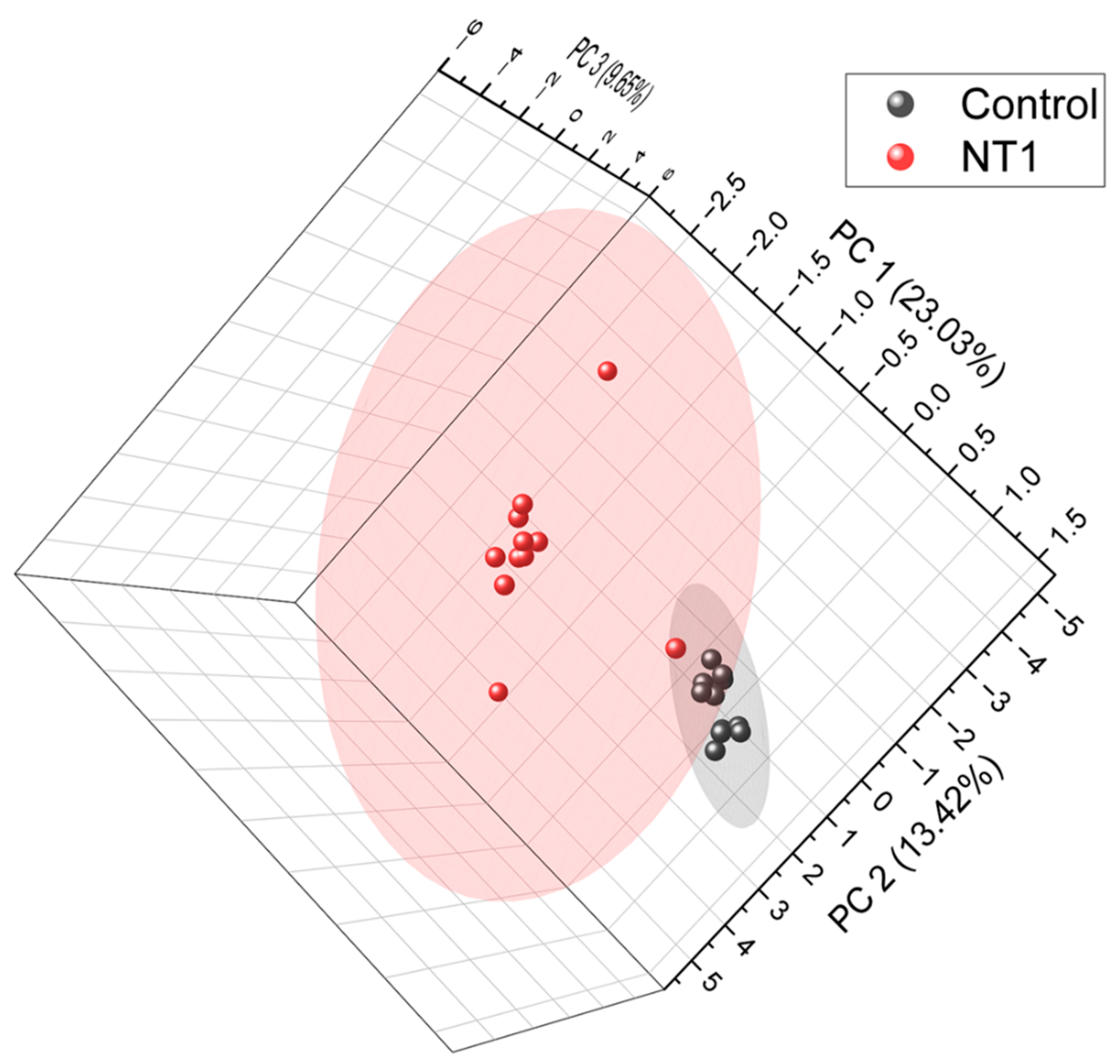
3.1. Unsupervised Principal Component Analysis of All Metabolites
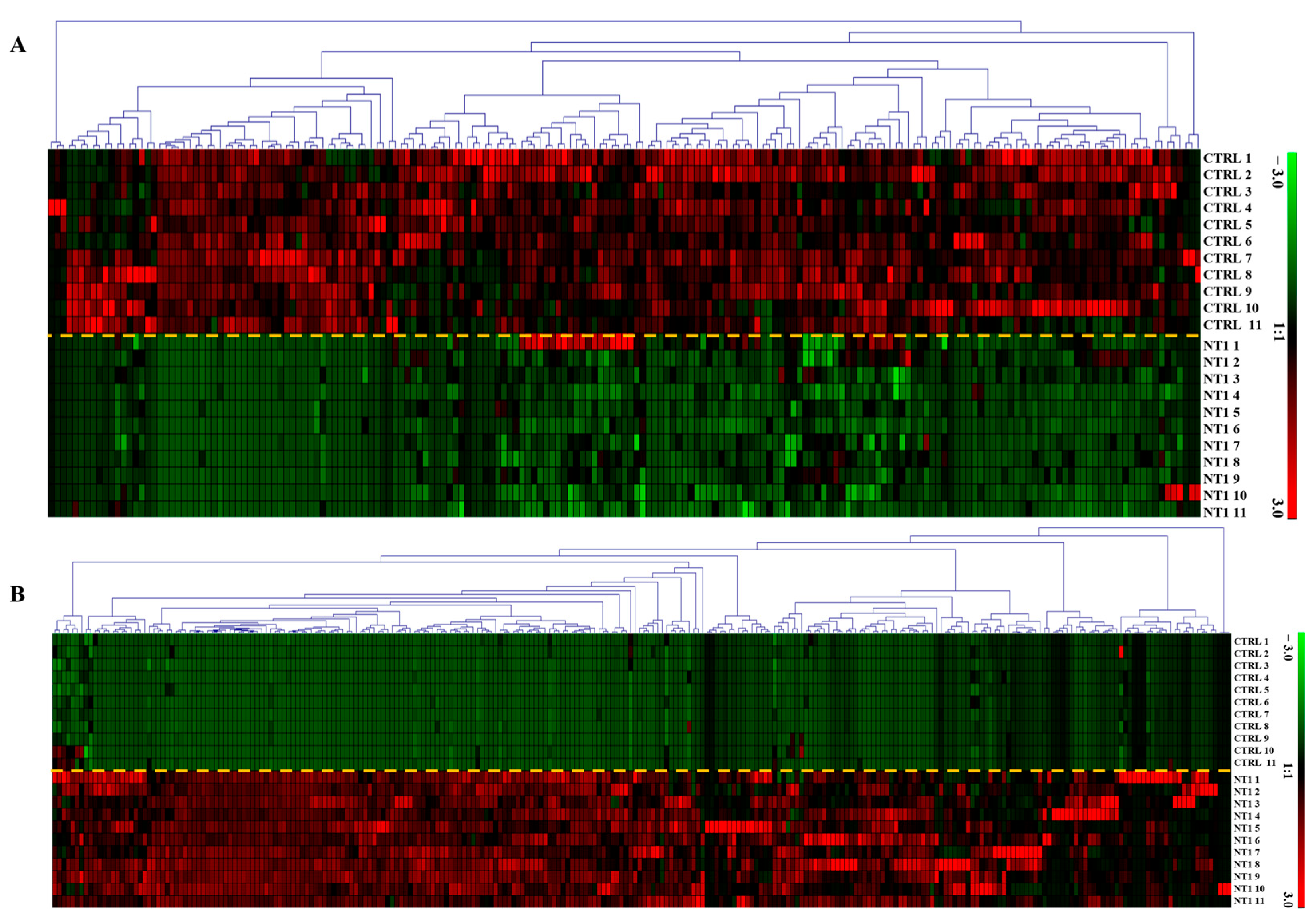
3.2. Heatmap of Differentially Abundant Metabolites
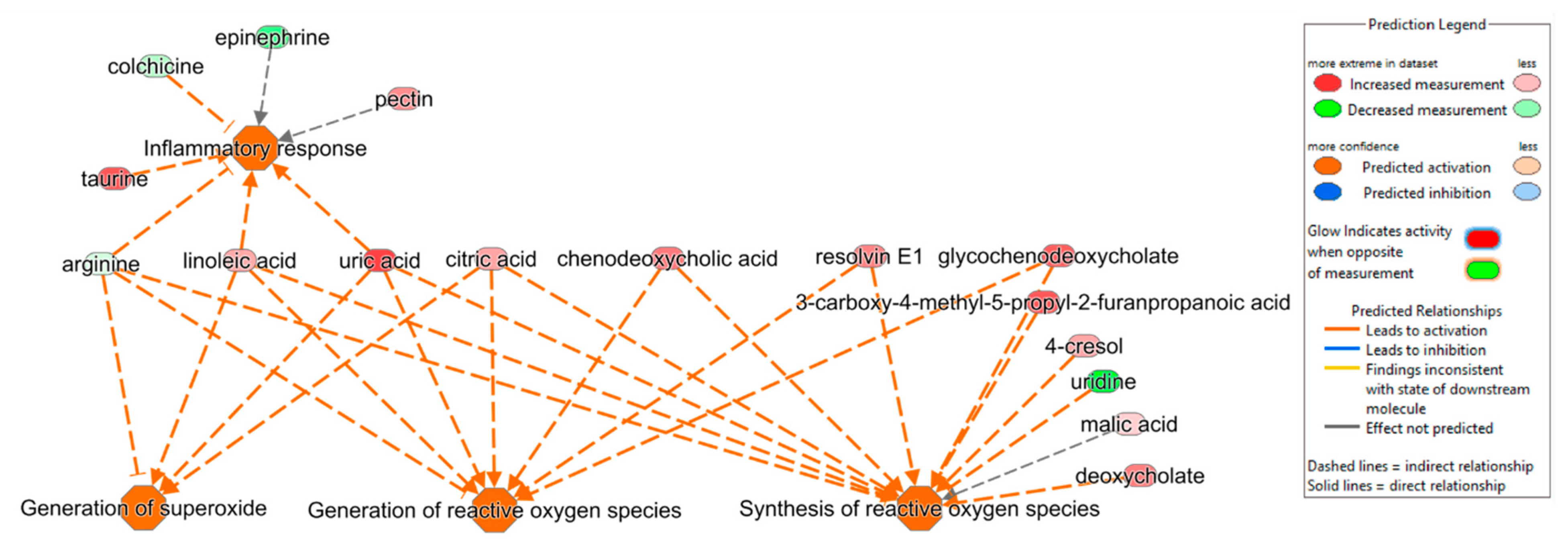
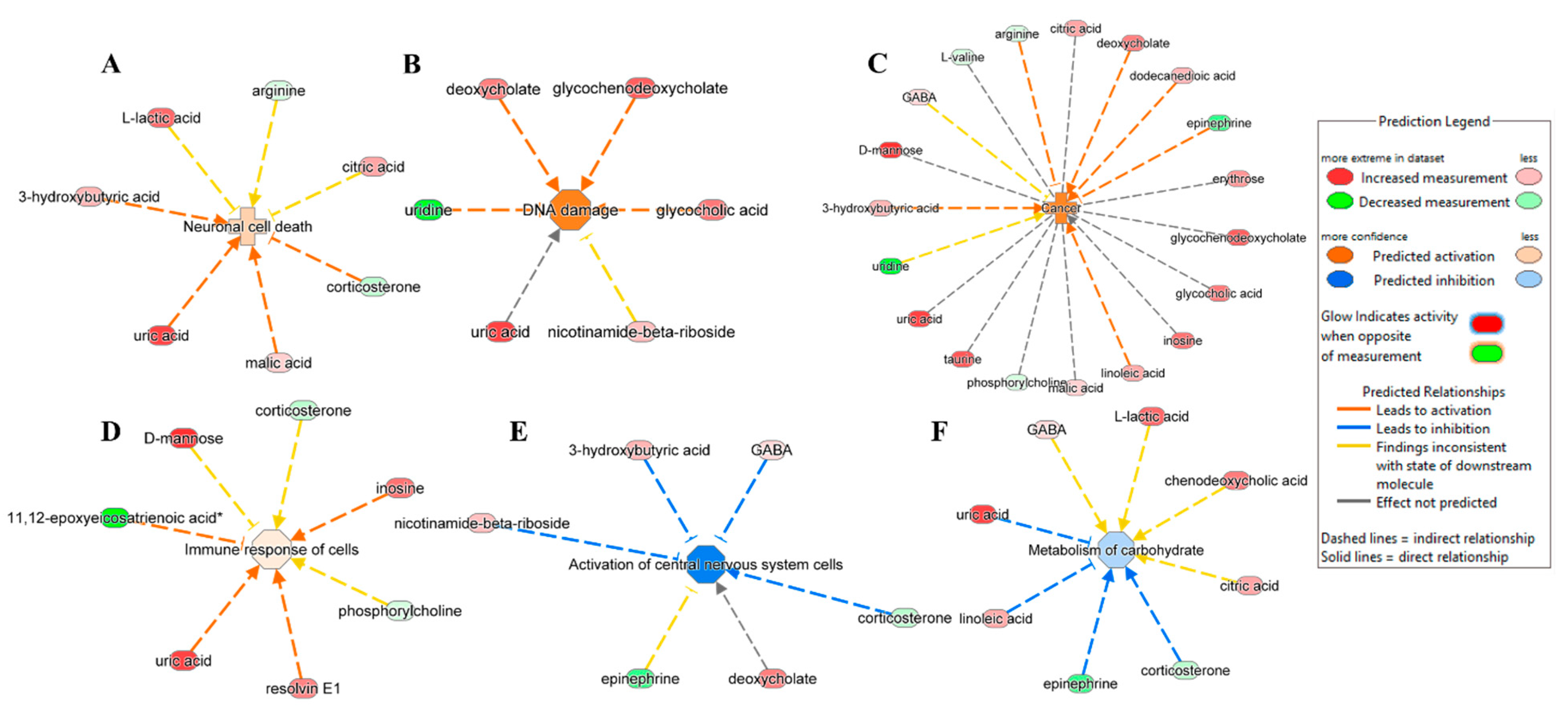
3.3. Ingenuity Pathway Analysis
3.4. Validation of DAMs by PRM
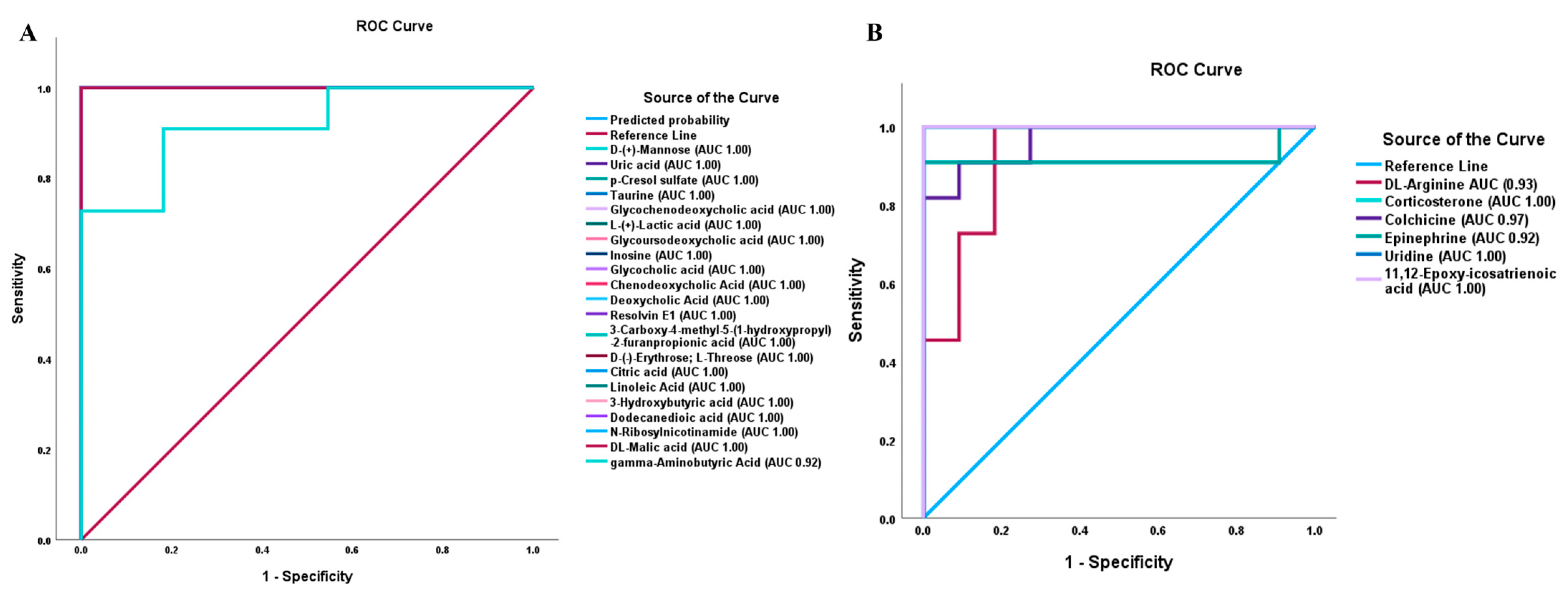
3.5. Box Plots and ROC Analysis of DAMs Associated with Important Biological Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NT1 | Narcolepsy type 1 |
| DCM | Dichloromethane |
| HLA | Human leukocyte antigen |
| CSF | Cerebrospinal fluid |
| MPA | Mobile phase A |
| MPB | Mobile phase B |
| HCD | High-energy collision dissociation |
| IPA | Ingenuity pathway analysis |
| HMDB | Human Metabolome Database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PRM | Parallel reaction monitoring |
| PCA | Principal component analysis |
| ROS | Reactive oxygen species |
| GABA | Gamma-amino-butyric acid |
| DAMs | Differentially abundant metabolites |
| ROC | Receiver Operating Characteristics |
| FC | Fold change |
| AUC | Area under the curve |
| EDS | Excessive daytime sleepiness |
| REM | Rapid eye movement |
| NO | Nitric oxide |
| CNS | Central nervous system |
References
- Siegel, J.M. Narcolepsy. Sci. Am. 2000, 282, 76–81. [Google Scholar] [CrossRef]
- Rahman, T.; Farook, O.; Heyat, B.B.; Siddiqui, M.M. An overview of narcolepsy. IARJSET 2016, 3, 85–87. [Google Scholar]
- Guilleminault, C. Narcolepsy. In Sleep Disorders Medicine; Elsevier: Amsterdam, The Netherlands, 1994; pp. 241–254. [Google Scholar]
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Primers 2017, 3, 16100. [Google Scholar] [CrossRef]
- Bassetti, C.L.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Peraita-Adrados, R.; Martínez-Orozco, F.J. Sleep disorder comorbidities in narcolepsy. In Narcolepsy: A Clinical Guide; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–175. [Google Scholar]
- Severin, E.; Mațotă, A.-M.; Bordeianu, A.; Jidovu, A. Exploring the Literature on Narcolepsy: Insights into the Sleep Disorder That Strikes during the Day. preprint 2023. [Google Scholar] [CrossRef]
- Vringer, M.; Zhou, J.; Gool, J.K.; Bijlenga, D.; Lammers, G.J.; Fronczek, R.; Schinkelshoek, M.S. Recent Insights into the Pathophysiology of Narcolepsy Type 1. Sleep Med. Rev. 2024, 78, 101993. [Google Scholar] [CrossRef]
