Capsaicin as a Microbiome Modulator: Metabolic Interactions and Implications for Host Health
Abstract
1. Introduction
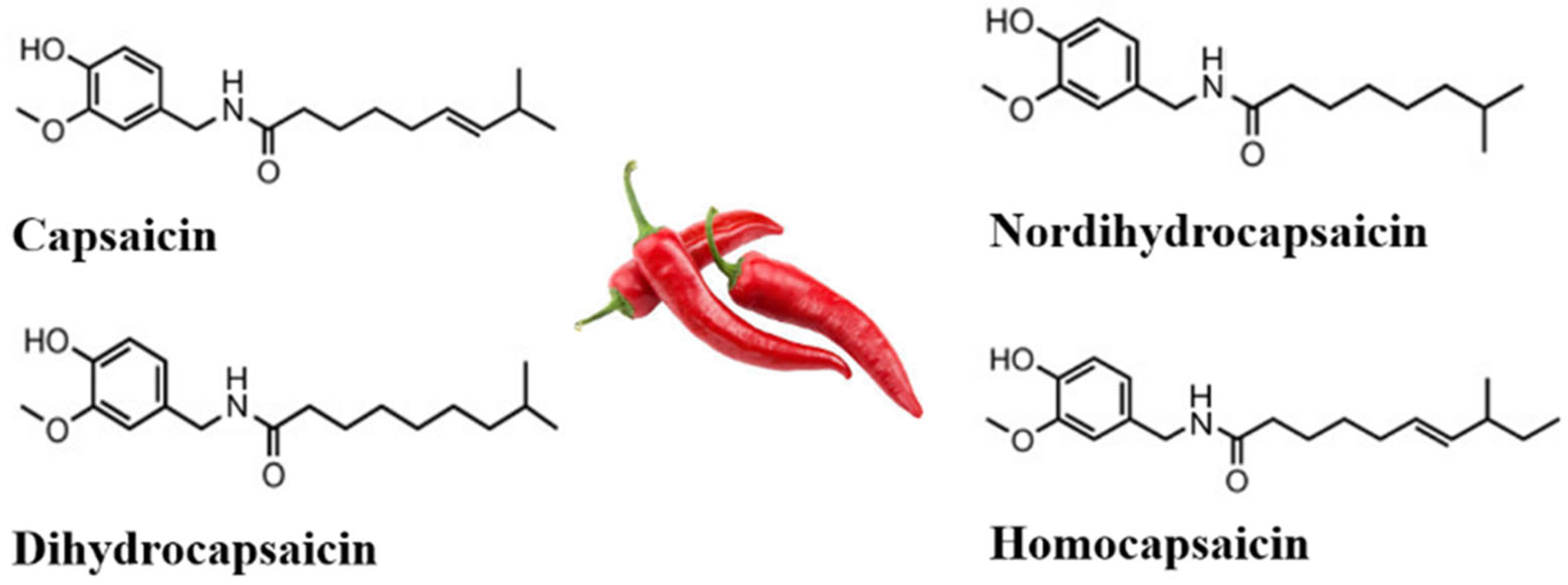
2. Biosynthesis, Consumption, and High-Pungency Pepper Varieties
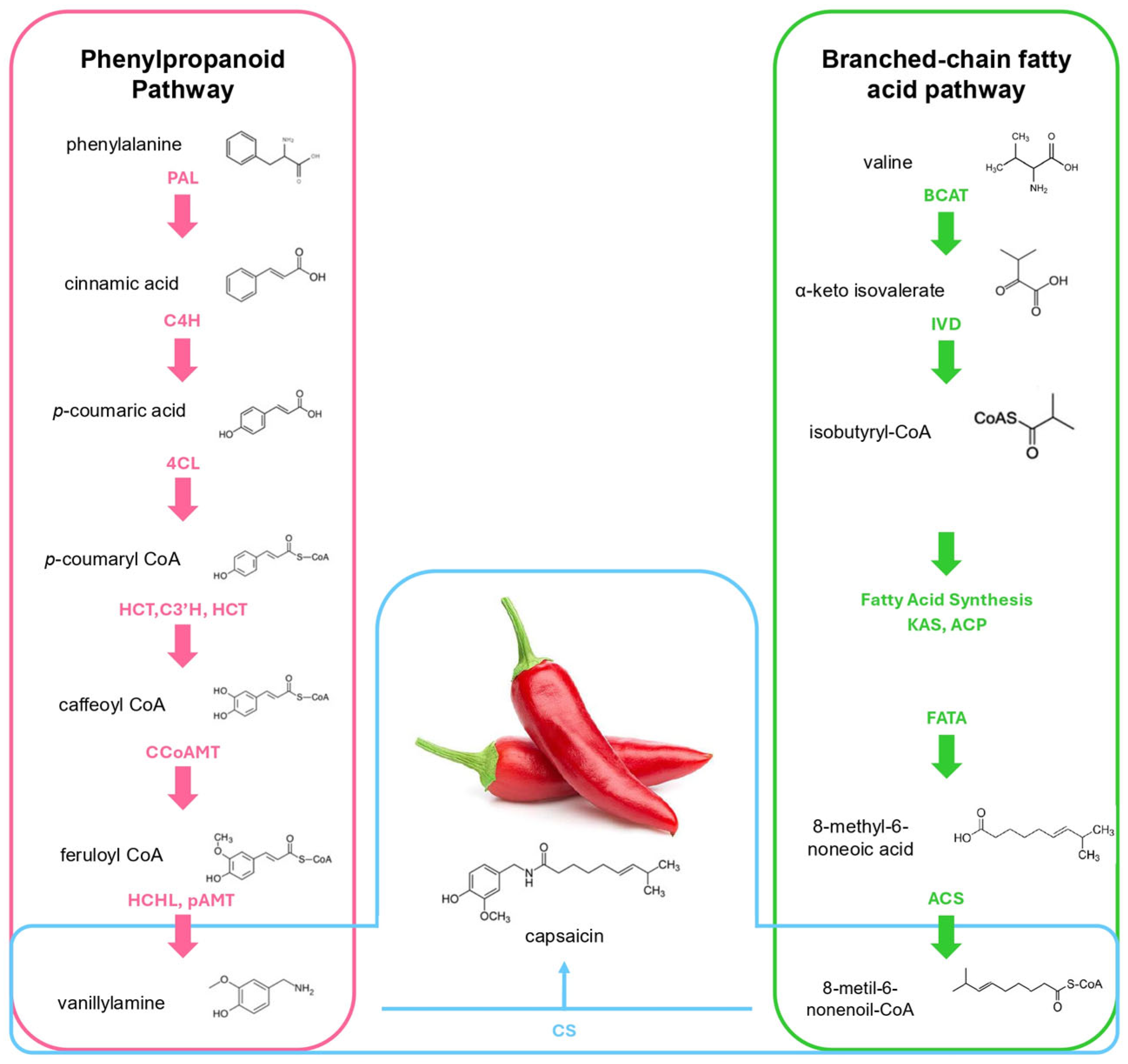
2.1. Capsaicin Biosynthesis
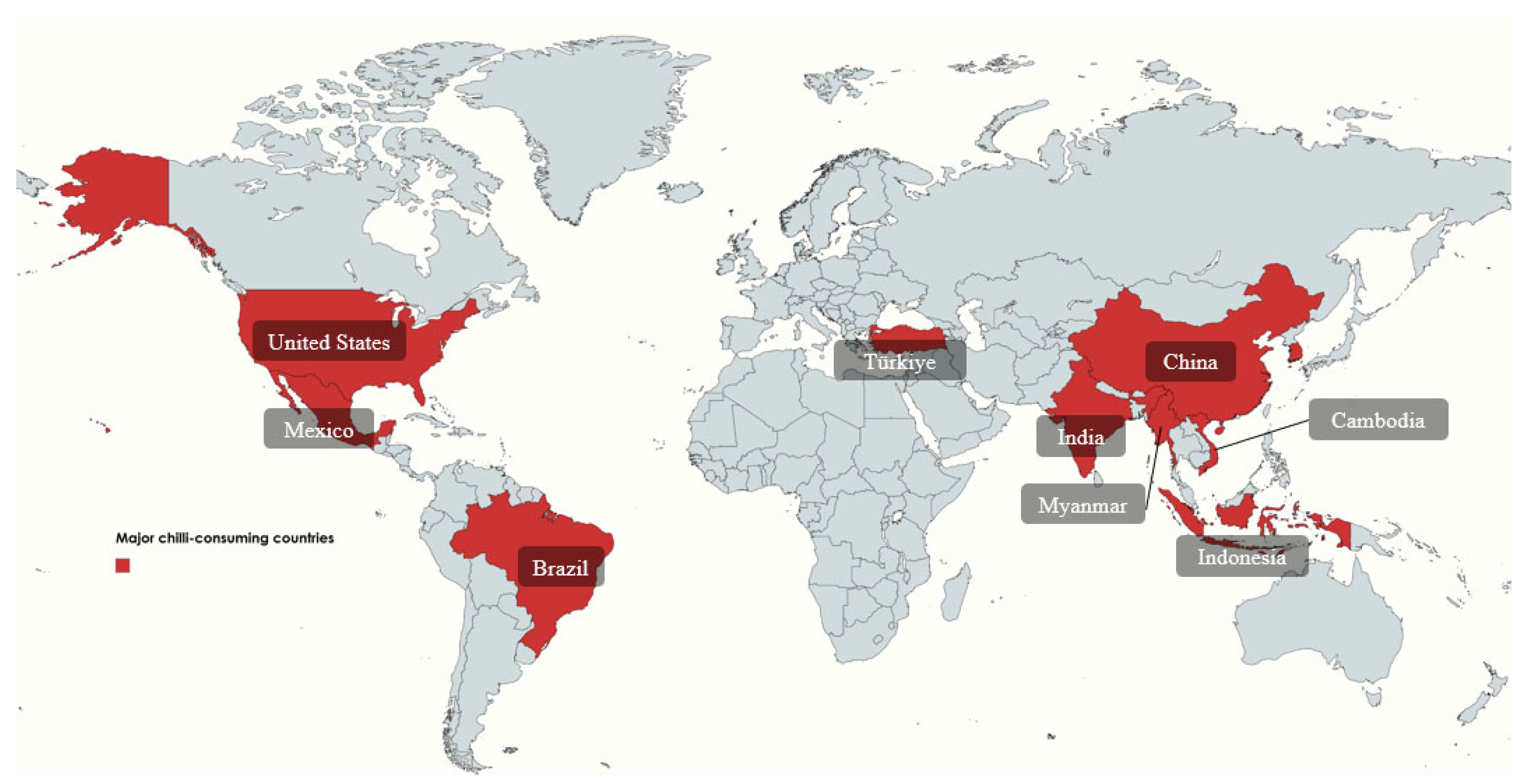
2.2. Chili Pepper Consumption
2.3. Pepper Varieties
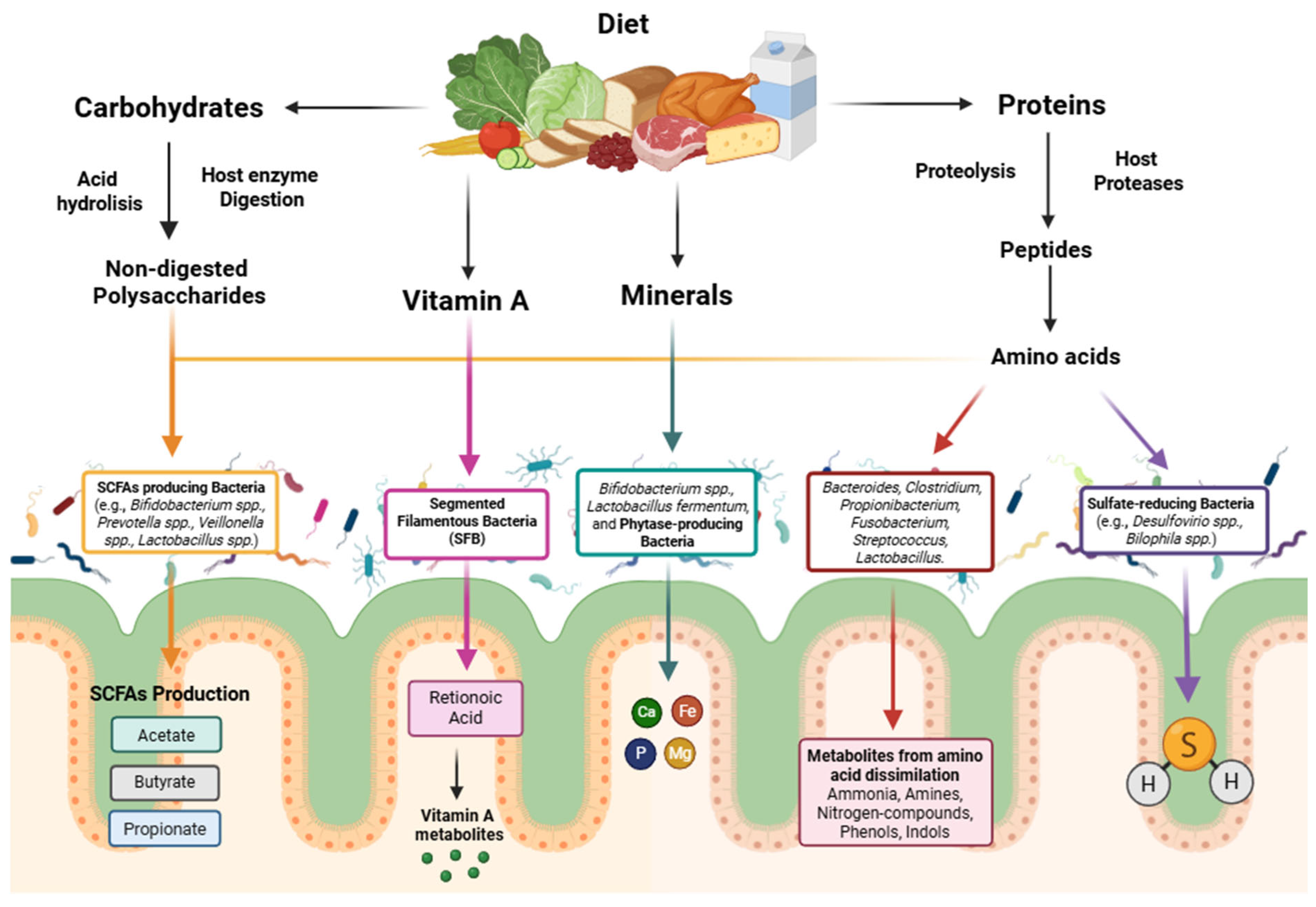
3. The Impact of Gut Microbiome in Host Health
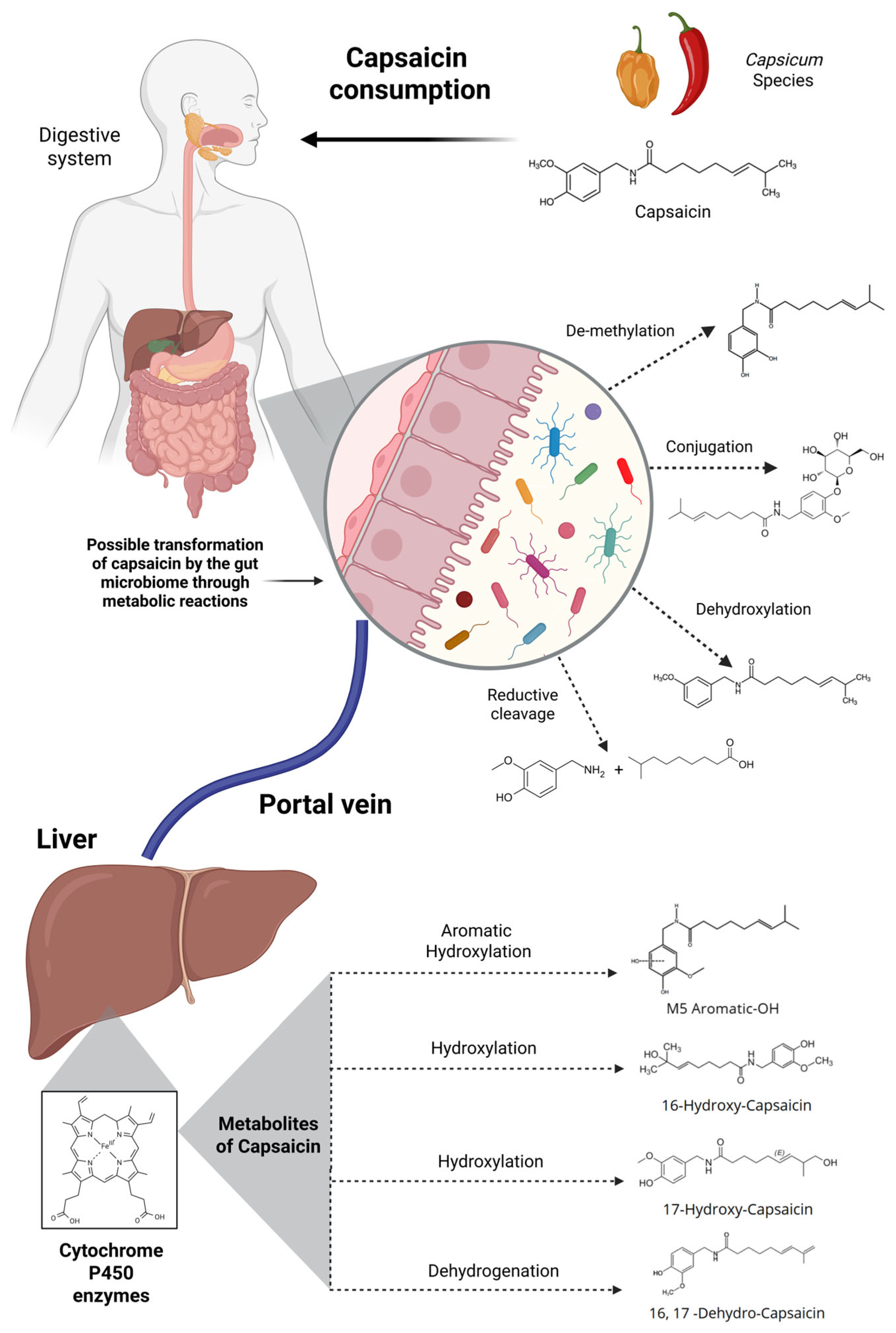
4. The Role of Host Metabolism and the Gut Microbiome in Capsaicin Biotransformation
4.1. Role of Gut Microbiota in Capsaicin Metabolism
4.2. Factors Influencing Capsaicin Bioavailability
4.3. Capsaicin Metabolism and the Central Nervous System
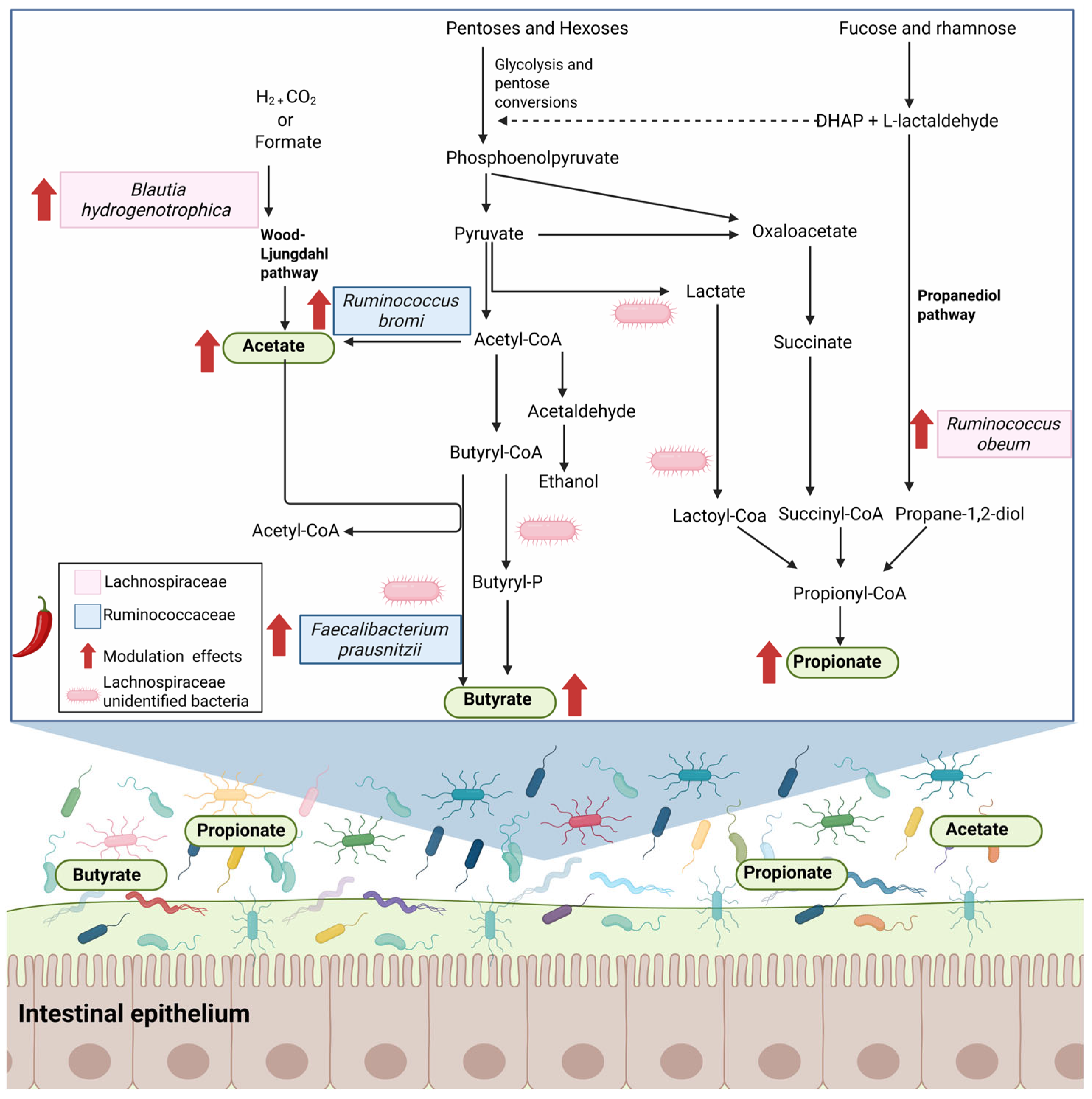
5. Effects of Capsaicin Intake on the Diversity and Composition of Gut Microbiota
6. Capsaicin’s Impact on Gene and Protein Modulation
6.1. Capsaicin’s Role in Cancer
6.2. Capsaicin’s Role in Liver Disease
6.3. Other Beneficial Impacts of Capsaicin
6.4. Negative Impacts Caused by Capsaicin Consumption
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maharjan, A.; Vasamsetti, B.M.K.; Park, J.-H. A Comprehensive Review of Capsaicin: Biosynthesis, Industrial Productions, Processing to Applications, and Clinical Uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, W.; Zhang, X.; Mao, J.; Zhang, Q.; Zhao, W.; Zhang, S.; Xie, J. Recent Advances in Analysis of Capsaicin and Its Effects on Metabolic Pathways by Mass Spectrometry. Front. Nutr. 2023, 10, 1227517. