Lipidomic Profiling Reveals HSD17B13 Deficiency-Associated Dysregulated Hepatic Phospholipid Metabolism in Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Liver Pathological Assessments
2.3. Determination of Liver Triglyceride (TG) and Cholesterol (TC) Levels
2.4. Glucose and Insulin Tolerance Tests
2.5. Lipidomic Analysis
2.5.1. Lipid Extraction
2.5.2. LC-MS/MS-Based Lipidomic Analysis
Chromatography
Mass Spectrometry
2.5.3. Statistical Analysis
3. Results
3.1. Liver Morphology of the Aged Hsd17b13 KO Mice
3.2. Liver Lipidome Is Altered in the Aged Mice Deficient in the HSD17B13 Enzyme
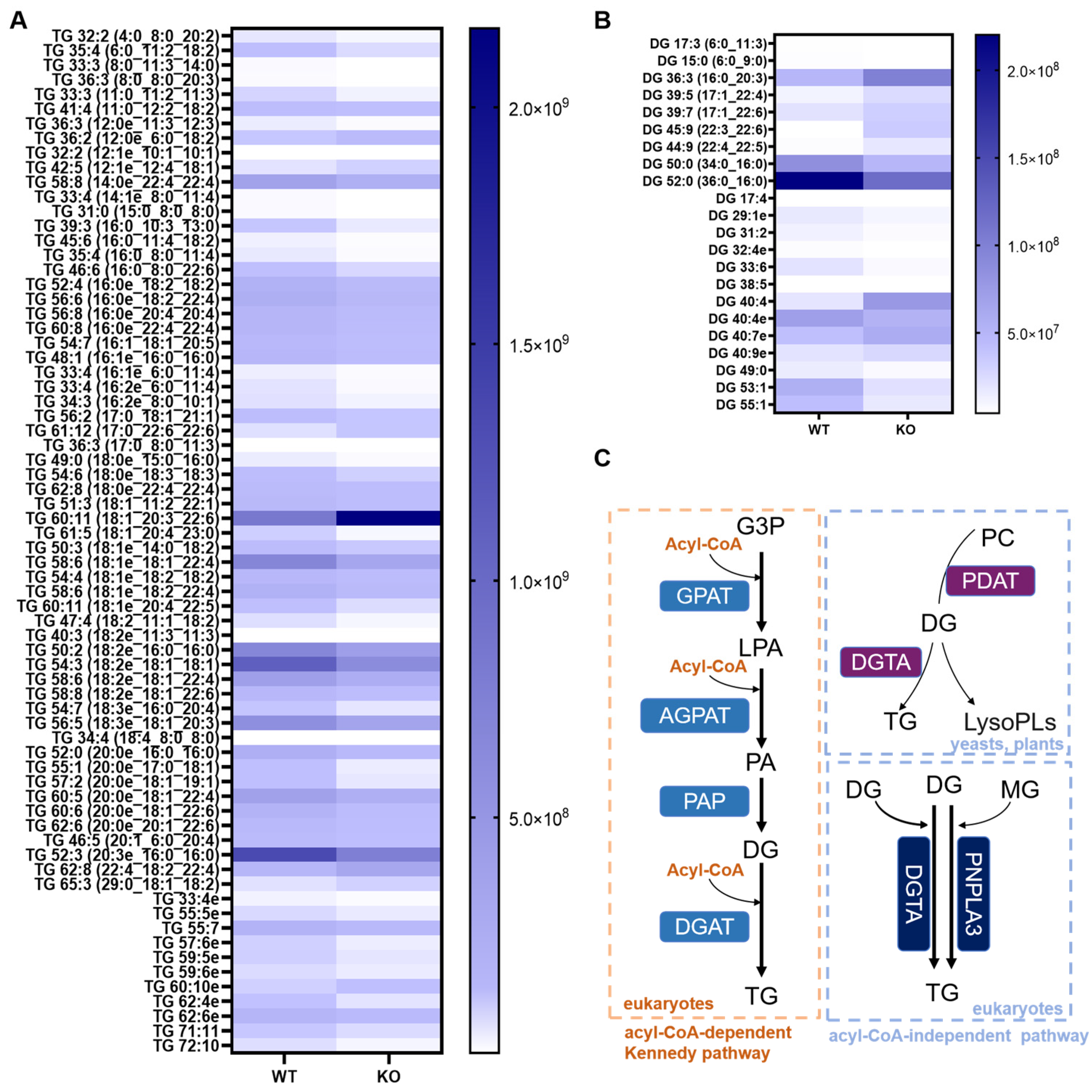
3.3. Altered TG and Diglyceride (DG) Metabolism Landscape in the Hsd17b13 KO Mice
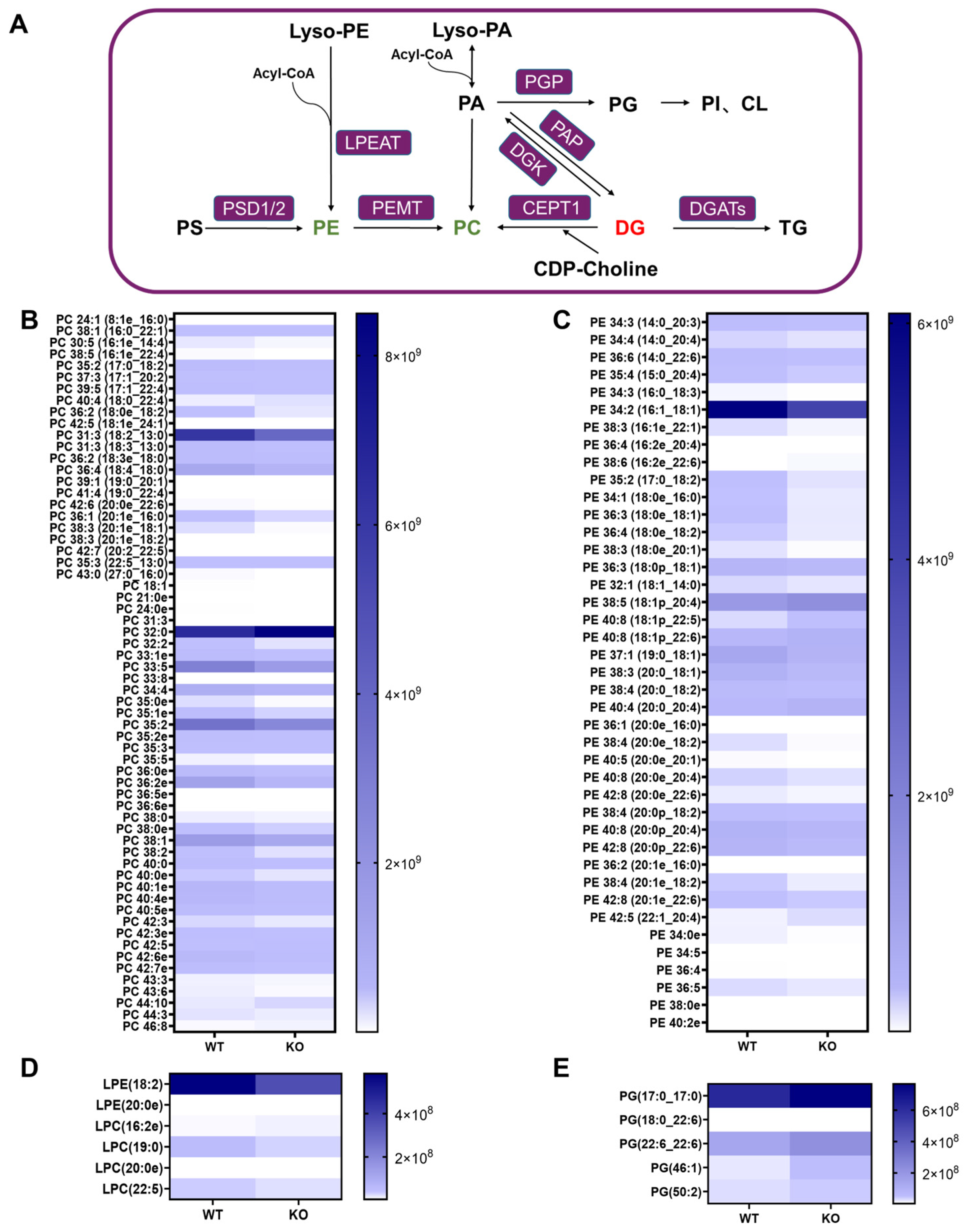
3.4. Altered Phospholipid Metabolism in the Hsd17b13 KO Mice
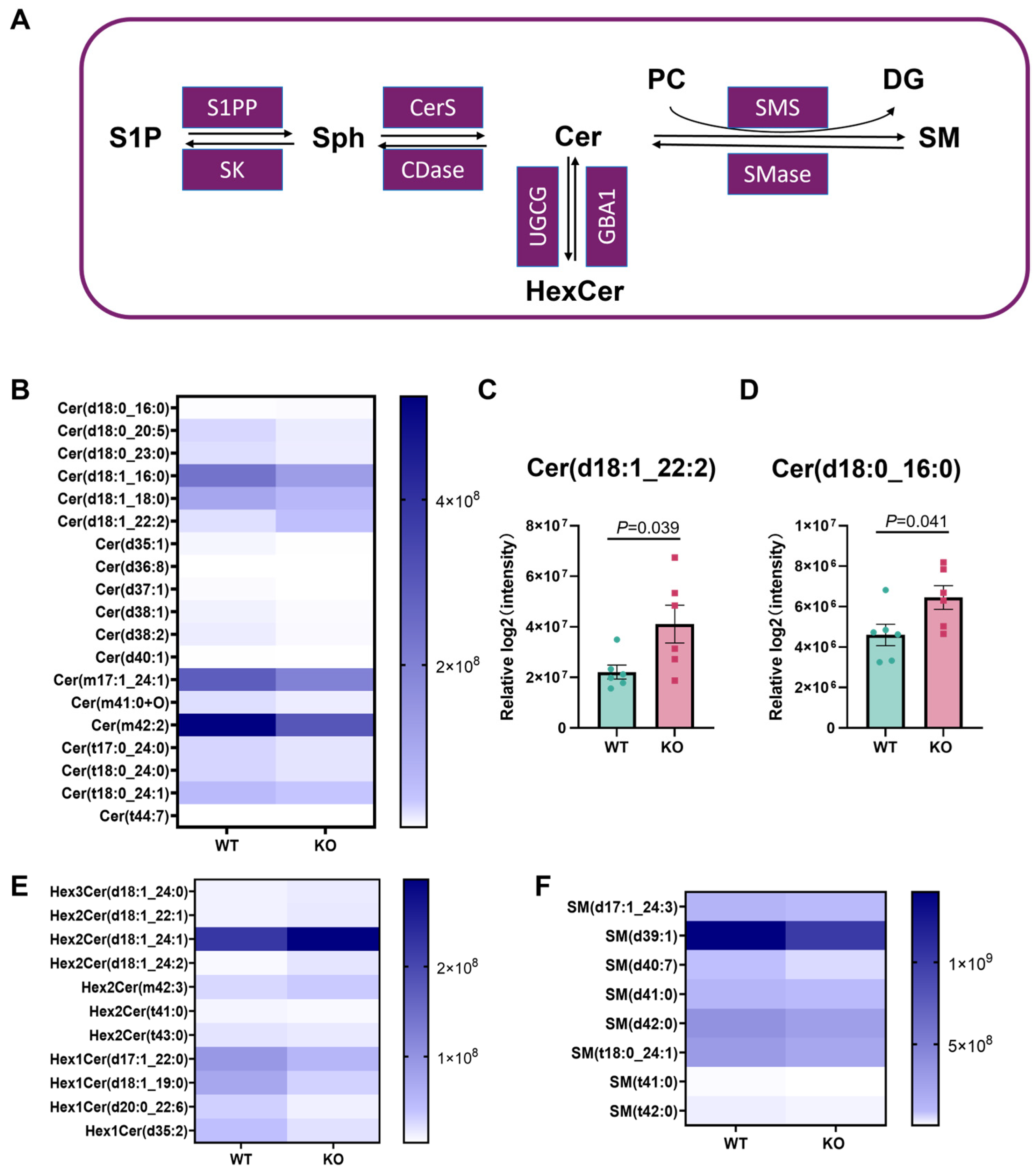
3.5. Cer Metabolic Remodeling in the Hsd17b13 KO Mice
3.6. Advanced Lipidomic Analysis of the Aged Hsd17b13 KO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, K.; Wang, L.; Jun, J.H.; Dutta, R.K.; Maeso-Díaz, R.; Oh, S.H.; Ko, D.C.; Diehl, A.M. Aging promotes metabolic dysfunction-associated steatotic liver disease by inducing ferroptotic stress. Nat. Aging 2024, 4, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Argentieri, M.A.; Xiao, S.; Bennett, D.; Winchester, L.; Nevado-Holgado, A.J.; Ghose, U.; Albukhari, A.; Yao, P.; Mazidi, M.; Lv, J.; et al. Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations. Nat. Med. 2024, 30, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, J.; Cai, J.; Li, R.; Sui, X.; Zhang, J.; Lu, T.; Chen, H.; Chen, G.; Li, H.; et al. Single-cell immune profiling of mouse liver aging reveals Cxcl2+ macrophages recruit neutrophils to aggravate liver injury. Hepatology 2024, 79, 589–605. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Sheedfar, F.; Di Biase, S.; Koonen, D.; Vinciguerra, M. Liver diseases and aging: Friends or foes? Aging Cell 2013, 12, 950–954. [Google Scholar] [CrossRef]
- Adhikary, S.; Esmeeta, A.; Dey, A.; Banerjee, A.; Saha, B.; Gopan, P.; Duttaroy, A.K.; Pathak, S. Impacts of gut microbiota alteration on age-related chronic liver diseases. Dig. Liver Dis. 2024, 56, 112–122. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Araki, M.; Motojima, K. 17beta-Hydroxysteroid dehydrogenase type 13 is a liver-specific lipid droplet-associated protein. Biochem. Biophys. Res. Commun. 2008, 370, 235–238. [Google Scholar] [CrossRef]
- Zhang, H.B.; Su, W.; Xu, H.; Zhang, X.Y.; Guan, Y.F. HSD17B13: A Potential Therapeutic Target for NAFLD. Front. Mol. Biosci. 2021, 8, 824776. [Google Scholar] [CrossRef]
