Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study
Abstract
1. Introduction
2. Materials and Methods
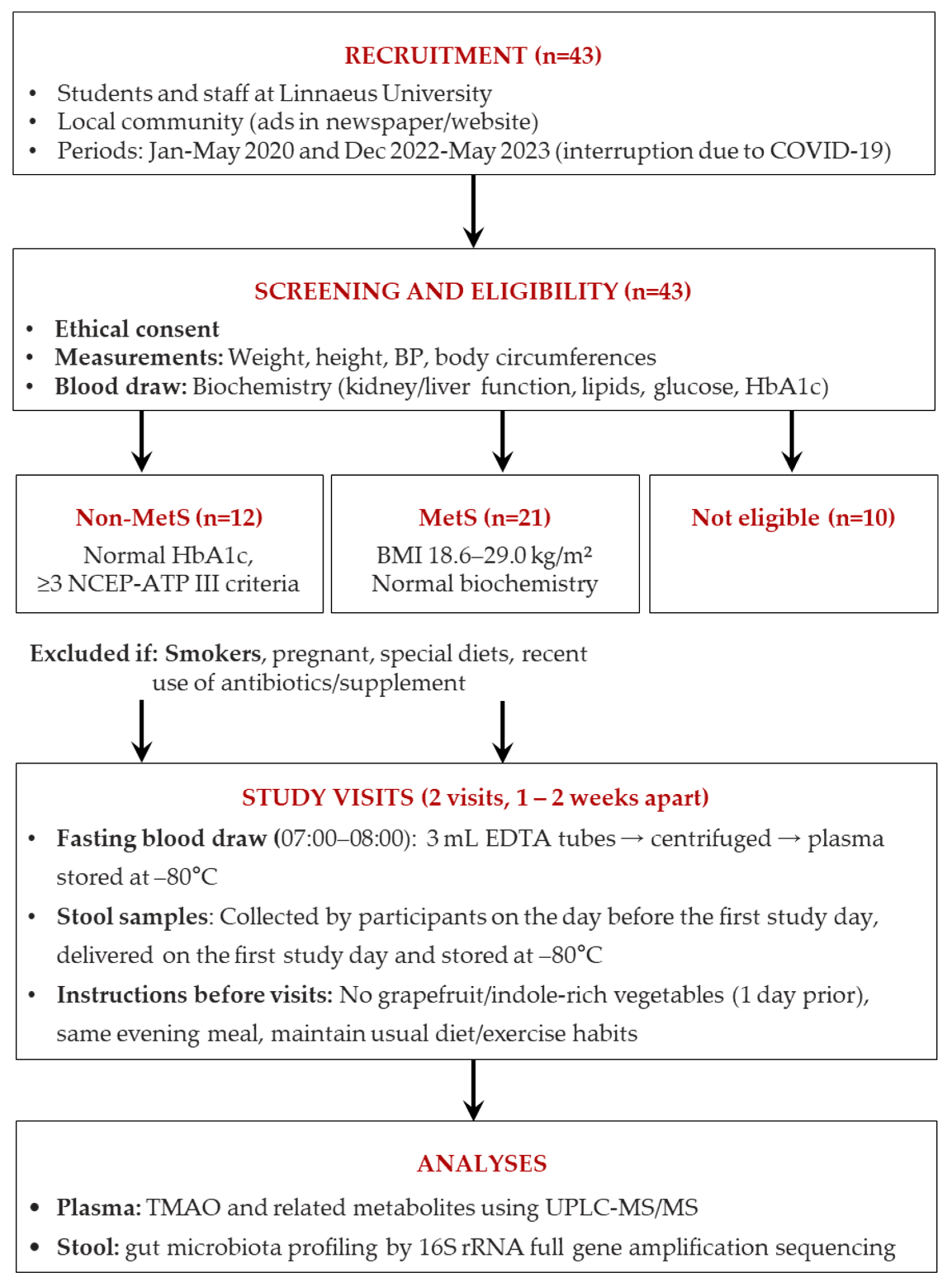
2.1. Subjects
2.2. Study Visits and Blood and Feces Sample Collection
2.3. Clinical Analyses
2.3.1. Blood Chemistry
2.3.2. Quantification of TMAO and Related Metabolites
2.3.3. Microbiota Analysis
Feces DNA Extraction
Bacteria 16S rRNA Full-Gene Amplicon Sequencing
Taxonomic Annotation
2.4. Statistical Analyses
3. Results
3.1. Clinical Parameters
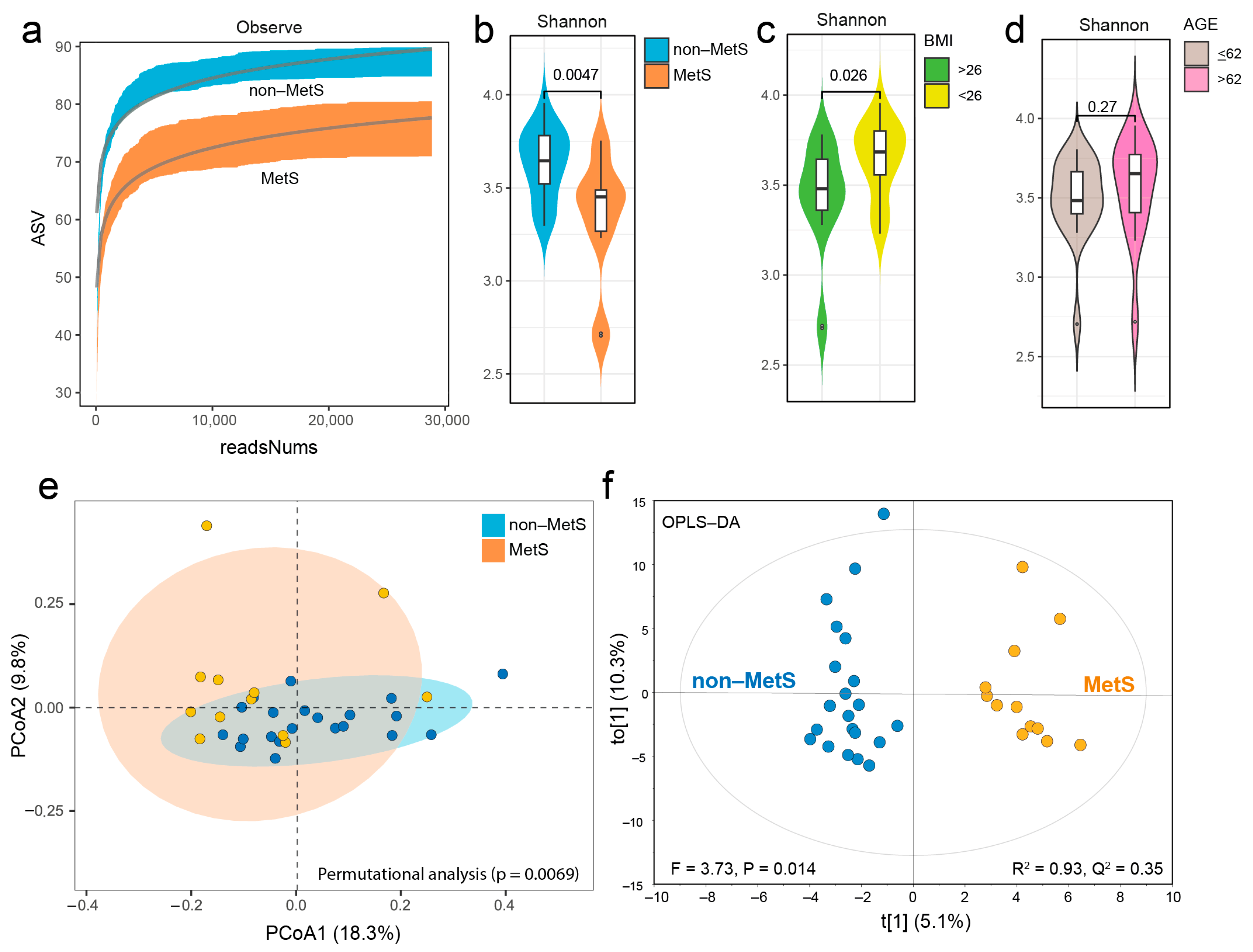
3.2. Gut Microbiota
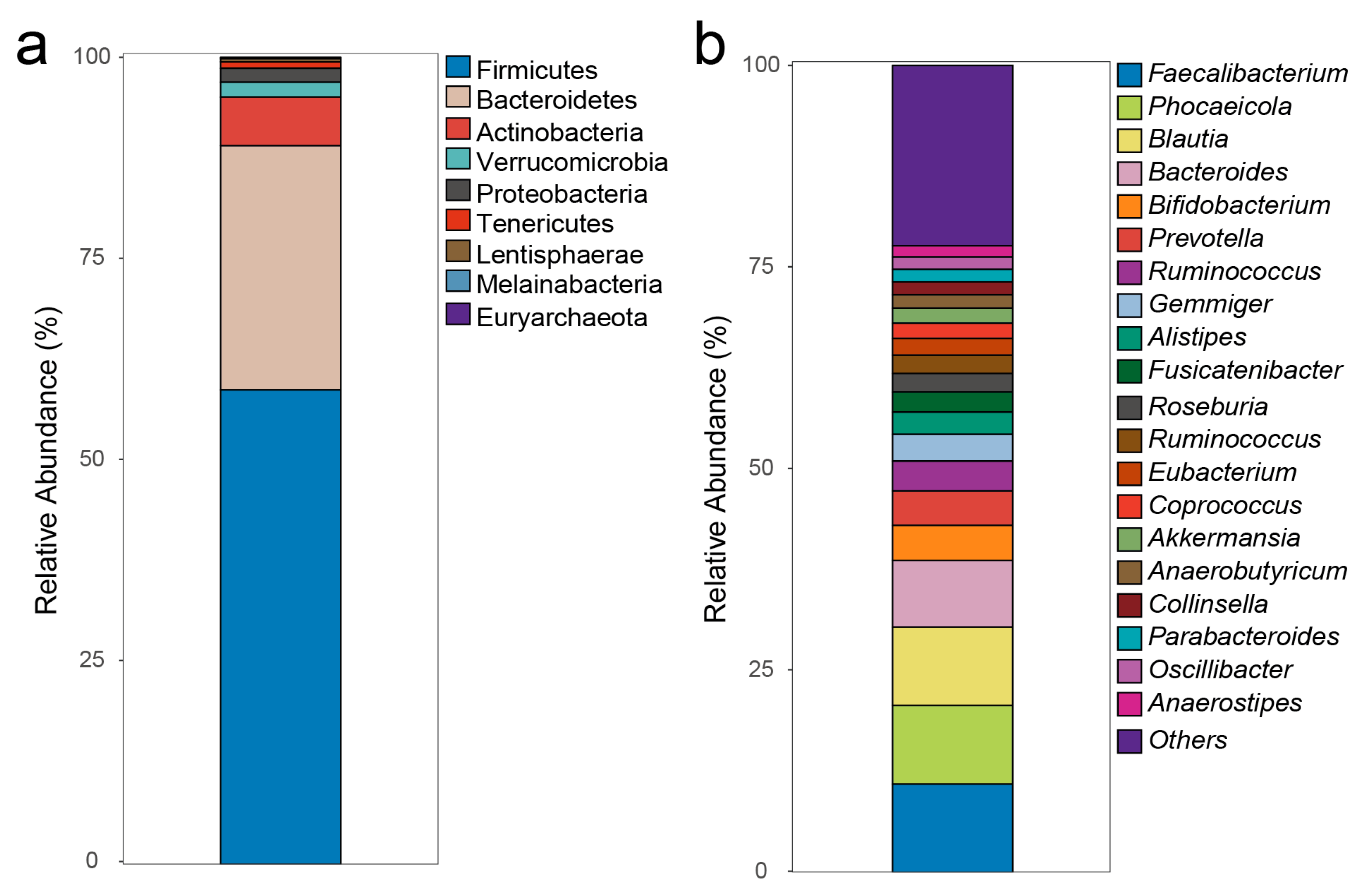
3.2.1. Gut Microbiota Composition
3.2.2. Gut Microbiota Composition of Non-MetS and MetS Subjects
3.2.3. Taxonomic Differences Between Non-MetS and MetS Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP III | Adult Treatment Panel III |
| CntA/B | A two-component Rieske-type oxygenase/reductase system converts carnitine to TMA |
| CutC | Glycyl radical enzyme converts choline to TMA (choline TMA-lyase) |
| CutD | CutC-activating protein (the activator protein for choline TMA-lyase) |
| YeaW/X | A two-component Rieske-type oxygenase/reductase system converts betaine to TMA |
| CVD | Cardiovascular disease |
| FMO3 | Flavin-containing monooxygenase 3 |
| MetS | Metabolic syndrome |
| MUC2 | Mucin 2, oligomeric mucus/gel-forming |
| NCEP | National Cholesterol Education Program |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PLS | Partial Least Squares Discriminant Analysis |
| rho | Spearman correlation coefficients |
| SCFAs | Short-chain fatty acids |
| SD | Standard deviation |
| T2D | Type 2 diabetes |
| TMAO | Trimethylamine N-oxide |
| TMA | Trimethylamine |
References