- Barateau, L.; Lopez, R.; Dauvilliers, Y. Management of narcolepsy. Curr. Treat. Options Neurol. 2016, 18, 43. [Google Scholar] [CrossRef]
- Heidbreder, A.; Dirks, C.; Ramm, M. Therapy for Cataplexy. Curr. Treat. Options Neurol. 2020, 22, 13. [Google Scholar] [CrossRef]
- Rogers, A.A.; Aiani, L.M.; Blanpain, L.T.; Yuxian, S.; Moore, R.; Willie, J.T. Deep brain stimulation of hypothalamus for narcolepsy-cataplexy in mice. Brain Stimul. 2020, 13, 1305–1316. [Google Scholar] [CrossRef]
- Carter, M.E.; Brill, J.; Bonnavion, P.; Huguenard, J.R.; Huerta, R.; de Lecea, L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Natl. Acad. Sci. USA 2012, 109, E2635–E2644. [Google Scholar] [CrossRef]
- Ono, D.; Yamanaka, A. Hypothalamic regulation of the sleep/wake cycle. Neurosci. Res. 2017, 118, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, L.; Zhang, B.; Zhu, W.; Dai, W.; Li, S.; Xu, H. The mechanism of different orexin/hypocretin neuronal projections in wakefulness and sleep. Brain Res. 2025, 1850, 149408. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; Liguori, R.; Plazzi, G.; Pizza, F. Reviewing the clinical implications of treating narcolepsy as an autoimmune disorder. In Nature and Science of Sleep; Taylor & Francis: Oxford, UK, 2021; pp. 557–577. [Google Scholar]
- Han, F.; Lin, L.; Schormair, B.; Pizza, F.; Plazzi, G.; Ollila, H.M.; Nevsimalova, S.; Jennum, P.; Knudsen, S.; Winkelmann, J. HLA DQB1* 06: 02 negative narcolepsy with hypocretin/orexin deficiency. Sleep 2014, 37, 1601–1608. [Google Scholar] [CrossRef]
- Ollila, H.M.; Ravel, J.M.; Han, F.; Faraco, J.; Lin, L.; Zheng, X.; Plazzi, G.; Dauvilliers, Y.; Pizza, F.; Hong, S.C.; et al. HLA-DPB1 and HLA class I confer risk of and protection from narcolepsy. Am. J. Hum. Genet. 2015, 96, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Capittini, C.; De Silvestri, A.; Terzaghi, M.; Scotti, V.; Rebuffi, C.; Pasi, A.; Manni, R.; Martinetti, M.; Tinelli, C. Correlation between HLA-DQB1*06:02 and narcolepsy with and without cataplexy: Approving a safe and sensitive genetic test in four major ethnic groups. A systematic meta-analysis. Sleep Med. 2018, 52, 150–157. [Google Scholar] [CrossRef]