- Liu, S.; Huang, C.; Li, D.; Ren, W.; Zhang, H.; Qi, M.; Li, X.; Yu, L. Molecular cloning and expression analysis of a new gene for short-chain dehydrogenase/reductase 9. Acta Biochim. Pol. 2007, 54, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, Y.; Jia, X.; Wu, W.; Li, L.; Tian, X.; Li, S.; Wang, C.; Xu, H.; Cao, J.; et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2014, 111, 11437–11442. [Google Scholar] [CrossRef]
- Ma, Y.; Belyaeva, O.V.; Brown, P.M.; Fujita, K.; Valles, K.; Karki, S.; de Boer, Y.S.; Koh, C.; Chen, Y.; Du, X.; et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated With Histological Features of Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1504–1519. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sakuma, I.; Gaspar, R.C.; Mooring, M.; Nasiri, A.; Kahn, M.; Zhang, X.M.; Zhang, D.; Sammalkorpi, H.; Penttilä, A.K.; et al. Inhibition of HSD17B13 protects against liver fibrosis by inhibition of pyrimidine catabolism in nonalcoholic steatohepatitis. Proc. Natl. Acad. Sci. USA 2023, 120, e2217543120. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, H.; Smith, J.; Chu, C.H.; Mercer, S.E.; Boehm, S.; McDonald, I.; Zinker, B.; Cheng, D. Evaluation of antisense oligonucleotide therapy targeting Hsd17b13 in a fibrosis mice model. J. Lipid Res. 2024, 65, 100514. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wu, S.; Yang, Y.; Guo, Y.; Zhang, H.; Su, J.; Chen, L.; Mao, Z.; Lan, R.; Cao, R.; et al. Phosphorylation of 17β-hydroxysteroid dehydrogenase 13 at serine 33 attenuates nonalcoholic fatty liver disease in mice. Nat. Commun. 2022, 13, 6577. [Google Scholar] [CrossRef] [PubMed]