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.; Haslberger, A.G. Gut Microbiota of Obese, Type 2 Diabetic Individuals is Enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after Weight Loss. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Z.; Bao, G.; Xue, C.; Zheng, W.; Fu, R.; Zhang, M.; Ding, J.; Yang, F.; Sun, B. Causal effect between gut microbiota and metabolic syndrome in European population: A bidirectional mendelian randomization study. Cell Biosci. 2024, 14, 67. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal Microbiota-Dependent Phosphatidylcholine Metabolites, Diastolic Dysfunction, and Adverse Clinical Outcomes in Chronic Systolic Heart Failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Dai, L.; Avesani, C.M.; Kublickiene, K.; Stenvinkel, P. The dietary source of trimethylamine N-oxide and clinical outcomes: An unexpected liaison. Clin. Kidney J. 2023, 16, 1804–1812. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Jin, S.; Lv, J.; Li, M.; Feng, N. The gut microbiota derived metabolite trimethylamine N-oxide: Its important role in cancer and other diseases. Biomed. Pharmacother. 2024, 177, 117031. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.-X.; Lv, E.-H.; Wen, P.-B.; Liu, X.; Wang, Y.-T.; Cai, X.-C.; Tian, J.-Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine Is a Proatherogenic Intermediate in Gut Microbial Metabolism of L-Carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Campo, A.M.-d.; Bodea, S.; Hamer, H.A.; Marks, J.A.; Haiser, H.J.; Turnbaugh, P.J.; Balskus, E.P. Characterization and Detection of a Widely Distributed Gene Cluster That Predicts Anaerobic Choline Utilization by Human Gut Bacteria. mBio 2015, 6, e00042-15. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta-analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Roi, C.M.; Pescari, D.; Velea-Barta, O.-A.; Mozos, I.; Stoian, D. Connections between serum Trimethylamine N-Oxide (TMAO), a gut-derived metabolite, and vascular biomarkers evaluating arterial stiffness and subclinical atherosclerosis in children with obesity. Front. Endocrinol. 2023, 14, 1253584. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, J.; Fu, Q.; Xu, X.; Wei, S.; Yang, S.; Chen, B. The associations between TMAO-related metabolites and blood lipids and the potential impact of rosuvastatin therapy. Lipids Health Dis. 2022, 21, 60. [Google Scholar] [CrossRef]
- Ringel, C.; Dittrich, J.; Gaudl, A.; Schellong, P.; Beuchel, C.F.; Baber, R.; Beutner, F.; Teren, A.; Engel, C.; Wirkner, K.; et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis 2021, 335, 62–67. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, C.-H.; Wang, J.-H.; Hsu, B.-G. Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients. Int. J. Environ. Res. Public Health 2022, 19, 8710. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Hefni, M.E.; Witthöft, C.M. Development and Application of a UPLC–MRM–MS Method for Quantifying Trimethylamine, Trimethylamine-N-Oxide, and Related Metabolites in Individuals with and Without Metabolic Syndrome. Separations 2025, 12, 53. [Google Scholar] [CrossRef]
- Esberg, A.; Fries, N.; Haworth, S.; Johansson, I. Saliva microbiome profiling by full-gene 16S rRNA Oxford Nanopore Technology versus Illumina MiSeq sequencing. NPJ Biofilms Microbiomes 2024, 10, 149. [Google