- Ohno, K.; Sakurai, T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front. Neuroendocrinol. 2008, 29, 70–87. [Google Scholar] [CrossRef]
- Mignot, E.; Zeitzer, J.; Pizza, F.; Plazzi, G. Sleep problems in narcolepsy and the role of hypocretin/orexin deficiency. Orexin Syst. Basic Sci. Role Sleep Pathol. 2021, 45, 103–116. [Google Scholar]
- Vinayavekhin, N.; Homan, E.A.; Saghatelian, A. Exploring Disease through Metabolomics. ACS Chem. Biol. 2010, 5, 91–103. [Google Scholar] [CrossRef]
- Baker, S.A.; Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A Global Biochemical Approach to Drug Response and Disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.H.; Lee, H.S.; Choi, G.; Jung, Y.S.; Ryu, D.; Park, H.S.; Hwang, G.S. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy 2013, 43, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Thomas, N.; Gooley, P.R.; Armstrong, C.W. Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research. Metabolites 2022, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.G.; Oh, J.; Keasling, A.; Colson, K.L.; Hamann, M.T. The utility of metabolomics in natural product and biomarker characterization. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2014, 1840, 3460–3474. [Google Scholar] [CrossRef] [PubMed]
- Pouralijan Amiri, M.; Khoshkam, M.; Salek, R.M.; Madadi, R.; Faghanzadeh Ganji, G.; Ramazani, A. Metabolomics in early detection and prognosis of acute coronary syndrome. Clin. Chim. Acta 2019, 495, 43–53. [Google Scholar] [CrossRef]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, L.; Liu, N.; Yuan, Y.; Deng, L.; Ding, Y.; Wang, F.; Zhou, J.; Xie, L. Serum metabolomics study of narcolepsy type 1 based on ultra-performance liquid chromatography-tandem mass spectrometry. Amino Acids 2023, 55, 1247–1259. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Barateau, L.; Middleton, B.; van der Veen, D.R.; Skene, D.J. Metabolomics Signature of Patients With Narcolepsy. Neurology 2022, 98, e493–e505. [Google Scholar] [CrossRef]
- Shimada, M.; Miyagawa, T.; Kodama, T.; Toyoda, H.; Tokunaga, K.; Honda, M. Metabolome analysis using cerebrospinal fluid from narcolepsy type 1 patients. Sleep 2020, 43, zsaa095. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar] [CrossRef]
