Physiological and Molecular Responses of Underutilized Genotype AHK-200 of Vegetable Melon (Cucumis melo var. melo) Against Drought Stress: Gas Exchange, Antioxidant Activity, and Gene Expression
Abstract
1. Introduction
2. Materials
2.1. Materials and Treatments
2.2. Shoot Weight Measurement
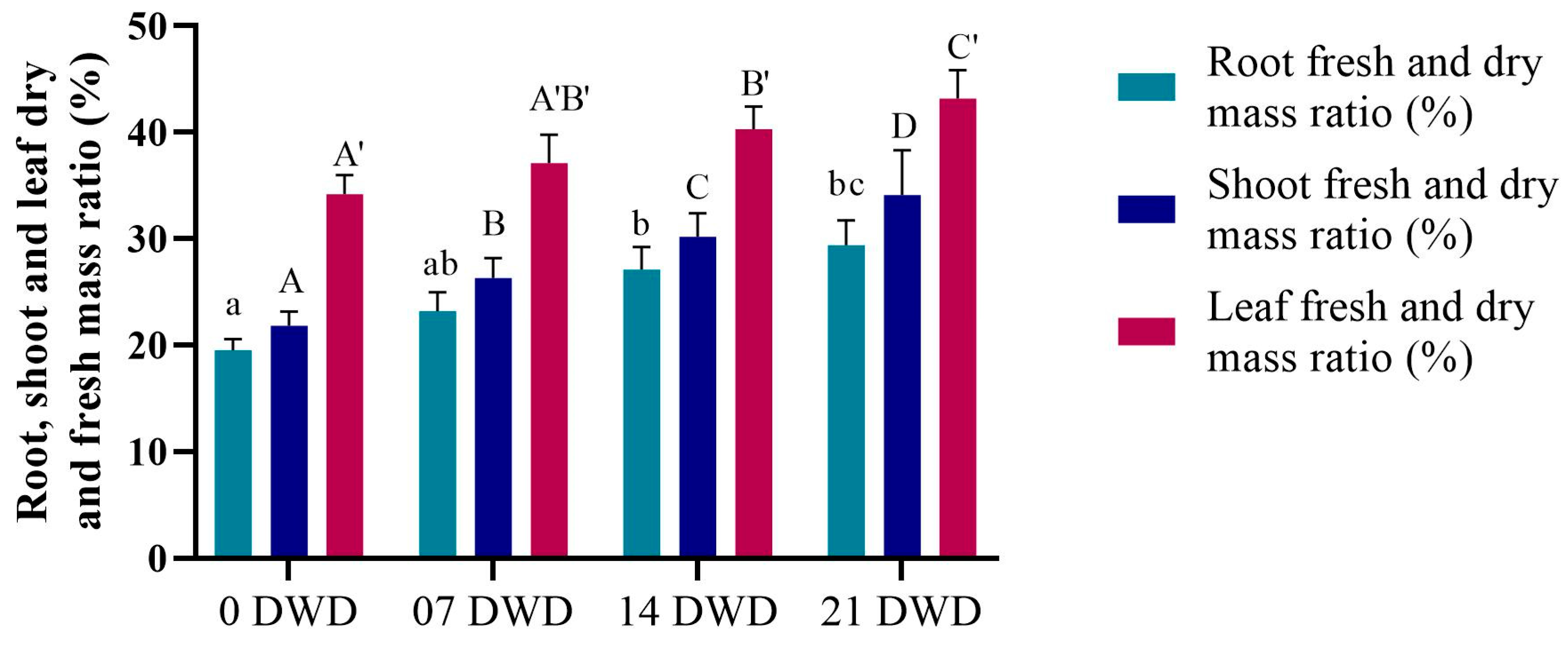
2.3. Root Length and Dry-to-Fresh Mass Ratio
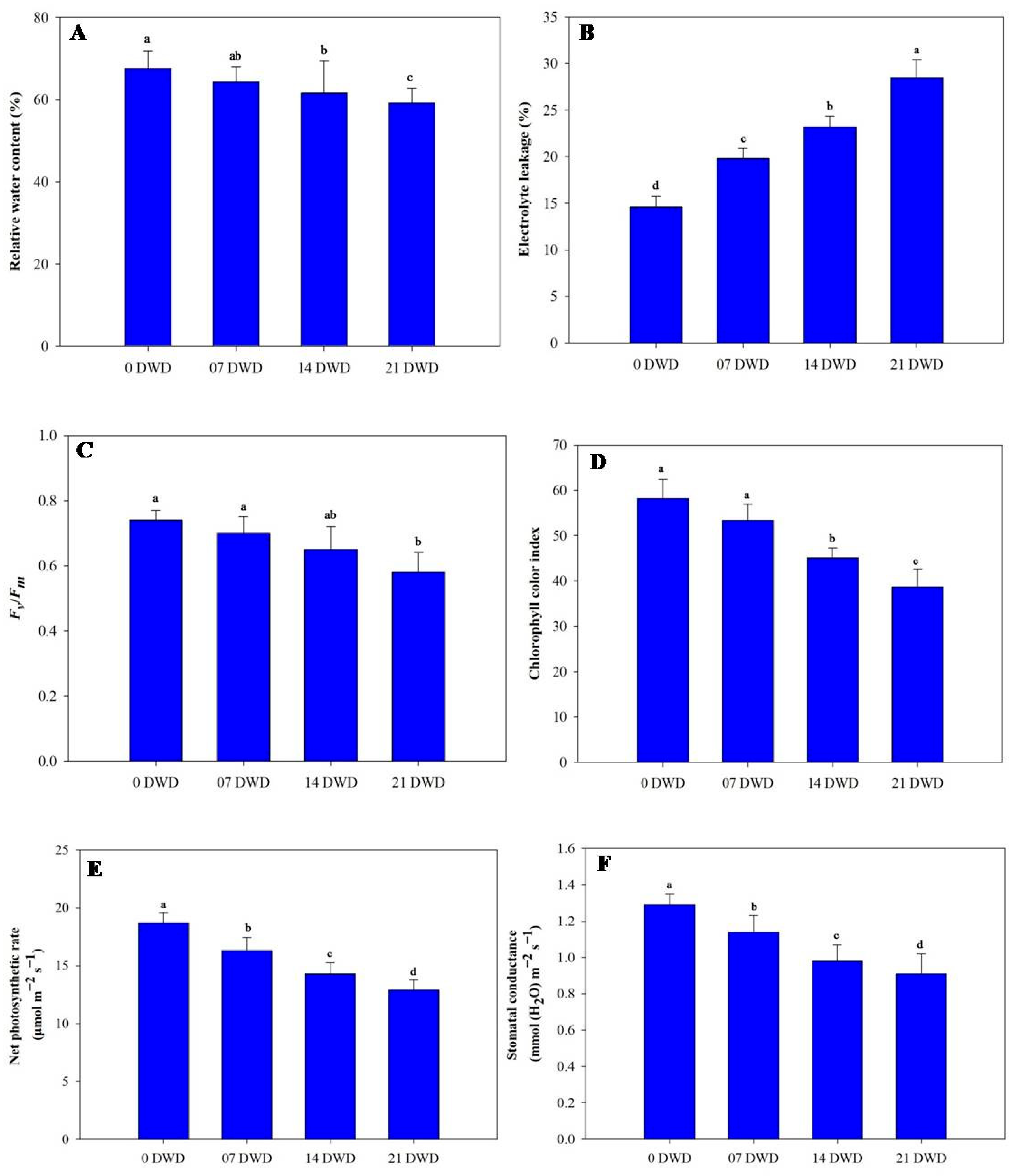
2.4. Net Photosynthetic Rate (PN) and Stomatal Conductance (gs)
2.5. Chlorophyll Fluorescence (Fv/Fm) and Chlorophyll Color Index (CCI)
2.6. Relative Water Content (RWC) and Electrolyte Leakage (EL)
2.7. Chlorophyll (Chl) and Carotenoid (Car) Measurement
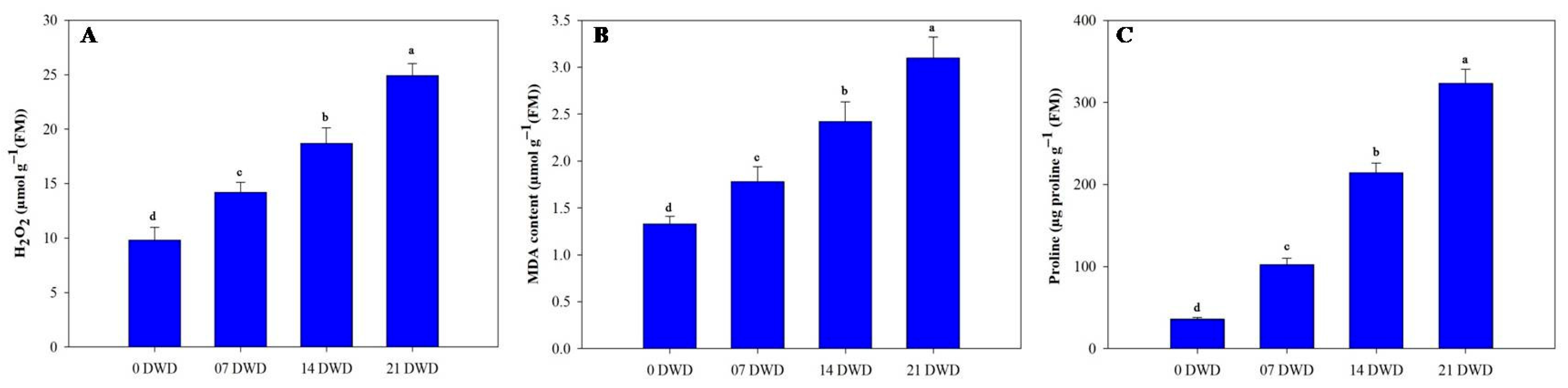
2.8. Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), and Proline Content
2.9. Antioxidant Enzyme Activity Determination
2.10. Physiochemical and Nutritional Properties of Soil
2.11. Elemental Analysis
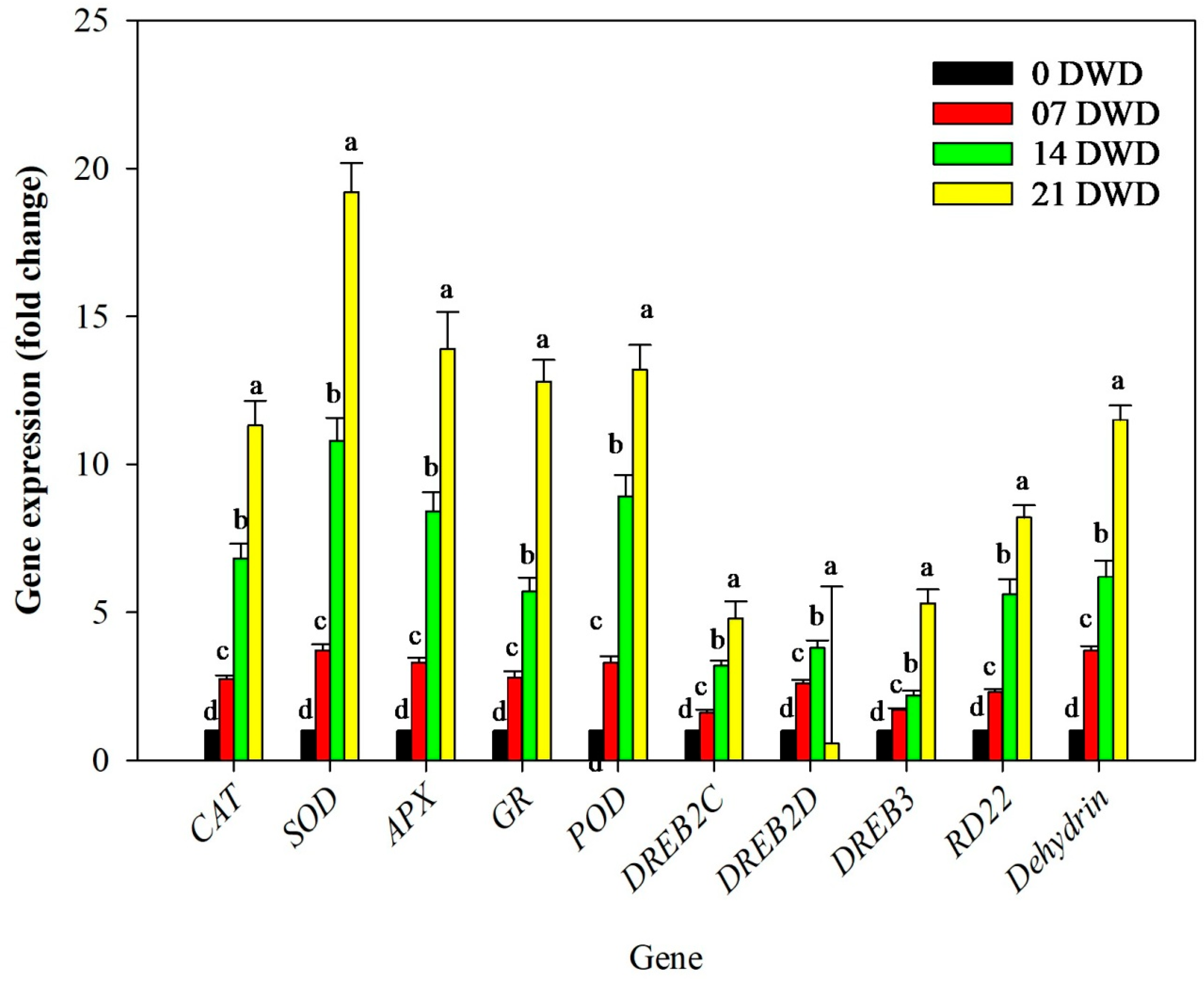
2.12. Selection of Genes and Their Expression Analysis
2.13. Statistical Analysis
3. Results
3.1. Soil Parameters
3.2. Morphological Parameters
3.3. Physiological Parameters
3.4. Biochemical Parameters
3.5. Antioxidant Enzyme Activity
3.6. Electrolyte Concentration
3.7. Gene Expression Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, W.A.; Atri, N.; Yang, L.; Singh, B.; Pandey, S. Genetic diversity in muskmelon based on SSR markers and morphological traits under well-watered and water-deficit condition. Biocatal. Agric. Biotechnol. 2020, 26, 101630. [Google Scholar] [CrossRef]
- Pandey, S.; Ansari, W.A.; Jha, A.; Bhatt, K.V.; Singh, B. Evaluation of melons and indigenous Cucumis spp. genotypes for drought tolerance. II Int. Symp. Underutilized Plant Species Crops Future-Beyond FoodSecur. 2011, 979, 335–339. [Google Scholar] [CrossRef]