- Vanherle, S.; Loix, M.; Miron, V.E.; Hendriks, J.J.A.; Bogie, J.F.J. Lipid metabolism, remodelling and intercellular transfer in the CNS. Nat. Rev. Neurosci. 2025, 26, 214–231. [Google Scholar] [CrossRef]
- Zhu, Y.; Tong, X.; Xue, J.; Qiu, H.; Zhang, D.; Zheng, D.Q.; Tu, Z.C.; Ye, C. Phospholipid biosynthesis modulates nucleotide metabolism and reductive capacity. Nat. Chem. Biol. 2025, 21, 35–46. [Google Scholar] [CrossRef]
- Tavasoli, M.; Lahire, S.; Reid, T.; Brodovsky, M.; McMaster, C.R. Genetic diseases of the Kennedy pathways for membrane synthesis. J. Biol. Chem. 2020, 295, 17877–17886. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, D.; Yang, X.; Liu, D.; Shan, H.; Luo, J.; Li, X.; Yin, Y. Plasma lipidomic analysis reveals disruption of ether phosphatidylcholine biosynthesis and facilitates early detection of hepatitis B-related hepatocellular carcinoma. Lipids Health Dis. 2025, 24, 69. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar]
- Li, Z.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewell, L.; Mason, A.; Vance, D.E. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, J.; Dai, Z.; Wang, Q.; Qiao, R.; Huang, Y.; Ma, B.; Jiang, J.; Zhu, C.; Su, W.; et al. Expression and localization of HSD17B13 along mouse urinary tract. Am. J. Physiol. Renal Physiol. 2024, 327, F146–F157. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011.