- Sanni, A.; Goli, M.; Zhao, J.; Wang, J.; Barsa, C.; El Hayek, S.; Talih, F.; Lanuzza, B.; Kobeissy, F.; Plazzi, G.; et al. LC-MS/MS-Based Proteomics Approach for the Identification of Candidate Serum Biomarkers in Patients with Narcolepsy Type 1. Biomolecules 2023, 13, 420. [Google Scholar] [CrossRef]
- Onigbinde, S.; Gutierrez Reyes, C.D.; Sandilya, V.; Chukwubueze, F.; Oluokun, O.; Sahioun, S.; Oluokun, A.; Mechref, Y. Optimization of glycopeptide enrichment techniques for the identification of clinical biomarkers. Expert Rev. Proteom. 2024, 21, 431–462. [Google Scholar] [CrossRef]
- Donohoo, K.B.; Wang, J.; Goli, M.; Yu, A.; Peng, W.; Hakim, M.A.; Mechref, Y. Advances in mass spectrometry-based glycomics-An update covering the period 2017–2021. Electrophoresis 2022, 43, 119–142. [Google Scholar] [CrossRef]
- Xu, T.; Hu, C.; Xuan, Q.; Xu, G. Recent advances in analytical strategies for mass spectrometry-based lipidomics. Anal. Chim. Acta 2020, 1137, 156–169. [Google Scholar] [CrossRef]
- Chen, C.J.; Lee, D.Y.; Yu, J.; Lin, Y.N.; Lin, T.M. Recent advances in LC-MS-based metabolomics for clinical biomarker discovery. Mass Spectrom. Rev. 2023, 42, 2349–2378. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.E.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Griffin, J.L.; Atherton, H.; Shockcor, J.; Atzori, L. Metabolomics as a tool for cardiac research. Nat. Rev. Cardiol. 2011, 8, 630–643. [Google Scholar] [CrossRef]
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2012, 8, 22–33. [Google Scholar] [CrossRef]
- Schumacher-Schuh, A.; Bieger, A.; Borelli, W.V.; Portley, M.K.; Awad, P.S.; Bandres-Ciga, S. Advances in proteomic and metabolomic profiling of neurodegenerative diseases. Front. Neurol. 2022, 12, 792227. [Google Scholar] [CrossRef]
- Quintero Escobar, M.; Pontes, J.G.d.M.; Tasic, L. Metabolomics in degenerative brain diseases. Brain Res. 2021, 1773, 147704. [Google Scholar] [CrossRef]
- Shao, Y.; Le, W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol. Neurodegener. 2019, 14, 3. [Google Scholar] [CrossRef]
- Hassan-Smith, G.; Wallace, G.R.; Douglas, M.R.; Sinclair, A.J. The role of metabolomics in neurological disease. J. Neuroimmunol. 2012, 248, 48–52. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Krishnan, K.R.R. Metabolomics: A Global Biochemical Approach to the Study of Central Nervous System Diseases. Neuropsychopharmacology 2009, 34, 173–186. [Google Scholar] [CrossRef]