Scholar] [CrossRef]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2013, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Nickols, W.A.; Kuntz, T.; Shen, J.; Maharjan, S.; Mallick, H.; Franzosa, E.A.; Thompson, K.N.; Nearing, J.T.; Huttenhower, C. MaAsLin 3: Refining and extending generalized multivariable linear models for meta-omic association discovery. bioRxiv 2024, 628459. [Google Scholar] [CrossRef]
- RStudioTeam. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Cha, Y.S. Effects of L-carnitine on obesity, diabetes, and as an ergogenic aid. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 306–308. [Google Scholar]
- Lent-Schochet, D.; Silva, R.; McLaughlin, M.; Huet, B.; Jialal, I. Changes to trimethylamine-N-oxide and its precursors in nascent metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2018, 35. [Google Scholar] [CrossRef]
- Gao, X.; Tian, Y.; Randell, E.; Zhou, H.; Sun, G. Unfavorable Associations Between Serum Trimethylamine N-Oxide and L-Carnitine Levels with Components of Metabolic Syndrome in the Newfoundland Population. Front. Endocrinol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Naghipour, S.; Cox, A.J.; Fisher, J.J.; Plan, M.; Stark, T.; West, N.; Peart, J.N.; Headrick, J.P.; Du Toit, E.F. Circulating TMAO, the gut microbiome and cardiometabolic disease risk: An exploration in key precursor disorders. Diabetol. Metab. Syndr. 2024, 16, 133. [Google Scholar] [CrossRef]
- Reddy, P.; Leong, J.; Jialal, I. Amino acid levels in nascent metabolic syndrome: A contributor to the pro-inflammatory burden. J. Diabetes Complicat. 2018, 32, 465–469. [Google Scholar] [CrossRef]
- Yang, Y.-N.; Wang, Q.-C.; Xu, W.; Yu, J.; Zhang, H.; Wu, C. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed. Pharmacother. 2022, 155, 113749. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Pugar, E.M.G.d.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5, e00857-19. [Google Scholar] [CrossRef] [PubMed]