- Narváez-Rivas, M.; Zhang, Q. Comprehensive untargeted lipidomic analysis using core-shell C30 particle column and high field orbitrap mass spectrometer. J. Chromatogr. A 2016, 1440, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Keller, M.A.; Sailer, S.; Seppi, D.; Golderer, G.; Werner-Felmayer, G.; Zoeller, R.A.; Watschinger, K. A novel assay for the introduction of the vinyl ether double bond into plasmalogens using pyrene-labeled substrates. J. Lipid Res. 2018, 59, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Colas, R.A.; Walker, M.E.; Serhan, C.N. Lipid Mediator Metabolomics Via LC-MS/MS Profiling and Analysis. Methods Mol. Biol. 2018, 1730, 59–72. [Google Scholar]
- Dasilva, G.; Muñoz, S.; Lois, S.; Medina, I. Non-Targeted LC-MS/MS Assay for Screening Over 100 Lipid Mediators from ARA, EPA, and DHA in Biological Samples Based on Mass Spectral Fragmentations. Molecules 2019, 24, 2276. [Google Scholar] [CrossRef]
- Olund Villumsen, S.; Benfeitas, R.; Knudsen, A.D.; Gelpi, M.; Høgh, J.; Thomsen, M.T.; Murray, D.; Ullum, H.; Neogi, U.; Nielsen, S.D. Integrative Lipidomics and Metabolomics for System-Level Understanding of the Metabolic Syndrome in Long-Term Treated HIV-Infected Individuals. Front. Immunol. 2021, 12, 742736. [Google Scholar] [CrossRef]
- Molenaar, M.R.; Jeucken, A.; Wassenaar, T.A.; van de Lest, C.H.A.; Brouwers, J.F.; Helms, J.B. LION/web: A web-based ontology enrichment tool for lipidomic data analysis. Gigascience 2019, 8, giz061. [Google Scholar] [CrossRef]
- Adam, M.; Heikelä, H.; Sobolewski, C.; Portius, D.; Mäki-Jouppila, J.; Mehmood, A.; Adhikari, P.; Esposito, I.; Elo, L.L.; Zhang, F.P.; et al. Hydroxysteroid (17β) dehydrogenase 13 deficiency triggers hepatic steatosis and inflammation in mice. Faseb J. 2018, 32, 3434–3447. [Google Scholar] [CrossRef] [PubMed]
- Koutsari, C.; Lazaridis, K.N. Emerging genes associated with the progression of nonalcoholic fatty liver disease. Hepatology 2010, 52, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Siloto, R.M.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 2012, 51, 350–377. [Google Scholar] [CrossRef]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef]
- DeLong, C.J.; Shen, Y.J.; Thomas, M.J.; Cui, Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Hajduch, E.; Lachkar, F.; Ferré, P.; Foufelle, F. Roles of Ceramides in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef]
- Ma, Y.; Brown, P.M.; Lin, D.D.; Ma, J.; Feng, D.; Belyaeva, O.V.; Podszun, M.C.; Roszik, J.; Allen, J.N.; Umarova, R.; et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Deficiency Does Not Protect Mice From Obesogenic Diet Injury. Hepatology 2021, 73, 1701–1716. [Google Scholar] [CrossRef]
- Liang, B.; Fu, L.; Liu, P. Regulation of lipid droplet dynamics and lipid homeostasis by hydroxysteroid dehydrogenase proteins. Trends Cell Biol. 2025, 35, 153–165. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Liang, D.; Song, K.; Kong, X.; He, M.; Liao, X.; Huang, Z.; Kang, A.; Bai, R.; et al. Roles of lipid droplets and related proteins in metabolic diseases. Lipids Health Dis. 2024, 23, 218. [Google Scholar] [CrossRef]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef]
