Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus
Abstract
1. Introduction
2. TXNIP: Roles and Mechanisms of Action
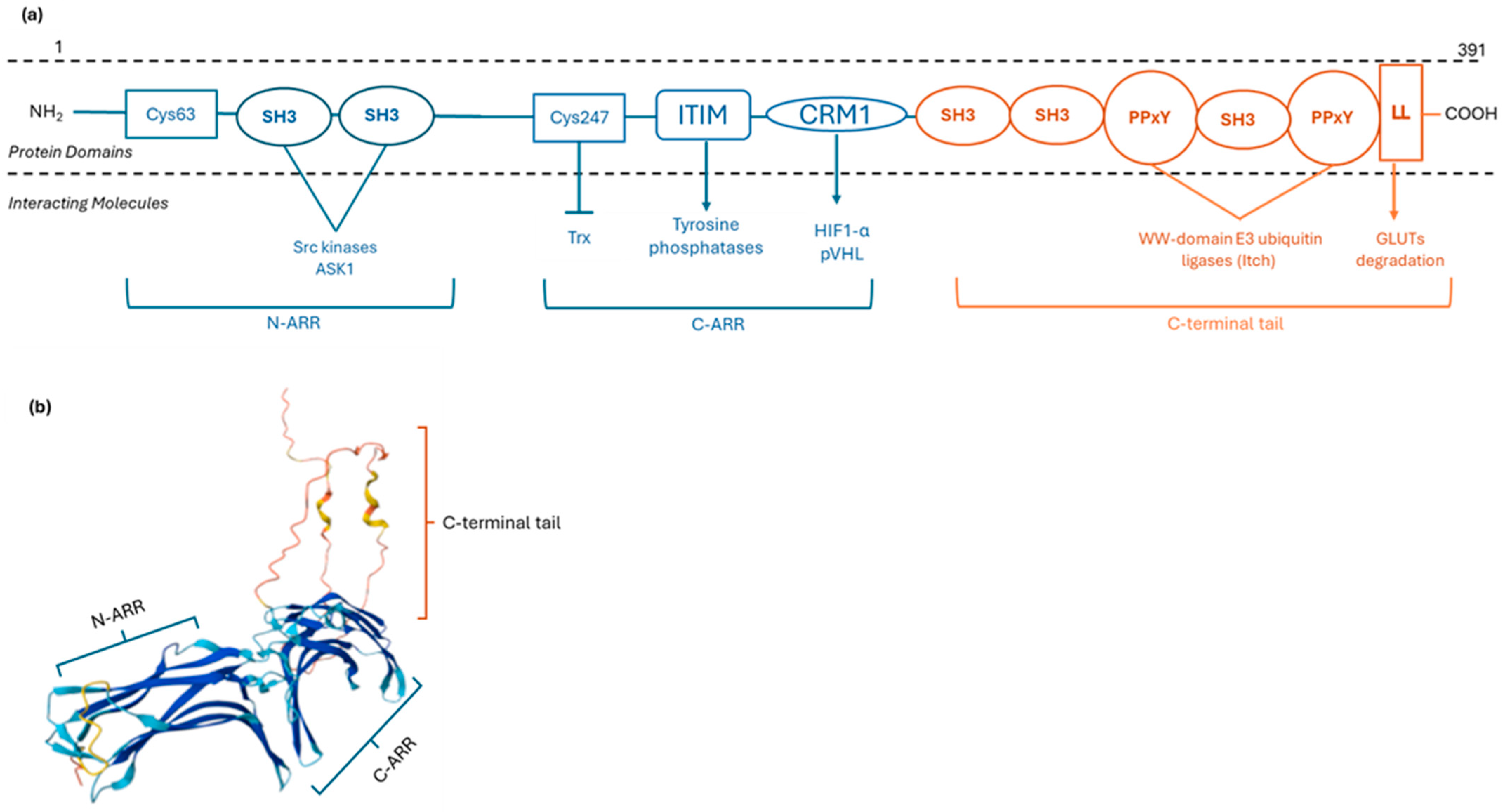
2.1. TXNIP Structure
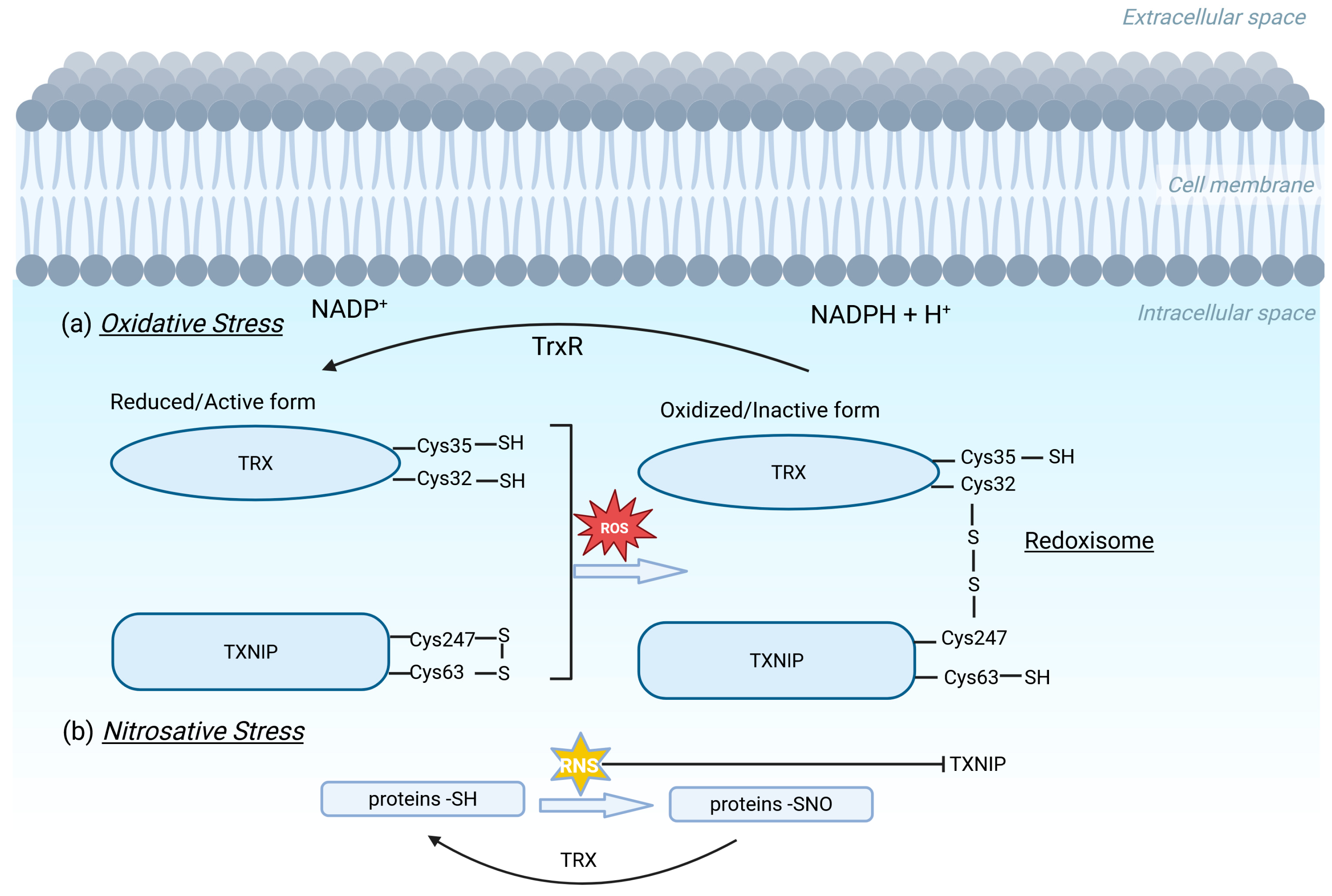
2.2. TXNIP/TRX Redox System
2.3. TXNIP and Mechanisms of Action
3. The Role of TXNIP in Diabetes
4. Gestational Diabetes Mellitus
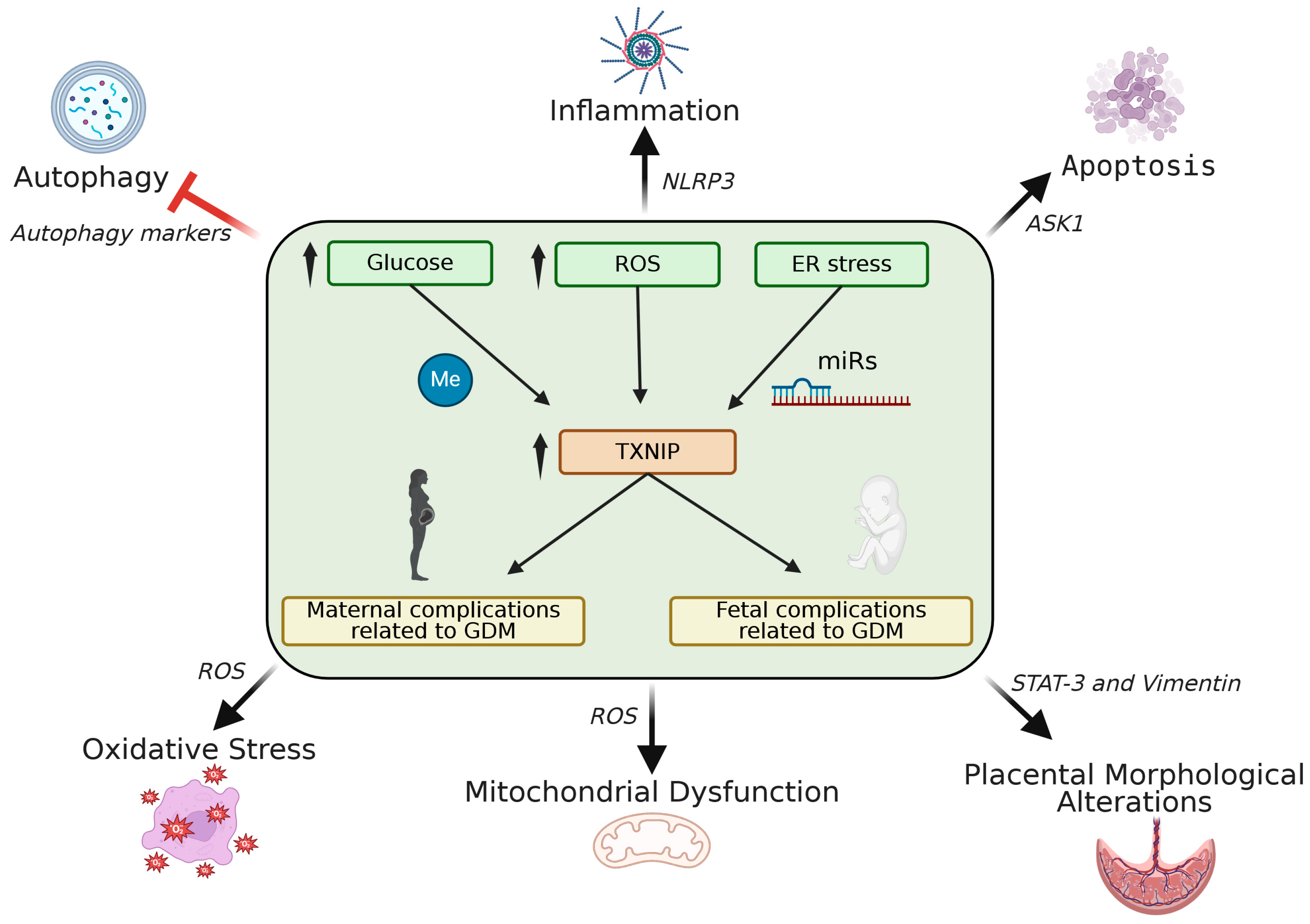
5. The Role of TXNIP in GDM Pathophysiology
5.1. TXNIP Expression in GDM
5.2. Mechanisms of TXNIP Action in GDM
5.3. TXNIP and Trophoblast Dysfunction in GDM
5.4. TXNIP and Placental Morphology in GDM
5.5. TXNIP Methylation Status in GDM
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Park, S.J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Masutani, H.; Yoshihara, E.; Masaki, S.; Chen, Z.; Yodoi, J. Thioredoxin binding protein (TBP)-2/Txnip and alpha-arrestin proteins in cancer and diabetes mellitus. J. Clin. Biochem. Nutr. 2012, 50, 23–34. [Google Scholar] [CrossRef]
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Neelaveni, K.; Kenchey, H.; Thakur, S.S. An insight into major signaling pathways and protein-protein interaction networks involved in the pathogenesis of gestational diabetes mellitus. Proteomics 2022, 22, e2100200. [Google Scholar] [CrossRef]
- Chen, K.S.; DeLuca, H.F. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta 1995, 1263, 1–9. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Margosian, M.R.; Sheth, S.S.; Lusis, A.J.; Parks, E.J. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip-/- mice. J. Nutr. 2004, 134, 1475–1480. [Google Scholar] [CrossRef]
- Advani, A.; Gilbert, R.E.; Thai, K.; Gow, R.M.; Langham, R.G.; Cox, A.J.; Connelly, K.A.; Zhang, Y.; Herzenberg, A.M.; Christensen, P.K.; et al. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 730–741. [Google Scholar] [CrossRef]
- Spindel, O.N.; World, C.; Berk, B.C. Thioredoxin interacting protein: Redox dependent and independent regulatory mechanisms. Antioxid. Redox Signal. 2012, 16, 587–596. [Google Scholar] [CrossRef]
- Chutkow, W.A.; Patwari, P.; Yoshioka, J.; Lee, R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008, 283, 2397–2406. [Google Scholar] [CrossRef]