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of Capsaicin and Its Implications for Drug Delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fan, W.; Li, D.; Hao, Z.; Liu, P.; Liu, C.; Cui, K.; Zhang, W.; Liu, X.; Zhang, Q.; et al. Metabolic Pathways, Pharmacokinetic, and Brain Neurochemicals Effects of Capsaicin: Comprehensively Insights from In Vivo Studies. Phytomedicine 2024, 135, 156212. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. MBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Preparation, Pungency, and Bioactivity of Capsaicin: A Review. Int. J. Food Sci. Technol. 2024, 59, 6659–6687. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. Capsaicin: Effects on the Pathogenesis of Hepatocellular Carcinoma. Molecules 2019, 24, 2350. [Google Scholar] [CrossRef]
- Takeda, Y.; Dai, P. Capsaicin Directly Promotes Adipocyte Browning in the Chemical Compound-Induced Brown Adipocytes Converted from Human Dermal Fibroblasts. Sci. Rep. 2022, 12, 6612. [Google Scholar] [CrossRef]
- Ates, B.; Öner, Ç.; Akbulut, Z.; Çolak, E. Capsaicin Alters the Expression of Genetic and Epigenetic Molecules in Hepatocellular Carcinoma Cell. Int. J. Mol. Cell. Med. 2023, 11, 236–243. [Google Scholar] [CrossRef]
- Szallasi, A. Capsaicin and Cancer: Guilty as Charged or Innocent until Proven Guilty? Temperature 2023, 10, 35–49. [Google Scholar] [CrossRef]