- Klemm, R.W.; Carvalho, P. Lipid Droplets Big and Small: Basic Mechanisms That Make Them All. Annu. Rev. Cell Dev. Biol. 2024, 40, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Khot, V.; Chavan-Gautam, P.; Joshi, S. Proposing interactions between maternal phospholipids and the one carbon cycle: A novel mechanism influencing the risk for cardiovascular diseases in the offspring in later life. Life Sci. 2015, 129, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Song, I.; Lee, S.; You, M.; Kwon, J. Protective Effects of Phosphatidylcholine against Hepatic and Renal Cell Injury from Advanced Glycation End Products. Medicina 2022, 58, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Huang, X.; Yuan, M.; Wang, J.; Jia, R.; Wang, T.; Tan, Y.; Zhu, S.; Xu, Q.; Wu, X. HSD17B13 liquid-liquid phase separation promotes leukocyte adhesion in chronic liver inflammation. J. Mol. Cell Biol. 2024, 16, mjae018. [Google Scholar] [CrossRef]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef]
- Dorighello, G.; McPhee, M.; Halliday, K.; Dellaire, G.; Ridgway, N.D. Differential contributions of phosphotransferases CEPT1 and CHPT1 to phosphatidylcholine homeostasis and lipid droplet biogenesis. J. Biol. Chem. 2023, 299, 104578. [Google Scholar] [CrossRef]
- Horibata, Y.; Ando, H.; Sugimoto, H. Locations and contributions of the phosphotransferases EPT1 and CEPT1 to the biosynthesis of ethanolamine phospholipids. J. Lipid Res. 2020, 61, 1221–1231. [Google Scholar] [CrossRef]
- Presa, N.; Dominguez-Herrera, A.; van der Veen, J.N.; Vance, D.E.; Gómez-Muñoz, A. Implication of phosphatidylethanolamine N-methyltransferase in adipocyte differentiation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165853. [Google Scholar] [CrossRef]
- Kim, Y.C.; Seok, S.; Byun, S.; Kong, B.; Zhang, Y.; Guo, G.; Xie, W.; Ma, J.; Kemper, B.; Kemper, J.K. AhR and SHP regulate phosphatidylcholine and S-adenosylmethionine levels in the one-carbon cycle. Nat. Commun. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Feng, Y.; Zhang, X.; Guan, Y.; Su, W. Lipidomic Profiling Reveals HSD17B13 Deficiency-Associated Dysregulated Hepatic Phospholipid Metabolism in Aged Mice. Metabolites 2025, 15, 353. https://doi.org/10.3390/metabo15060353
Zhang C, Feng Y, Zhang X, Guan Y, Su W. Lipidomic Profiling Reveals HSD17B13 Deficiency-Associated Dysregulated Hepatic Phospholipid Metabolism in Aged Mice. Metabolites. 2025; 15(6):353. https://doi.org/10.3390/metabo15060353
Chicago/Turabian StyleZhang, Cong, Yingxin Feng, Xiaoyan Zhang, Youfei Guan, and Wen Su. 2025. "Lipidomic Profiling Reveals HSD17B13 Deficiency-Associated Dysregulated Hepatic Phospholipid Metabolism in Aged Mice" Metabolites 15, no. 6: 353. https://doi.org/10.3390/metabo15060353
APA StyleZhang, C., Feng, Y., Zhang, X., Guan, Y., & Su, W. (2025). Lipidomic Profiling Reveals HSD17B13 Deficiency-Associated Dysregulated Hepatic Phospholipid Metabolism in Aged Mice. Metabolites, 15(6), 353. https://doi.org/10.3390/metabo15060353