- Samadia, D.K.; Haldhar, S.M.; Ram, H.; Verma, A.K.; Gurjar, P.S. Kachri melon (a non-dessert form of Cucumis melo) diversity, germplasm utilization and varietal development under hot arid climate: Approaches and realization. J. Agric. Ecol. 2024, 18, 14–27. [Google Scholar] [CrossRef]
- Samadia, D.K.; Haldhar, S.M. Strategies and advancements for improvement in arid vegetables. Indian J. Arid. Hortic. 2018, 13, 11–18. [Google Scholar]
- Samadia, D.K.; Haldhar, S.M. Mateera, watermelon (Citrullus lanatus) germplasm utilization for improving fruit quality and marketable harvest under hot arid climate of India: Approaches and out-put. J. Agric. Ecol. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Samadia, D.K. Studies on genetic variability and scope of improvement in round melon under hot arid conditions. Indian J. Arid. Hortic. 2007, 64, 58–62. [Google Scholar]
- Samadia, D.K.; Haldhar, S.M. Scope and strategies for genetic improvement in vegetable crop-plants under high temperature and abiotic stressed climate of Rajasthan: A gap analysis. J. Agric. Ecol. 2019, 8, 1–18. [Google Scholar] [CrossRef]
- Haldhar, S.M.; Samadia, D.K.; Bhargava, R.; Choudhary, B.R.; Singh, D. Host plant accessions determine bottom-up effect of snapmelon (Cucumis melo var. momordica) against melon fly (Bactroceracucurbitae (Coquillett)). Breed. Sci. 2018, 68, 499–507. [Google Scholar] [CrossRef]
- Samadia, D.K.; Pareek, O.P. Kachari: Arid region cucurbit vegetable for processing industry. In Proceedings of the 4th Agricultural Science Congress, Jaipur, India, 21–24 February 1999. [Google Scholar]
- Kausar, A.; Ashraf, M.Y.; Ali, I.; Niaz, M.; Abbass, Q. Evaluation of sorghum varieties/lines for salt tolerance using physiological indices as screening tool. Pak. J. Bot. 2012, 44, 47–52. [Google Scholar]