- Kopta, T.; Sekara, A.; Pokluda, R.; Ferby, V.; Caruso, G. Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants 2020, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Li, L.; Donovan-Mak, M.; Chen, Z.-H.; Panchal, S.K. Capsicum Waste as a Sustainable Source of Capsaicinoids for Metabolic Diseases. Foods 2023, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, W.; Zhang, L.; Wu, D.; Sun, P.; Huang, C.; Fu, G.; Deng, Q.; Wang, Z.; Cheng, S. MYB24 Negatively Regulates the Biosynthesis of Lignin and Capsaicin by Affecting the Expression of Key Genes in the Phenylpropanoid Metabolism Pathway in Capsicum Chinense. Molecules 2023, 28, 2644. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Wei, L.; Kang, R.; Chen, L.; Zhang, M.; Tan, E.K.; Liu, W. Capsaicin Functions as Drosophila Ovipositional Repellent and Causes Intestinal Dysplasia. Sci. Rep. 2020, 10, 9963. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Chu, Y.; Cohen, B.E.; Chuang, H. A Single TRPV1 Amino Acid Controls Species Sensitivity to Capsaicin. Sci. Rep. 2020, 10, 8038. [Google Scholar] [CrossRef]
- Jang, W.; Oh, M.; Cho, E.-H.; Baek, M.; Kim, C. Drosophila Pain Sensitization and Modulation Unveiled by a Novel Pain Model and Analgesic Drugs. PLoS One 2023, 18, e0281874. [Google Scholar] [CrossRef] [PubMed]