- Bonomo, R.; Cavaletti, G.; Skene, D.J. Metabolomics markers in neurology: Current knowledge and future perspectives for therapeutic targeting. Expert Rev. Neurother. 2020, 20, 725–738. [Google Scholar] [CrossRef]
- Nybo, S.E.; Lamberts, J.T. Integrated use of LC/MS/MS and LC/Q-TOF/MS targeted metabolomics with automated label-free microscopy for quantification of purine metabolites in cultured mammalian cells. Purinergic Signal. 2019, 15, 17–25. [Google Scholar] [CrossRef]
- Russell, K.L.; Rodman, H.R.; Pak, V.M. Sleep insufficiency, circadian rhythms, and metabolomics: The connection between metabolic and sleep disorders. Sleep Breath. 2023, 27, 2139–2153. [Google Scholar] [CrossRef]
- Yoon, S.J.; Long, N.P.; Jung, K.-H.; Kim, H.M.; Hong, Y.J.; Fang, Z.; Kim, S.J.; Kim, T.J.; Anh, N.H.; Hong, S.-S.; et al. Systemic and Local Metabolic Alterations in Sleep-Deprivation-Induced Stress: A Multiplatform Mass-Spectrometry-Based Lipidomics and Metabolomics Approach. J. Proteome Res. 2019, 18, 3295–3304. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.T.; Purba, W.; Daramola, O.; Bhuiyan, M.M.A.A.; Nwaiwu, J.; Fowowe, M.; Wang, J.; Hamdy, N.A.; Agami, M.A.; El-Feky, A.Y. Positive impact of DPP-4 or SGLT2 inhibitors on mild cognitive impairment in type 2 diabetes patients on metformin therapy: A metabolomic mechanistic insight. Biomed. Pharmacother. 2025, 182, 117771. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard Jr, J.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Yu, L.; Zhuang, Q.-X.; Zhu, J.-N.; Wang, J.-J. Central functions of the orexinergic system. Neurosci. Bull. 2013, 29, 355–365. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; text revision; American Academy of Sleep Medicine: Darien, IL, USA, 2023. [Google Scholar]
- Atashi, M.; Reyes, C.D.G.; Sandilya, V.; Purba, W.; Ahmadi, P.; Hakim, M.A.; Kobeissy, F.; Plazzi, G.; Moresco, M.; Lanuzza, B.; et al. LC-MS/MS Quantitation of HILIC-Enriched N-glycopeptides Derived from Low-Abundance Serum Glycoproteins in Patients with Narcolepsy Type 1. Biomolecules 2023, 13, 1589. [Google Scholar] [CrossRef]