- Ansari, W.A.; Atri, N.; Singh, B.; Kumar, P.; Pandey, S. Morpho-physiological and biochemical responses of muskmelon genotypes to different degree of water deficit. Photosynthetica 2018, 56, 1019–1030. [Google Scholar] [CrossRef]
- Khare, N.; Goyary, D.; Singh, N.K.; Shah, P.; Rathore, M.; Anandhan, S.; Sharma, D.; Arif, M.; Ahmed, Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2010, 103, 267–277. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UVVIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12- transformed transgenic tomato plants. Plant Physiol. Bioch. 2012, 61, 108–114. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxides in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH changes during legume growth and application of plant material. Biol. Fertil. Soils 1996, 23, 236–242. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Pentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; Volume 498, pp. 151–154. [Google Scholar]
- Salam, A.K.; Desvia, Y.; Sutanto, E.; Syam, T.; Nugroho, S.G.; Kimura, M. Activities of soil enzymes in different land-use systems in middle terrace areas of Lampung Province, South Sumatra, Indonesia. Soil Sci. Plant Nutri. 1999, 45, 89–99. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A Rapid Procedure for the Estimation of Available Nitrogen in Soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture; US Government Printing Office: Washington DC, USA, 1954. [Google Scholar]
- Kirkbright, G.F.; Sargent, M. Atomic Absorption and Fluorescence Spectroscopy; Academic Press Inc.: London, UK, 1974; ISBN 0-12-409750-2. [Google Scholar]
- Bhati, K.K.; Aggarwal, S.; Sharma, S.; Mantri, S.; Singh, S.P.; Bhalla, S.; Kaur, J.; Tiwari, S.; Roy, J.K.; Tuli, R.; et al. Differential expression of structural genes for the late phase of phytic acid biosynthesis in developing seeds of wheat (Triticum aestivum L.). Plant Sci. 2014, 224, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crops Res. 2014, 165, 15–24. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [CrossRef]