- Maloney, G.S.; Kochevenko, A.; Tieman, D.M.; Tohge, T.; Krieger, U.; Zamir, D.; Taylor, M.G.; Fernie, A.R.; Klee, H.J. Characterization of the Branched-Chain Amino Acid Aminotransferase Enzyme Family in Tomato. Plant Physiol. 2010, 153, 925–936. [Google Scholar] [CrossRef]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular Biology of Capsaicinoid Biosynthesis in Chili Pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef]
- Park, M.-E.; Kim, H.U. Applications and Prospects of Genome Editing in Plant Fatty Acid and Triacylglycerol Biosynthesis. Front. Plant Sci. 2022, 13, 969844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, C.; Ye, Q.; Liu, C.; Wan, H.; Ruan, M.; Zhou, G.; Wang, R.; Li, Z.; Diao, M.; et al. The Influence of Different Factors on the Metabolism of Capsaicinoids in Pepper (Capsicum Annuum L.). Plants 2024, 13, 2887. [Google Scholar] [CrossRef] [PubMed]
- Siebert, E.; Lee, S.-Y.; Prescott, M.P. Chili Pepper Preference Development and Its Impact on Dietary Intake: A Narrative Review. Front. Nutr. 2022, 9, 1039207. [Google Scholar] [CrossRef]
- Mujoko, T. Chili Plants: Nutrition Content and Local Varieties as a Genetic Resources. Nusant. Sci. Technol. Proc. 2021, 5–9. [Google Scholar] [CrossRef]
- Secretaria de Agricultura y Desarrollo Rural (SADER) México, Entre Los Principales Productores de Chile Verde En El Mundo: Agricultura. Available online: https://www.gob.mx/agricultura/prensa/mexico-entre-los-principales-productores-de-chile-verde-en-el-mundo-agricultura?idiom=es (accessed on 12 March 2025).