- Schaus, S.R.; Pereira, G.V.; Luis, A.S.; Madlambayan, E.; Terrapon, N.; Ostrowski, M.P.; Jin, C.; Henrissat, B.; Hansson, G.C.; Martens, E.C. Ruminococcus torques is a keystone degrader of intestinal mucin glycoprotein, releasing oligosaccharides used by Bacteroides thetaiotaomicron. mBio 2024, 15, e0003924. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria with Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Off. J. Am. Coll. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Shi, X.; Gao, B.; Srivastava, A.; Izzi, Z.; Abdalla, Y.; Shen, W.; Raj, D. Alterations of gut microbial pathways and virulence factors in hemodialysis patients. Front. Cell. Infect. Microbiol. 2022, 12, 904284. [Google Scholar] [CrossRef]
- Du, L.; Zhang, K.; Liang, L.; Yang, Y.; Lu, D.; Zhou, Y.; Ren, T.; Fan, J.; Zhang, H.; Wang, Y.; et al. Multi-omics analyses of the gut microbiota and metabolites in children with metabolic dysfunction-associated steatotic liver disease. mSystems 2025, 10, e01148-24. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.; Cai, X.; Lee, J.; Bundy, J.D.; Jovanovich, A.; Chen, J.; Deo, R.; Lash, J.P.; Anderson, A.H.; Go, A.S.; et al. Deoxycholic Acid and Risks of Cardiovascular Events, ESKD, and Mortality in CKD: The CRIC Study. Kidney Med. 2022, 4, 100387. [Google Scholar] [CrossRef]
- Org, E.; Blum, Y.; Kasela, S.; Mehrabian, M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Wang, Z.; Ala-Korpela, M.; Hazen, S.L.; et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017, 18, 70. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ivey, K.L.; Wang, D.D.; Wilkinson, J.E.; Franke, A.; Lee, K.H.; Chan, A.; Huttenhower, C.; Hu, F.B.; et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: Findings from a longitudinal cohort of US men. Gut 2022, 71, 724–733. [Google Scholar] [CrossRef]