- Hui, S.T.; Andres, A.M.; Miller, A.K.; Spann, N.J.; Potter, D.W.; Post, N.M.; Chen, A.Z.; Sachithanantham, S.; Jung, D.Y.; Kim, J.K.; et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc. Natl. Acad. Sci. USA 2008, 105, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ge, J.; Kim, D.; Lee, J.J.; Choi, Y.J.; Chen, W.; Bowman, J.W.; Foo, S.S.; Chang, L.C.; Liang, Q.; et al. TXNIP-mediated crosstalk between oxidative stress and glucose metabolism. PLoS ONE 2024, 19, e0292655. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014, 4, 514. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakamura, H.; Nishiyama, A.; Hosoi, F.; Masutani, H.; Wada, H.; Yodoi, J. Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging. Free Radic. Res. 2000, 33, 851–855. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Matsui, M.; Oshima, M.; Oshima, H.; Takaku, K.; Maruyama, T.; Yodoi, J.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996, 178, 179–185. [Google Scholar] [CrossRef]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef]
- Biaglow, J.E.; Miller, R.A. The thioredoxin reductase/thioredoxin system: Novel redox targets for cancer therapy. Cancer Biol. Ther. 2005, 4, 6–13. [Google Scholar] [CrossRef]
- Ye, X.; Zuo, D.; Yu, L.; Zhang, L.; Tang, J.; Cui, C.; Bao, L.; Zan, K.; Zhang, Z.; Yang, X.; et al. ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem. Biophys. Res. Commun. 2017, 485, 499–505. [Google Scholar] [CrossRef]
- Morita, S.; Villalta, S.A.; Feldman, H.C.; Register, A.C.; Rosenthal, W.; Hoffmann-Petersen, I.T.; Mehdizadeh, M.; Ghosh, R.; Wang, L.; Colon-Negron, K.; et al. Targeting ABL-IRE1alpha Signaling Spares ER-Stressed Pancreatic beta Cells to Reverse Autoimmune Diabetes. Cell Metab. 2017, 25, 883–897.e8. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.F.; Hong, S.Y.; He, H.; Zheng, L.; Hagen, T.; Luo, Y.; Yu, F.X. A potential mechanism of metformin-mediated regulation of glucose homeostasis: Inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cell Signal. 