- Liu, F.; Zhao, J.; Sun, H.; Xiong, C.; Sun, X.; Wang, X.; Wang, Z.; Jarret, R.; Wang, J.; Tang, B.; et al. Genomes of Cultivated and Wild Capsicum Species Provide Insights into Pepper Domestication and Population Differentiation. Nat. Commun. 2023, 14, 5487. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili Peppers (Capsicum spp.): The Spice Not Only for Cuisine Purposes: An Update on Current Knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A Comparative Overview on Chili Pepper (Capsicum Genus) and Sichuan Pepper (Zanthoxylum Genus): From Pungent Spices to Pharma-Foods. Trends Food Sci. Technol. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.-C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.-N. Gut Microbiota, Dietary Phytochemicals, and Benefits to Human Health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Tang, X.; Cui, S.; Zhang, Q.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods 2022, 11, 686. [Google Scholar] [CrossRef]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.-M. Capsaicin and Gut Microbiota in Health and Disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Anders, B.; Anders, M.; Kreuzer, M.; Zinn, S.; Fricker, L.; Maier, C.; Wolters, M.; Köhm, M.; Behrens, F.; Walter, C. Sensory Testing and Topical Capsaicin Can Characterize Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2022, 41, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Cirilli, I.; Marcheggiani, F.; Mabhida, S.E.; Ziqubu, K.; Ntamo, Y.; Jack, B.; Nyambuya, T.M.; Hanser, S.; et al. Capsaicin, Its Clinical Significance in Patients with Painful Diabetic Neuropathy. Biomed. Pharmacother. 2022, 153, 113439. [Google Scholar] [CrossRef]
- Guedes, V.; Castro, J.P.; Brito, I. Topical Capsaicin for Pain in Osteoarthritis: A Literature Review. Reumatol. Clínica 2018, 14, 40–45. [Google Scholar] [CrossRef]
- McCormack, P.L. Capsaicin Dermal Patch. Drugs 2010, 70, 1831–1842. [Google Scholar] [CrossRef]
- Mahalak, K.K.; Bobokalonov, J.; Firrman, J.; Williams, R.; Evans, B.; Fanelli, B.; Soares, J.W.; Kobori, M.; Liu, L. Analysis of the Ability of Capsaicin to Modulate the Human Gut Microbiota In Vitro. Nutrients 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem Across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Sperandio, V. A Complex Relationship: The Interaction among Symbiotic Microbes, Invading Pathogens, and Their Mammalian Host. Mucosal Immunol. 2011, 4, 133–138. [Google Scholar] [CrossRef]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.-C. Gut Microbiota Diversity According to Dietary Habits and Geographical Provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Mobeen, F.; Sharma, V.; Prakash, T. Enterotype Variations of the Healthy Human Gut Microbiome in Different Geographical Regions. Bioinformation 2018, 14, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, L.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Armet, A.M.; Deehan, E.C.; O’Sullivan, A.F.; Mota, J.F.; Field, C.J.; Prado, C.M.; Lucey, A.J.; Walter, J. Rethinking Healthy Eating in Light of the Gut Microbiome. Cell Host Microbe 2022, 30, 764–785. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Butel, M.-J.; Waligora-Dupriet, A.-J.; Wydau-Dematteis, S. The Developing Gut Microbiota and Its Consequences for Health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Watts, A.M.; West, N.P.; Zhang, P.; Smith, P.K.; Cripps, A.W.; Cox, A.J. The Gut Microbiome of Adults with Allergic Rhinitis is Characterised by Reduced Diversity and an Altered Abundance of Key Microbial Taxa Compared to Controls. Int. Arch. Allergy Immunol. 2021, 182, 94–105. [Google Scholar] [CrossRef]
- Aguilera, A.C.; Dagher, I.A.; Kloepfer, K.M. Role of the Microbiome in Allergic Disease Development. Curr. Allergy Asthma Rep. 2020, 20, 44. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.-M.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the Gut Microbiome and Risk of Asthma in Childhood. Nat. Commun. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Karačić, A.; Renko, I.; Krznarić, Ž.; Klobučar, S.; Liberati Pršo, A.-M. The Association between the Firmicutes/Bacteroidetes Ratio and Body Mass among European Population with the Highest Proportion of Adults with Obesity: An Observational Follow-Up Study from Croatia. Biomedicines 2024, 12, 2263. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Koenig, D.; Engelbrecht, K.; Doney, L.; Hards, K.; Al, K.F.; Reid, G.; Burton, J.P. Emerging Connections Between Gut Microbiome Bioenergetics and Chronic Metabolic Diseases. Cell Rep. 2021, 37, 110087. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between Bacterial and Fungal Communities in the Human Gut Microbiota and Their Implications for Nutritional Status and Body Weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Baars, D.P.; Fondevila, M.F.; Meijnikman, A.S.; Nieuwdorp, M. The Central Role of the Gut Microbiota in the Pathophysiology and Management of Type 2 Diabetes. Cell Host Microbe 2024, 32, 1280–1300. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, F.; Bhosle, A.; Dong, D.; Mehta, R.; Ghazi, A.; Zhang, Y.; Liu, Y.; Rinott, E.; Ma, S.; et al. Strain-Specific Gut Microbial Signatures in Type 2 Diabetes Identified in a Cross-Cohort Analysis of 8,117 Metagenomes. Nat. Med. 2024, 30, 2265–2276. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Sinha, A.K.; Laursen, M.F.; Licht, T.R. Regulation of Microbial Gene Expression: The Key to Understanding Our Gut Microbiome. Trends Microbiol. 2025, 33, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sankarasubramanian, J.; Ahmad, R.; Avuthu, N.; Singh, A.B.; Guda, C. Gut Microbiota and Metabolic Specificity in Ulcerative Colitis and Crohn’s Disease. Front. Med. 2020, 7, 606298. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.; Dunkel, A.; Waldschmitt, N.; Raj, A.C.D.; Lagkouvardos, I.; Corraliza, A.M.; Mayorgas, A.; Martinez-Medina, M.; Reiter, S.; Schloter, M.; et al. Integrated Microbiota and Metabolite Profiles Link Crohn’s Disease to Sulfur Metabolism. Nat. Commun. 2020, 11, 4322. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut Microbiome Lipid Metabolism and Its Impact on Host Physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.; Álvarez-Quintero, R.; Velásquez-Mejía, E.; Sierra, J.; Corrales-Agudelo, V.; Carmona, J.; Abad, J.; Escobar, J. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Solas, M. Dysbiosis and Alzheimer’s Disease: Cause or Treatment Opportunity? Cell. Mol. Neurobiol. 2022, 42, 377–387. [Google Scholar] [CrossRef]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The Gut Microbiome in Alzheimer’s Disease: What We Know and What Remains to Be Explored. Mol. Neurodegener. 2023, 18, 9. [Google Scholar] [CrossRef]