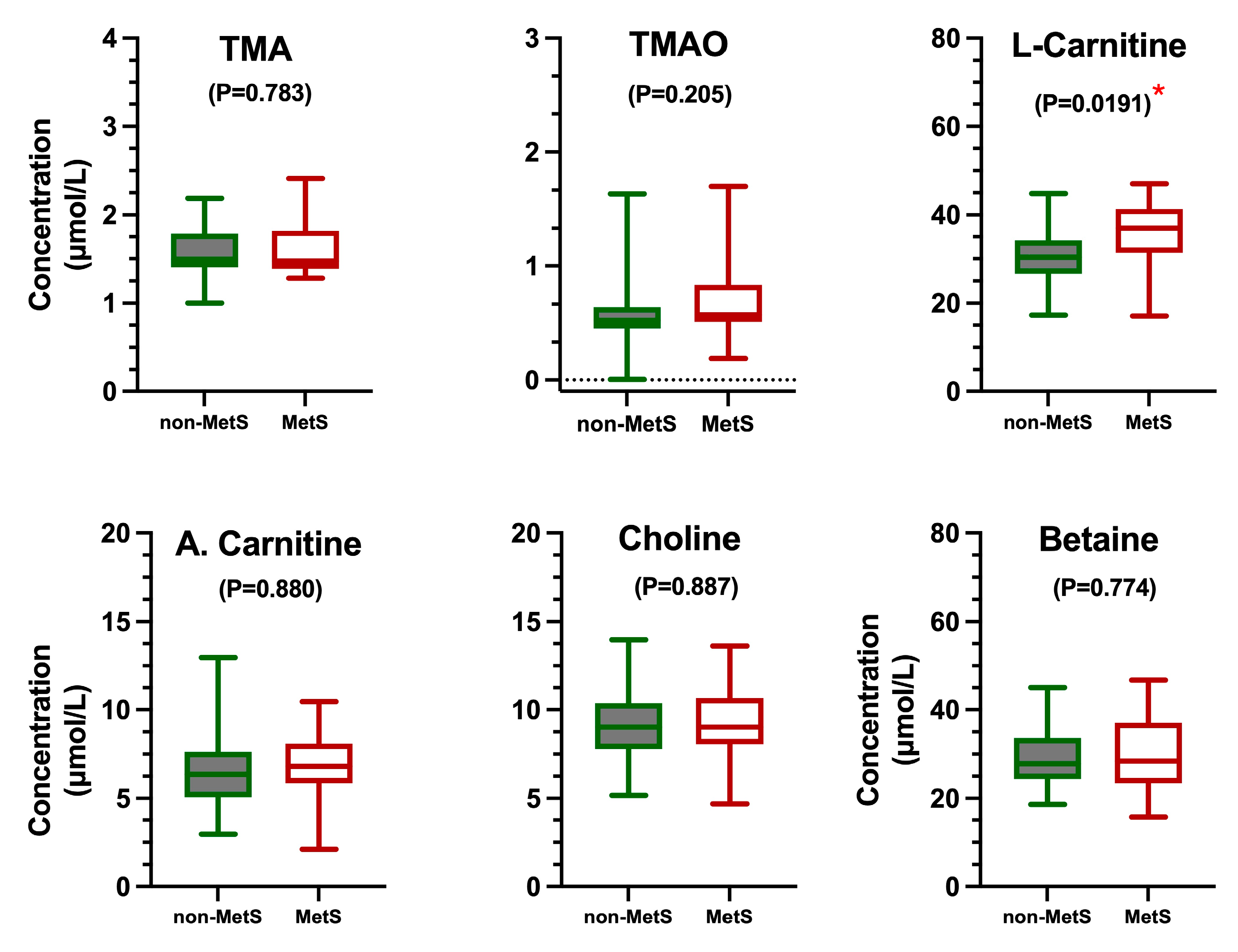
| Variable | Non-MetS (n = 21) | MetS (n = 12) | Unpaired T Test |
|---|---|---|---|
| Age (years) # | 57 (13.2) | 63 (8.7) | 0.1772 ns |
| Waist circumference (cm) | 87 (9.5) | 111 (12.5) | <0.0001 *** |
| Weight (kg) | 73 (10.9) | 95 (18.7) | 0.0002 *** |
| Height (m) | 1.71 (0.07) | 1.67 (0.11) | 0.2401 ns |
| BMI (kg/m2) | 24.8 (3.2) | 33.5 (5.1) | <0.0001 *** |
| P-glucose (mmol/L) | 5.3 (0.38) | 6.0 (0.45) | <0.0001 *** |
| HbA1c (mmol/L) # | 35.1 (2.2) | 39.1 (5.3) | 0.0045 ** |
| P-cholesterol (mmol/L) | 5.8 (1.12) | 5.1 (1.04) | 0.0934 ns |
| P-HDL-cholesterol (mmol/L) | 1.9 (0.47) | 1.3 (0.42) | 0.0013 ** |
| P-triglycerides (mmol/L) # | 0.98 (0.49) | 1.91 (0.73) | 0.0001 *** |
| Variable | TMAO | Carnitine | Choline | TMA | ||||
|---|---|---|---|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| Waist circumference | 0.18 | 0.15 ns | 0.61 | <0.0001 *** | 0.13 | 0.31 ns | −0.05 | 0.67 ns |
| Weight | 0.25 | 0.05 * | 0.60 | <0.0001 *** | 0.21 | 0.09 ns | −0.08 | 0.53 ns |
| BMI | 0.25 | 0.04 * | 0.59 | <0.0001 *** | 0.16 | 0.20 ns | −0.06 | 0.64 ns |
| P-glucose | 0.11 | 0.37 ns | 0.37 | <0.0001 *** | 0.00 | 0.99 ns | −0.20 | 0.11 ns |
| HbA1c | 0.19 | 0.13 ns | 0.37 | <0.0001 *** | −0.08 | 0.51 ns | 0.29 | 0.02 * |
| P-cholesterol | −0.11 | 0.38 ns | −0.17 | 0.17 ns | 0.16 | 0.19 ns | −0.14 | 0.28 ns |
| P-HDL cholesterol | −0.06 | 0.63 ns | −0.42 | <0.0001 *** | −0.07 | 0.57 ns | 0.13 | 0.29 ns |