- Sanni, A.; Hakim, M.A.; Goli, M.; Adeniyi, M.; Talih, F.; Lanuzza, B.; Kobeissy, F.; Plazzi, G.; Moresco, M.; Mondello, S.; et al. Serum N-Glycan Profiling of Patients with Narcolepsy Type 1 Using LC-MS/MS. ACS Omega 2024, 9, 32628–32638. [Google Scholar] [CrossRef]
- Berczi, I.; Stephano, A.Q. Chapter 8—Vasopressin, the Acute Phase Response and Healing. In Insights to Neuroimmune Biology, 2nd ed.; Berczi, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 185–199. [Google Scholar]
- Finamore, P.; Scarlata, S.; Cardaci, V.; Antonelli Incalzi, R. Exhaled breath analysis in obstructive sleep apnea syndrome: A review of the literature. Medicina 2019, 55, 538. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Qian, Y.; Guan, J.; Yi, H.; Zou, J.; Wang, Y.; Meng, L.; Zhao, A.; Yin, S.; et al. Metabolomics Profiling for Obstructive Sleep Apnea and Simple Snorers. Sci. Rep. 2016, 6, 30958. [Google Scholar] [CrossRef]
- Tasali, E.; Ip, M.S. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc. Am. Thorac. Soc. 2008, 5, 207–217. [Google Scholar] [CrossRef]
- Fritsche, K.L. Too much linoleic acid promotes inflammation—Doesn’t it? Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 173–175. [Google Scholar] [CrossRef]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.G.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef]
- Rhee, S.G. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhuang, Z.; Wang, W.; He, L.; Wu, H.; Cao, Y.; Pan, F.; Zhao, J.; Hu, Z.; Sekhar, C. OGG1 is essential in oxidative stress induced DNA demethylation. Cell. Signal. 2016, 28, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxid. Redox Signal. 2021, 34, 402–420. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Mohammadi, S.; Dolatshahi, M.; Zare-Shahabadi, A.; Rahmani, F. Untangling narcolepsy and diabetes: Pathomechanisms with eyes on therapeutic options. Brain Res. 2019, 1718, 212–222. [Google Scholar] [CrossRef]
- Virarkar, M.; Alappat, L.; Bradford, P.G.; Awad, A.B. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013, 53, 1157–1167. [Google Scholar] [CrossRef]
- Guix, F.; Uribesalgo, I.; Coma, M.; Munoz, F. The physiology and pathophysiology of nitric oxide in the brain. Prog. Neurobiol. 2005, 76, 126–152. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Kori, M.; Aydın, B.; Unal, S.; Arga, K.Y.; Kazan, D. Metabolic Biomarkers and Neurodegeneration: A Pathway Enrichment Analysis of Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. OMICS J. Integr. Biol. 2016, 20, 645–661. [Google Scholar] [CrossRef]