- van Harten, A.C.; Wiste, H.J.; Weigand, S.D.; Mielke, M.M.; Kremers, W.K.; Eichenlaub, U.; Dyer, R.B.; Algeciras-Schimnich, A.; Knopman, D.S.; Jack, C.R.; et al. Detection of Alzheimer’s Disease Amyloid Beta 1-42, P-tau, and T-tau Assays. Alzheimer’s Dement. 2022, 18, 635–644. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Stone, W.J.; Factor, S.A.; Molho, E.; Zabetian, C.P.; Standaert, D.G.; Payami, H. Exploring Human-Genome Gut-Microbiome Interaction in Parkinson’s Disease. npj Park. Dis. 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s Disease Implicates the Gut Microbiome in Multiple Disease Mechanisms. Nat. Commun. 2022, 13, 6958. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.-T.S.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered Composition and Function of Intestinal Microbiota in Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A Robust Microbiome Signature for Autism Spectrum Disorder across Different Studies Using Machine Learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand Spiciness: Mechanism of TRPV1 Channel Activation by Capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Collins, S.L.; Patterson, A.D. The Gut Microbiome: An Orchestrator of Xenobiotic Metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction Between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin Induces Mitochondrial Dysfunction and Apoptosis in Anaplastic Thyroid Carcinoma Cells via TRPV1-Mediated Mitochondrial Calcium Overload. Cell. Signal. 2020, 75, 109733. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Liu, H.; Zhang, X.; Wu, B. Glucuronidation of Capsaicin by Liver Microsomes and Expressed UGT Enzymes: Reaction Kinetics, Contribution of Individual Enzymes and Marked Species Differences. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the Mystery of Capsaicin: A Tool to Understand and Treat Pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, L.; Yu, F.; Li, N.; Deng, J.; Zhang, J.; Xiong, S.; Hu, X. Capsaicin: A Spicy Way in Liver Disease. Front. Pharmacol. 2024, 15, 1451084. [Google Scholar] [CrossRef]
- Chan, S.H.; Azlan, A.; Ismail, A.; Shafie, N.H. Capsaicin: Current Understanding in Therapeutic Effects, Drug Interaction, and Bioavailability. Malays. J. Med. Health Sci. 2020, 16, 219–227. [Google Scholar]
- Gong, T.; Wang, H.; Liu, S.; Zhang, M.; Xie, Y.; Liu, X. Capsaicin Regulates Lipid Metabolism through Modulation of Bile Acid/Gut Microbiota Metabolism in High-Fat-Fed SD Rats. Food Nutr. Res. 2022, 66, 8289. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Fan, J.; Feng, Z.; Song, X. Pharmacological Activity of Capsaicin: Mechanisms and Controversies (Review). Mol. Med. Rep. 2024, 29, 38. [Google Scholar] [CrossRef]
- Domene, C.; Darré, L.; Oakes, V.; Gonzalez-Resines, S. A Potential Route of Capsaicin to Its Binding Site in the TRPV1 Ion Channel. J. Chem. Inf. Model. 2022, 62, 2481–2489. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Mózsik, G. Capsaicin, The Vanilloid Receptor TRPV1 Agonist in Neuroprotection: Mechanisms Involved and Significance. Neurochem. Res. 2023, 48, 3296–3315. [Google Scholar] [CrossRef]
- Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. Protective Role of Capsaicin in Neurological Disorders: An Overview. Neurochem. Res. 2022, 47, 1513–1531. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The Interplay between Diet and the Gut Microbiome: Implications for Health and Disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The Oral–Gut Microbiome Axis in Health and Disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Wang, F.; Huang, X.; Chen, Y.; Zhang, D.; Chen, D.; Chen, L.; Lin, J. Study on the Effect of Capsaicin on the Intestinal Flora through High-Throughput Sequencing. ACS Omega 2020, 5, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Zhang, S.-Y.; Wen, R.; Zhang, T.-N.; Yang, N. Role of Histone Deacetylases and Their Inhibitors in Neurological Diseases. Pharmacol. Res. 2024, 208, 107410. [Google Scholar] [CrossRef]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent Action of Butyrate over β-Hydroxybutyrate as Histone Deacetylase Inhibitor, Transcriptional Modulator and Anti-Inflammatory Molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, B.; Friesen, M.; Liu, S.; Zhu, C.; Yang, C. Capsaicin Attenuates Lipopolysaccharide-Induced Inflammation and Barrier Dysfunction in Intestinal Porcine Epithelial Cell Line-J2. Front. Physiol. 2021, 12, 715469. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Wang, Y. Capsaicin Reduces Obesity by Reducing Chronic Low-Grade Inflammation. Int. J. Mol. Sci. 2024, 25, 8979. [Google Scholar] [CrossRef]
- Shen, W.; Shen, M.; Zhao, X.; Zhu, H.; Yang, Y.; Lu, S.; Tan, Y.; Li, G.; Li, M.; Wang, J.; et al. Anti-Obesity Effect of Capsaicin in Mice Fed with High-Fat Diet is Associated with an Increase in Population of the Gut Bacterium Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and Function of Akkermansia Muciniphila in Microbiome Ecology, Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Zhou, Y.; Shi, Q.; Li, Y.; Wang, H.; Liao, L. Capsaicin Modulates Akkermansia Muciniphila Abundance by Enhancing MUCIN2 Levels in Mice Fed with High-Fat Diets. Food Nutr. Res. 2024, 68, 10–29219. [Google Scholar] [CrossRef]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y.; et al. Capsaicin Shapes Gut Microbiota and Pre-Metastatic Niche to Facilitate Cancer Metastasis to Liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef]