2012, 24, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, W.; Guo, H.; Zhao, L.; Yue, L.; Li, X.; Feng, D.; Luo, J.; Wu, X.; Cui, W.; et al. Bakuchiol Attenuates Oxidative Stress and Neuron Damage by Regulating Trx1/TXNIP and the Phosphorylation of AMPK After Subarachnoid Hemorrhage in Mice. Front. Pharmacol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wei, M.; Zhang, B.; Kang, X.; Liu, D.; Zheng, W.; Pan, X.; Quan, Y.; Liao, D.; Shen, J. MicroRNA-33 regulates the NLRP3 inflammasome signaling pathway in macrophages. Mol. Med. Rep. 2018, 17, 3318–3327. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, F.; Qu, K.; Liu, C.; Zhang, J. TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook. Oxidative Med. Cell. Longev. 2022, 2022, 7805115. [Google Scholar] [CrossRef]
- Bedarida, T.; Baron, S.; Vibert, F.; Ayer, A.; Henrion, D.; Thioulouse, E.; Marchiol, C.; Beaudeux, J.L.; Cottart, C.H.; Nivet-Antoine, V. Resveratrol Decreases TXNIP mRNA and Protein Nuclear Expressions with an Arterial Function Improvement in Old Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 720–729. [Google Scholar] [CrossRef]
- Forrester, M.T.; Seth, D.; Hausladen, A.; Eyler, C.E.; Foster, M.W.; Matsumoto, A.; Benhar, M.; Marshall, H.E.; Stamler, J.S. Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J. Biol. Chem. 2009, 284, 36160–36166. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Miyahara, H.; Hasegawa, K.; Yashiro, M.; Ohara, T.; Fujisawa, M.; Yoshimura, T.; Matsukawa, A.; Tsukahara, H. Thioredoxin interacting protein protects mice from fasting induced liver steatosis by activating ER stress and its downstream signaling pathways. Sci. Rep. 2022, 12, 4819. [Google Scholar] [CrossRef]
- Fang, S.; Jin, Y.; Zheng, H.; Yan, J.; Cui, Y.; Bi, H.; Jia, H.; Zhang, H.; Wang, Y.; Na, L.; et al. High glucose condition upregulated Txnip expression level in rat mesangial cells through ROS/MEK/MAPK pathway. Mol. Cell. Biochem. 2011, 347, 175–182. [Google Scholar] [CrossRef]
- Panse, M.; Kluth, O.; Lorza-Gil, E.; Kaiser, G.; Muhlbauer, E.; Schurmann, A.; Haring, H.U.; Ullrich, S.; Gerst, F. Palmitate and insulin counteract glucose-induced thioredoxin interacting protein (TXNIP) expression in insulin secreting cells via distinct mechanisms. PLoS ONE 2018, 13, e0198016. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef]