- Fleszar, M.G.; Wiśniewski, J.; Zboch, M.; Diakowska, D.; Gamian, A.; Krzystek-Korpacka, M. Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes. Sci. Rep. 2019, 9, 13764. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Santosh, W.; Kumar, S.; Christlet, H.T.T. Metabolic profiling of Parkinson’s disease: Evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 2009, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.P. Biochemical changes in the brain and metabolism as risk factors of neurological disorders. World J. Neurosci. 2022, 12, 45–56. [Google Scholar] [CrossRef]
- Arlt, W.; Allolio, B. Adrenal insufficiency. Lancet 2003, 361, 1881–1893. [Google Scholar] [CrossRef]
- Daramola, O.; Gutierrez Reyes, C.D.; Chávez-Reyes, J.; Marichal-Cancino, B.A.; Nwaiwu, J.; Onigbinde, S.; Adeniyi, M.; Solomon, J.; Bhuiyan, M.M.A.A.; Mechref, Y. Metabolomic Changes in Rat Serum after Chronic Exposure to Glyphosate-Based Herbicide. Metabolites 2024, 14, 50. [Google Scholar] [CrossRef]
- Altaweraqi, R.A.; Yao, S.Y.; Smith, K.M.; Cass, C.E.; Young, J.D. HPLC reveals novel features of nucleoside and nucleobase homeostasis, nucleoside metabolism and nucleoside transport. Biochim. Biophys. Acta (BBA)-Biomembranes 2020, 1862, 183247. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inokuchi, T.; Ka, T.; Yamamoto, A.; Takahashi, S.; Tsutsumi, Z.; Tamada, D.; Okuda, C.; Moriwaki, Y. Relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides Nucleotides Nucleic Acids 2010, 29, 504–508. [Google Scholar] [CrossRef]
- Banerjee, P.S.; Lagerlöf, O.; Hart, G.W. Roles of O-GlcNAc in chronic diseases of aging. Mol. Asp. Med. 2016, 51, 1–15. [Google Scholar] [CrossRef]
- Roach, P.J.; Depaoli-Roach, A.A.; Hurley, T.D.; Tagliabracci, V.S. Glycogen and its metabolism: Some new developments and old themes. Biochem. J. 2012, 441, 763–787. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.-l.; Zhang, Y.; Zhang, J.; Deng, Z.; Wu, X.; Yin, Y. Dynamic oral administration of uridine affects the diurnal rhythm of bile acid and cholesterol metabolism-related genes in mice. Biol. Rhythm. Res. 2019, 50, 543–552. [Google Scholar] [CrossRef]