- Su, M.; She, Y.; Deng, M.; Guo, Y.; Li, Y.; Liu, G.; Zhang, H.; Sun, B.; Liu, D. The Effect of Capsaicin on Growth Performance, Antioxidant Capacity, Immunity and Gut Micro-Organisms of Calves. Animals 2023, 13, 2309. [Google Scholar] [CrossRef]
- Kim, B.-T.; Kim, K.-M.; Kim, K.-N. The Effect of Ursodeoxycholic Acid on Small Intestinal Bacterial Overgrowth in Patients with Functional Dyspepsia: A Pilot Randomized Controlled Trial. Nutrients 2020, 12, 1410. [Google Scholar] [CrossRef]
- Garcia-Lozano, M.; Haynes, J.; Lopez-Ortiz, C.; Natarajan, P.; Peña-Garcia, Y.; Nimmakayala, P.; Stommel, J.; Alaparthi, S.B.; Sirbu, C.; Balagurusamy, N.; et al. Effect of Pepper-Containing Diets on the Diversity and Composition of Gut Microbiome of Drosophila Melanogaster. Int. J. Mol. Sci. 2020, 21, 945. [Google Scholar] [CrossRef]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia Muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Fu, J. Advances in Antiobesity Mechanisms of Capsaicin. Curr. Opin. Pharmacol. 2021, 61, 1–5. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Ding, G.; Deng, L.; He, Y.; Zhang, Q.; Wang, J.; Chen, X. The Possible Effects of Chili Peppers on ADHD in Relation to the Gut Microbiota. Front. Nutr. 2025, 12, 1551650. [Google Scholar] [CrossRef]
- Li, J.; Liao, X.; Yin, X.; Deng, Z.; Hu, G.; Zhang, W.; Jiang, F.; Zhao, L. Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice. Nutrients 2022, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.S.; Yue, Y.; Li, C.; Yang, H.-J.; Zhang, T.; Wu, X.; Jeong, D.Y.; Park, S. Moderate Capsaicin-Containing Kochujang Alleviates Memory Impairment through the Gut-Brain Axis in Rats with Scopolamine-Induced Amnesia. Biomed. Pharmacother. 2024, 178, 117091. [Google Scholar] [CrossRef]
- Owolo, O.; Audu, H.J.; Afolayan, A.O.; Ayeni, F.A. Pepper Power: Short-Term Impact of Pepper Consumption on the Gut Bacteriome Composition in Healthy Volunteers. PeerJ 2024, 12, e18707. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, N.; Peng, W.; Yang, Q.; Ren, J.; Yang, H.; Chen, L.; Chen, Y.; Guo, J. Effects of Capsaicin on Glucose Uptake and Consumption in Hepatocytes. Molecules 2023, 28, 5258. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Isoda, H. Capsaicin Induced the Upregulation of Transcriptional and Translational Expression of Glycolytic Enzymes Related to Energy Metabolism in Human Intestinal Epithelial Cells. J. Agric. Food Chem. 2009, 57, 11148–11153. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.-D.; Caruntu, A.; Scheau, A.-E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Liu, D.; Zhao, T.; Lu, Z.; Zhu, L.; Cao, L.; Yang, J.; Jin, J.; Cai, Y. Capsaicin Reactivates HMOF in Gastric Cancer Cells and Induces Cell Growth Inhibition. Cancer Biol. Ther. 2016, 17, 1117–1125. [Google Scholar] [CrossRef]
- Chinreddy, S.R.; Mashozhera, N.T.; Rashrash, B.; Flores-Iga, G.; Nimmakayala, P.; Hankins, G.R.; Harris, R.T.; Reddy, U.K. Unraveling TRPV1’s Role in Cancer: Expression, Modulation, and Therapeutic Opportunities with Capsaicin. Molecules 2024, 29, 4729. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Yuan, T.-M.; Liu, B.-H.; Liu, K.-L.; Wung, C.-H.; Chuang, S.-M. Capsaicin Potentiates Anticancer Drug Efficacy Through Autophagy-Mediated Ribophorin II Downregulation and Necroptosis in Oral Squamous Cell Carcinoma Cells. Front. Pharmacol. 2021, 12, 676813. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of Capsaicin as a Potential New Therapeutic Drug in Human Cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. [Google Scholar] [CrossRef]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Wu, I.-J.; Hong, J.-Y.; Liu, B.-H.; Liang, R.-Y.; Yuan, T.-M.; Chuang, S.-M. Capsaicin-Induced TRIB3 Upregulation Promotes Apoptosis in Cancer Cells. Cancer Manag. Res. 2018, 10, 4237–4248. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Cang, S.; Ma, P.; Song, Z. Capsaicin: A “Hot” KDM1A/LSD1 Inhibitor from Peppers. Bioorg. Chem. 2020, 103, 104161. [Google Scholar] [CrossRef]
- Qin, J.; Li, H.; Yu, W.; Wei, L.; Wen, B. Effect of Cold Exposure and Capsaicin on the Expression of Histone Acetylation and Toll-like Receptors in 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis. Environ. Sci. Pollut. Res. 2021, 28, 60981–60992. [Google Scholar] [CrossRef]
- Lin, M.-H.; Lee, Y.-H.; Cheng, H.-L.; Chen, H.-Y.; Jhuang, F.-H.; Chueh, P. Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (TNOX) and Sirtuin1 (SIRT1). Molecules 2016, 21, 849. [Google Scholar] [CrossRef]
- Hudáková, T.; Šemeláková, M.; Očenáš, P.; Kožurková, M.; Krochtová, K.; Sovová, S.; Tóthová, Z.; Guľášová, Z.; Popelka, P.; Solár, P. Chili Pepper Extracts, Capsaicin, and Dihydrocapsaicin as Potential Anticancer Agents Targeting Topoisomerases. BMC Complement. Med. Ther. 2024, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin Represses Transcriptional Activity of β-Catenin in Human Colorectal Cancer Cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef]
- Bitencourt, S.; Mesquita, F.; Basso, B.; Schmid, J.; Ferreira, G.; Rizzo, L.; Bauer, M.; Bartrons, R.; Ventura, F.; Rosa, J.L.; et al. Capsaicin Modulates Proliferation, Migration, and Activation of Hepatic Stellate Cells. Cell Biochem. Biophys. 2014, 68, 387–396. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-Β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Vrânceanu, M.; Hegheş, S.-C.; Cozma-Petruţ, A.; Banc, R.; Stroia, C.M.; Raischi, V.; Miere, D.; Popa, D.-S.; Filip, L. Plant-Derived Nutraceuticals Involved in Body Weight Control by Modulating Gene Expression. Plants 2023, 12, 2273. [Google Scholar] [CrossRef]
- Hu, J.; Lin, Z.; Yang, Y.; Christian, M.; Li, S.; Zheng, B.; Tan, B.K.; Lin, S. Preconceptional Capsaicin Intervention Mitigates Negative Effects of Paternal Obesity on Metabolic Characteristics in Male Offspring upon High-Fat Diet Challenge. J. Funct. Foods 2024, 116, 106137. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Cheng, K.; Zhang, L.; Wang, T. Capsaicin Alleviates the Intestinal Oxidative Stress via Activation of TRPV1/PKA/UCP2 and Keap1/Nrf2 Pathways in Heat-Stressed Mice. J. Funct. Foods 2023, 108, 105749. [Google Scholar] [CrossRef]