| P-triglycerides | 0.14 | 0.25 ns | 0.55 | <0.0001 *** | 0.10 | 0.41 ns | −0.01 | 0.96 ns |
| Systolic blood pressure | 0.05 | 0.68 ns | 0.15 | 0.22 ns | 0.06 | 0.63 ns | 0.03 | 0.84 ns |
| Diastolic blood pressure | −0.10 | 0.43 ns | 0.11 | 0.39 ns | 0.26 | 0.04 * | −0.17 | 0.18 ns |
| Variables | Adjusted for Age, Sex, Sampling Day | Not Adjusted for Age, Sex, Sampling Day | |||
|---|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | ||
| TMAO | Carnitine | 0.212 | 0.087 | 0.237 | 0.056 |
| TMAO | Choline | 0.163 | 0.192 | 0.300 | 0.014 |
| TMAO | TMA | 0.439 | <0.001 | 0.332 | 0.006 |
| Carnitine | Choline | 0.039 | 0.756 | 0.082 | 0.514 |
| Carnitine | TMA | 0.063 | 0.617 | 0.036 | 0.775 |
| Choline | TMA | 0.137 | 0.272 | 0.128 | 0.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefni, M.E.; Witthöft, C.M.; Hellström, P.; Johansson, I.; Esberg, A. Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study. Metabolites 2025, 15, 364. https://doi.org/10.3390/metabo15060364
Hefni ME, Witthöft CM, Hellström P, Johansson I, Esberg A. Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study. Metabolites. 2025; 15(6):364. https://doi.org/10.3390/metabo15060364
Chicago/Turabian StyleHefni, Mohammed E., Cornelia M. Witthöft, Patrik Hellström, Ingegerd Johansson, and Anders Esberg. 2025. "Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study" Metabolites 15, no. 6: 364. https://doi.org/10.3390/metabo15060364
APA StyleHefni, M. E., Witthöft, C. M., Hellström, P., Johansson, I., & Esberg, A. (2025). Plasma TMAO Concentrations and Gut Microbiota Composition in Subjects with and Without Metabolic Syndrome: Results from Pilot Study. Metabolites, 15(6), 364. https://doi.org/10.3390/metabo15060364