- Ishrat, T.; Mohamed, I.N.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Thioredoxin-interacting protein: A novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol. Neurobiol. 2015, 51, 766–778. [Google Scholar] [CrossRef]
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef]
- Chen, J.; Hui, S.T.; Couto, F.M.; Mungrue, I.N.; Davis, D.B.; Attie, A.D.; Lusis, A.J.; Davis, R.A.; Shalev, A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008, 22, 3581–3594. [Google Scholar] [CrossRef]
- Yoshihara, E.; Fujimoto, S.; Inagaki, N.; Okawa, K.; Masaki, S.; Yodoi, J.; Masutani, H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat. Commun. 2010, 1, 127. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Hara, T.; O’Sullivan-Murphy, B.; Kanekura, K.; Lu, S.; Hara, M.; Ishigaki, S.; Zhu, L.J.; Hayashi, E.; Hui, S.T.; et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012, 16, 265–273. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Luo, B.; Huang, F.; Liu, Y.; Liang, Y.; Wei, Z.; Ke, H.; Zeng, Z.; Huang, W.; He, Y. NLRP3 Inflammasome as a Molecular Marker in Diabetic Cardiomyopathy. Front. Physiol. 2017, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, Y.; Li, C.; Suh, J.; Sivapackiam, J.; Goncalves, T.M.; Jarad, G.; Zhao, G.; Urano, F.; Sharma, V.; et al. Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome. Proc. Natl. Acad. Sci. USA 2022, 119, e2116505119. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.Y.; Kim, S.K. Quercetin and Ascorbic Acid Suppress Fructose-Induced NLRP3 Inflammasome Activation by Blocking Intracellular Shuttling of TXNIP in Human Macrophage Cell Lines. Inflammation 2017, 40, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Devi, T.S.; Yumnamcha, T. The Role of Txnip in Mitophagy Dysregulation and Inflammasome Activation in Diabetic Retinopathy: A New Perspective. JOJ Ophthalmol. 2017, 4, 19080. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, R.; Tan, H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int. J. Biol. Sci. 2019, 15, 1345–1357. [Google Scholar] [CrossRef]
- Blouet, C.; Schwartz, G.J. Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice. J. Neurosci. 2011, 31, 6019–6027. [Google Scholar] [CrossRef]
- Minn, A.H.; Hafele, C.; Shalev, A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 2005, 146, 2397–2405. [Google Scholar] [CrossRef]