- Lu, M.; Lan, Y.; Xiao, J.; Song, M.; Chen, C.; Liang, C.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin Ameliorates the Redox Imbalance and Glucose Metabolism Disorder in an Insulin-Resistance Model via Circadian Clock-Related Mechanisms. J. Agric. Food Chem. 2019, 67, 10089–10096. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.-L.; Xiang, Y.; Tian, D.-Y.; Zhu, C.; Li, W.-W.; Liu, Y.-H.; Bu, X.-L.; Shen, L.-L.; Jin, W.-S.; et al. Capsaicin Consumption Reduces Brain Amyloid-Beta Generation and Attenuates Alzheimer’s Disease-Type Pathology and Cognitive Deficits in APP/PS1 Mice. Transl. Psychiatry 2020, 10, 230. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Mahajan, N.; Kaur, J.; Devi, K.; Dharavath, R.N.; Singh, R.P.; Kondepudi, K.K.; Bishnoi, M. Mucin Secretory Action of Capsaicin Prevents High Fat Diet-Induced Gut Barrier Dysfunction in C57BL/6 Mice Colon. Biomed. Pharmacother. 2022, 145, 112452. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Ding, H.; Yan, C.; Zhu, H.; Huang, S.; Chen, Z.-Y. Dietary Supplementation with Capsaicinoids Alleviates Obesity in Mice Fed a High-Fat-High-Fructose Diet. Food Funct. 2024, 15, 8572–8585. [Google Scholar] [CrossRef]
- Thongin, S.; Den-udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial Effects of Capsaicin and Dihydrocapsaicin on Endothelial Inflammation, Nitric Oxide Production and Antioxidant Activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef]
- Andretta, E.; Costa, A.; Ventura, E.; Quintiliani, M.; Damiano, S.; Giordano, A.; Morrione, A.; Ciarcia, R. Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients 2024, 16, 3758. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, H.; Aburjai, T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Kwon, Y. Estimation of Dietary Capsaicinoid Exposure in Korea and Assessment of Its Health Effects. Nutrients 2021, 13, 2461. [Google Scholar] [CrossRef]
- Deng, R.; Yu, S.; Ruan, X.; Liu, H.; Zong, G.; Cheng, P.; Tao, R.; Chen, W.; Wang, A.; Zhao, Y.; et al. Capsaicin Orchestrates Metastasis in Gastric Cancer via Modulating Expression of TRPV1 Channels and Driving Gut Microbiota Disorder. Cell Commun. Signal. 2023, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lv, Y.; Zha, W.; Hong, X.; Luo, Q. Chili Consumption and Risk of Gastric Cancer: A Meta-Analysis. Nutr. Cancer 2021, 73, 45–54. [Google Scholar] [CrossRef] [PubMed]

| Model | Effect | Description | Reference |
|---|---|---|---|
| C57BL/6 Mice | Anti-obesity effect | Capsaicin modulated the secretion of mucus in the gut from high-diet obesity-induced mice, which significantly contributed to the anti-obesity effects that capsaicin has, such as metabolism augmentation and energy expenditure. | [136] |
| C57BL/6J Mice | Anti-obesity effect | The effect of supplementation with capsaicinoids on mice fed with a high-fat-high-fructose diet reversed the obesity caused by this diet; additionally, it reduced hepatic lipid accumulation. | [137] |
| Primary Human Umbilical Vein Endothelial Cells | Anti-inflammatory activity | Capsaicin and hydrocapsaicin cardiovascular benefits were tested using primary human endothelial cells. | [138] |
| Antioxidant activity | |||
| MSTO-211H, NCI-H2052, NCI-H2452 and NCI-H28 Human Malignant Mesothelioma cell lines | Anticancer activity | Capsaicin demonstrated the ability to inhibit malignant mesothelioma (MM) cel0l growth by inducing S-phase cell cycle arrest, reducing motility and migration of MM cells, and inhibiting AKT and ERK1/2 activation. | [139] |
| MCF7 and MDA-MB-231 cell lines | Anticancer activity | Capsaicin-loaded nanoliposomes improved anticancer activities significantly on the different cell lines in comparison with capsaicin alone. | [140] |
| K562 cell line | |||
| PANC1 cell line | |||
| A375 cell line | |||
| Human | Abdominal pain | Data from the Korean population were analyzed, showing that even low doses of capsaicin can cause abdominal pain. On the other hand, the risk of gastric cancer still requires evaluation, but high-level consumers had major risks and were susceptible to H. pylori infection. | [141] |
| Gastric cancer risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corral-Guerrero, I.A.; Martínez-Medina, A.E.; Alvarado-Mata, L.Y.; Chávez, A.C.F.; Muñoz-García, R.; Luévanos-Escareño, M.P.; Sosa-Martínez, J.D.; Castro-Alonso, M.J.; Nimmakayala, P.; Reddy, U.K.; et al. Capsaicin as a Microbiome Modulator: Metabolic Interactions and Implications for Host Health. Metabolites 2025, 15, 372. https://doi.org/10.3390/metabo15060372
Corral-Guerrero IA, Martínez-Medina AE, Alvarado-Mata LY, Chávez ACF, Muñoz-García R, Luévanos-Escareño MP, Sosa-Martínez JD, Castro-Alonso MJ, Nimmakayala P, Reddy UK, et al. Capsaicin as a Microbiome Modulator: Metabolic Interactions and Implications for Host Health. Metabolites. 2025; 15(6):372. https://doi.org/10.3390/metabo15060372
Chicago/Turabian StyleCorral-Guerrero, Iván Artemio, Angela Elena Martínez-Medina, Litzy Yazmin Alvarado-Mata, Ana Cristina Figueroa Chávez, Roberto Muñoz-García, Miriam Paulina Luévanos-Escareño, Jazel Doménica Sosa-Martínez, María José Castro-Alonso, Padma Nimmakayala, Umesh K. Reddy, and et al. 2025. "Capsaicin as a Microbiome Modulator: Metabolic Interactions and Implications for Host Health" Metabolites 15, no. 6: 372. https://doi.org/10.3390/metabo15060372
APA StyleCorral-Guerrero, I. A., Martínez-Medina, A. E., Alvarado-Mata, L. Y., Chávez, A. C. F., Muñoz-García, R., Luévanos-Escareño, M. P., Sosa-Martínez, J. D., Castro-Alonso, M. J., Nimmakayala, P., Reddy, U. K., & Balagurusamy, N. (2025). Capsaicin as a Microbiome Modulator: Metabolic Interactions and Implications for Host Health. Metabolites, 15(6), 372. https://doi.org/10.3390/metabo15060372