- Ansari, W.A.; Krishna, R.; Yadav, P.S.; Chaubey, T.; Behera, T.K.; Bhat, K.V.; Pandey, S. Alteration in physio-chemical properties and gene expression pattern of snapmelon (Cucumis melo var. momordica) genotypes against drought stress. Plant Genet. Res. 2024, 22, 87–96. [Google Scholar] [CrossRef]
- Mirabad, A.A.; Lotfi, M.; Roozban, M.R. Impact of water-deficit stress on growth, yield and sugar content of cantaloupe (Cucumis melo L.). Proc. Int. Conf. 2013, 5, 2778–2782. [Google Scholar]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L.) plants. Physiol. Mol. Biol. Plants 2020, 26, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Seymen, M.; Yavuz, D.; Acar, B.; Metin, D.; Atakul, Z.; Kal, Ü. Morphophysiological and biochemical investigation of the potential of citron watermelon (Citrullus lanatus var. citroides) rootstock under different irrigation regimes. Hortic. Environ. Biotechnol. 2024, 65, 1–15. [Google Scholar] [CrossRef]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Koç, C.; Ulusu, F.; Ulusu, Y. Physio-biochemical responses of registered bread wheat (Triticum aestivum L.) genotypes to drought stress: Variations in antioxidant parameters and photosynthetic pigment amounts. Anatol. J. Bot. 2024, 8, 1–10. [Google Scholar] [CrossRef]
- Kusvuran, S. Effects of drought and salt stresses on growth, stomatal conductance, leaf water and osmotic potentials of melon genotypes (Cucumis melo L.). Afr. J. Agric. Res. 2012, 7, 775–781. [Google Scholar]
- Ansari, W.A.; Atri, N.; Singh, B.; Pandey, S. Changes in antioxidant enzyme activities and gene expression in two muskmelon genotypes under progressive water stress. Biol. Plantarum 2017, 61, 333–341. [Google Scholar] [CrossRef]