- Jiang, G.; Yuan, L.; Liu, X.; Wu, H.; Yu, H.; Zhang, W.; Zhang, S.; Huang, Y. Circadian rhythm in neurodegenerative disease: The role of RNA modifications and potential application of RNA-based therapeutics. Ageing Neurodegener. Dis. 2024, 4, 16. [Google Scholar] [CrossRef]
| Information | NT1 | Control |
|---|---|---|
| Number of subjects | 11 | 11 |
| Age (years) | 19–71 | 28–73 |
| Sex (Male/Female) | 7/4 | 7/4 |
| Presence of HLA-DQB1*0602 allele | 10 | 2 |
| Cerebrospinal fluid hypocretin level (pg/mL) | 0–119.6 | Not available |
| Body Mass Index | 20.2–28.6 | Not available |
| Parameter | Median (Interquartile Range) |
|---|---|
| Time in bed, min | 445.0 (399.0–500.0) |
| Total Sleep Time, min | 407.5 (348.0–461.0) |
| Sleep Onset Latency, min | 6.0 (4.5–10.0) |
| First REM Latency, min | 2.5 (1.0–56.5) |
| Number of Stage Shifts/Hour | 12.6 (11.9–14.7) |
| Parameter | Median (Interquartile Range) |
| Number of Awakenings/hour | 3.3 (1.9–4.7) |
| Sleep Efficiency, % | 89.6 (82.1–95.3) |
| Sleep Stage N1, % | 3.4 (1.7–7.0) |
| Sleep Stage N2, % | 47.3 (45.6–53.8) |
| Sleep Stage N3, % | 17.9 (14.9–23.2) |
| Sleep Stage R, % | 28.2 (25.5–28.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, M.A.; Purba, W.; Sanni, A.; Bhuiyan, M.M.A.A.; Talih, F.; Lanza, G.; Kobeissy, F.; Plazzi, G.; Pizza, F.; Ferri, R.; et al. Alteration of Metabolic Profile in Patients with Narcolepsy Type 1. Metabolites 2025, 15, 382. https://doi.org/10.3390/metabo15060382
Hakim MA, Purba W, Sanni A, Bhuiyan MMAA, Talih F, Lanza G, Kobeissy F, Plazzi G, Pizza F, Ferri R, et al. Alteration of Metabolic Profile in Patients with Narcolepsy Type 1. Metabolites. 2025; 15(6):382. https://doi.org/10.3390/metabo15060382
Chicago/Turabian StyleHakim, Md Abdul, Waziha Purba, Akeem Sanni, Md Mostofa Al Amin Bhuiyan, Farid Talih, Giuseppe Lanza, Firas Kobeissy, Giuseppe Plazzi, Fabio Pizza, Raffaele Ferri, and et al. 2025. "Alteration of Metabolic Profile in Patients with Narcolepsy Type 1" Metabolites 15, no. 6: 382. https://doi.org/10.3390/metabo15060382
APA StyleHakim, M. A., Purba, W., Sanni, A., Bhuiyan, M. M. A. A., Talih, F., Lanza, G., Kobeissy, F., Plazzi, G., Pizza, F., Ferri, R., & Mechref, Y. (2025). Alteration of Metabolic Profile in Patients with Narcolepsy Type 1. Metabolites, 15(6), 382. https://doi.org/10.3390/metabo15060382