- Stoltzman, C.A.; Peterson, C.W.; Breen, K.T.; Muoio, D.M.; Billin, A.N.; Ayer, D.E. Glucose sensing by MondoA:Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6912–6917. [Google Scholar] [CrossRef]
- Yu, F.X.; Chai, T.F.; He, H.; Hagen, T.; Luo, Y. Thioredoxin-interacting protein (Txnip) gene expression: Sensing oxidative phosphorylation status and glycolytic rate. J. Biol. Chem. 2010, 285, 25822–25830. [Google Scholar] [CrossRef]
- Zitman-Gal, T.; Green, J.; Pasmanik-Chor, M.; Oron-Karni, V.; Bernheim, J. Endothelial pro-atherosclerotic response to extracellular diabetic-like environment: Possible role of thioredoxin-interacting protein. Nephrol. Dial. Transplant. 2010, 25, 2141–2149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kibbe, C.; Chen, J.; Xu, G.; Jing, G.; Shalev, A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J. Biol. Chem. 2013, 288, 23194–23202. [Google Scholar] [CrossRef] [PubMed]
- Chau, G.C.; Im, D.U.; Kang, T.M.; Bae, J.M.; Kim, W.; Pyo, S.; Moon, E.Y.; Um, S.H. mTOR controls ChREBP transcriptional activity and pancreatic beta cell survival under diabetic stress. J. Cell Biol. 2017, 216, 2091–2105. [Google Scholar] [CrossRef] [PubMed]
- Gateva, A.T.; Assyov, Y.S.; Velikova, T.; Kamenov, Z.A. Higher levels of thioredoxin interacting protein (TXNIP) in patients with prediabetes compared to obese normoglycemic subjects. Diabetol. Metab. Syndr. 2019, 13, 734–737. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef]
- Bonner-Weir, S. Life and death of the pancreatic beta cells. Trends Endocrinol. Metab. 2000, 11, 375–378. [Google Scholar] [CrossRef]
- Rorsman, P.; Braun, M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 2013, 75, 155–179. [Google Scholar] [CrossRef]
- Hui, T.Y.; Sheth, S.S.; Diffley, J.M.; Potter, D.W.; Lusis, A.J.; Attie, A.D.; Davis, R.A. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J. Biol. Chem. 2004, 279, 24387–24393. [Google Scholar] [CrossRef]
- Chen, J.; Fontes, G.; Saxena, G.; Poitout, V.; Shalev, A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes 2010, 59, 440–447. [Google Scholar] [CrossRef]
- Parikh, H.; Carlsson, E.; Chutkow, W.A.; Johansson, L.E.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007, 4, e158. [Google Scholar] [CrossRef]