- Qian, C.L.; Zhao, Y.Y.; Mi, H.B.; Chen, X.H.; Guo, L.J.; Mao, L.C. Role of antioxidative system during the development and senescence of cucumber fruit. Biol. Plantarum 2012, 56, 793–797. [Google Scholar] [CrossRef]
- Cook, R.; Lupette, J.; Benning, C. The role of chloroplast membrane lipid metabolism in plant environmental responses. Cells 2021, 10, 706. [Google Scholar] [CrossRef]
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Prasad, R.; Verma, J.P.; Singh, M. Overexpression of AtDREB1 and BcZAT12 genes confers drought tolerance by reducing oxidative stress in double transgenic tomato (Solanum lycopersicum L.). Plant Cell Rep. 2021, 40, 2173–2190. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Hackenberg, M.; Shi, B.J.; Gustafson, P.; Langridge, P. A transgenic transcription factor (TaDREB3) in barley affects the expression of microRNAs and other small non-coding RNAs. PLoS ONE 2012, 7, e42030. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, M.; Zhang, J.; Tian, X.; Duan, L.; Li, Z. Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton. J. Exp. Bot. 2012, 63, 3741–3755. [Google Scholar] [CrossRef]
- Bellaloui, N.; Turley, R.B. Effects of fuzzless cottonseed phenotype on cottonseed nutrient composition in near isogenic cotton (Gossypium hirsutum L.) mutant lines under well-watered and water stress conditions. Front. Plant Sci. 2013, 4, 516. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Grau, O.; Chen, H.Y.; Janssens, I.A.; Ciais, P.; Piao, S.; Peñuelas, J. Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Change Biol. 2017, 23, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Nikju, M.B.; Mobasser, H.R.; Ganjali, H.R. Influence of variety on biological yield, harvest index, percent of protein in Zea mays. Biol. Forum 2015, 7, 662. [Google Scholar]
- Tadayyon, A.; Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro- and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant Nutr. 2018, 41, 304–310. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef]
- Nahar, K.; Gretzmacher, R. Response of shoot and root development of seven tomato cultivars in hydrophonic system under water stress. Acad. J. Plant Sci. 2011, 4, 57–63. [Google Scholar]
- Vafaie, A.; Ebadi, A.; Rastgou, B.; Moghadam, S.H. The effects of potassium and magnesium on yield and some physiological traits of safflower (Carthamus tinctorius). Int. J. Agric. Crop Sci. 2013, 5, 1895. [Google Scholar]
- Urbina, I.; Sardans, J.; Beierkuhnlein, C.; Jentsch, A.; Backhaus, S.; Grant, K.; Peñuelas, J. Shifts in the elemental composition of plants during a very severe drought. Environ. Exp. Bot. 2015, 111, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Afshar, R.M.; Hadi, H.; Pirzad, A. Effect of nano-iron on the yield and yield component of cowpea (Vigna unguiculata) under end season water deficit. Int. J. Agric. 2013, 3, 27. [Google Scholar]


| Treatments | EC (Mosiemens/cm) | pH | Organic Carbon (%) |
|---|---|---|---|
| 0 DWD | 198 ± 7.38 a | 7.1 ± 0.28 a | 0.92 ± 0.11 a |
| 07 DWD | 192 ± 11.25 a | 7.1 ± 0.49 a | 0.91 ± 0.08 a |
| 14 DWD | 185 ± 6.32 ab | 7.2 ± 0.52 a | 0.88 ± 0.05 a |
| 21 DWD | 172 ± 10.44 c | 7.1 ± 0.22 a | 0.87 ± 0.09 a |
| Treatments | Total Chlorophyll (mg g−1 (DM)) | Carotenoid (mg g−1 (DM)) |
|---|---|---|
| 0 DWD | 59.2 ± 7.38 a | 1.92 ± 0.09 a |
| 07 DWD | 55.1 ± 11.25 a | 1.73 ± 0.11 b |
| 14 DWD | 47.4 ± 6.32 b | 1.58 ± 0.08 c |
| 21 DWD | 41.0 ± 10.44 c | 1.48 ± 0.07 d |
| Treatments | Na (µg/g) | K (µg/g) | Mg (µg/g) | Fe (µg/g) | Ca (µg/g) |
|---|---|---|---|---|---|
| 0 DWD | 1108 ± 63.2 cd | 34 ± 2.43 c | 2024 ± 117.2 a | 342.2 ± 12.1 d | 314.4 ± 18.2 d |
| 07 DWD | 1371 ± 82.4 c | 43 ± 3.22 b | 1743 ± 73.2 b | 402.1 ± 27.3 c | 435.2 ± 23.9 c |
| 14 DWD | 2292 ± 143.2 b | 47 ± 2.53 ab | 1345 ± 88.7 c | 553.3 ± 44.2 b | 512.3 ± 36.7 b |
| 21 DWD | 3473 ± 182.2 a | 52 ± 3.32 a | 1008 ± 24.1 d | 509.7 ± 21.9 a | 544.2 ± 17.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, S.; Ansari, W.A.; Krishna, R.; Yadav, A.; Jaiswal, D.K.; Singh, B. Physiological and Molecular Responses of Underutilized Genotype AHK-200 of Vegetable Melon (Cucumis melo var. melo) Against Drought Stress: Gas Exchange, Antioxidant Activity, and Gene Expression. Metabolites 2025, 15, 359. https://doi.org/10.3390/metabo15060359
Pandey S, Ansari WA, Krishna R, Yadav A, Jaiswal DK, Singh B. Physiological and Molecular Responses of Underutilized Genotype AHK-200 of Vegetable Melon (Cucumis melo var. melo) Against Drought Stress: Gas Exchange, Antioxidant Activity, and Gene Expression. Metabolites. 2025; 15(6):359. https://doi.org/10.3390/metabo15060359
Chicago/Turabian StylePandey, Sudhakar, Waquar Akhter Ansari, Ram Krishna, Akhilesh Yadav, Durgesh Kumar Jaiswal, and Bijendra Singh. 2025. "Physiological and Molecular Responses of Underutilized Genotype AHK-200 of Vegetable Melon (Cucumis melo var. melo) Against Drought Stress: Gas Exchange, Antioxidant Activity, and Gene Expression" Metabolites 15, no. 6: 359. https://doi.org/10.3390/metabo15060359
APA StylePandey, S., Ansari, W. A., Krishna, R., Yadav, A., Jaiswal, D. K., & Singh, B. (2025). Physiological and Molecular Responses of Underutilized Genotype AHK-200 of Vegetable Melon (Cucumis melo var. melo) Against Drought Stress: Gas Exchange, Antioxidant Activity, and Gene Expression. Metabolites, 15(6), 359. https://doi.org/10.3390/metabo15060359