- Shalev, A.; Pise-Masison, C.A.; Radonovich, M.; Hoffmann, S.C.; Hirshberg, B.; Brady, J.N.; Harlan, D.M. Oligonucleotide microarray analysis of intact human pancreatic islets: Identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 2002, 143, 3695–3698. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Saxena, G.; Mungrue, I.N.; Lusis, A.J.; Shalev, A. Thioredoxin-interacting protein: A critical link between glucose toxicity and beta-cell apoptosis. Diabetes 2008, 57, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Guo, Y.; Moore, W.V.; He, L.G.; Zang, M.; Clements, M.A.; Yan, Y. Thioredoxin-interacting protein promotes high-glucose-induced macrovascular endothelial dysfunction. Biochem. Biophys. Res. Commun. 2017, 493, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Yoshioka, J.; Takahashi, T.; He, Z.; King, G.L.; Lee, R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004, 279, 30369–30374. [Google Scholar] [CrossRef]
- Kim, G.S.; Jung, J.E.; Narasimhan, P.; Sakata, H.; Chan, P.H. Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice. Neurobiol. Dis. 2012, 46, 440–449. [Google Scholar] [CrossRef]
- Nasoohi, S.; Parveen, K.; Ishrat, T. Metabolic Syndrome, Brain Insulin Resistance, and Alzheimer’s Disease: Thioredoxin Interacting Protein (TXNIP) and Inflammasome as Core Amplifiers. J. Alzheimers Dis. 2018, 66, 857–885. [Google Scholar] [CrossRef]
- Wang, B.F.; Yoshioka, J. The Emerging Role of Thioredoxin-Interacting Protein in Myocardial Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 219–229. [Google Scholar] [CrossRef]
- Qayyum, N.; Haseeb, M.; Kim, M.S.; Choi, S. Role of Thioredoxin-Interacting Protein in Diseases and Its Therapeutic Outlook. Int. J. Mol. Sci. 2021, 22, 2754. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Thielen, L.; Shalev, A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 75–80. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.G.; Upton, J.P.; Praveen, P.V.; Ghosh, R.; Nakagawa, Y.; Igbaria, A.; Shen, S.; Nguyen, V.; Backes, B.J.; Heiman, M.; et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012, 16, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Mehta, J.P.; Barron, N.; Doolan, P.; Jeppesen, P.B.; Clynes, M.; O’Driscoll, L. Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell. Physiol. Biochem. 2010, 25, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013, 19, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Filios, S.R.; Xu, G.; Chen, J.; Hong, K.; Jing, G.; Shalev, A. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates Zeb1 protein signaling and beta cell apoptosis. J. Biol. Chem. 2014, 289, 36275–36283. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; Murthy, D.S.J. Normal pregnancy- a state of insulin resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef]
- Ryan, E.A.; Enns, L. Role of gestational hormones in the induction of insulin resistance. J. Clin. Endocrinol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef]
- Holemans, K.; Aerts, L.; Van Assche, F.A. Lifetime consequences of abnormal fetal pancreatic development. J. Physiol. 2003, 547, 11–20. [Google Scholar] [CrossRef]
- Sunehag, A.; Ewald, U.; Larsson, A.; Gustafsson, J. Attenuated hepatic glucose production but unimpaired lipolysis in newborn infants of mothers with diabetes. Pediatr. Res. 1997, 42, 492–497. [Google Scholar] [CrossRef][Green Version]
- Ahlsson, F.S.; Diderholm, B.; Ewald, U.; Gustafsson, J. Lipolysis and insulin sensitivity at birth in infants who are large for gestational age. Pediatrics 2007, 120, 958–965. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Group, H.S.C.R. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes 2009, 58, 453–459. [Google Scholar] [CrossRef]
- Sacks, D.A.; Hadden, D.R.; Maresh, M.; Deerochanawong, C.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Coustan, D.R.; Hod, M.; Oats, J.J.; et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012, 35, 526–528. [Google Scholar] [CrossRef]
- Colomiere, M.; Permezel, M.; Riley, C.; Desoye, G.; Lappas, M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur. J. Endocrinol. 2009, 160, 567–578. [Google Scholar] [CrossRef]
- Desoye, G.; Hauguel-de Mouzon, S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 2007, 30, S120–S126. [Google Scholar] [CrossRef]
- Gaccioli, F.; Lager, S.; Powell, T.L.; Jansson, T. Placental transport in response to altered maternal nutrition. J. Dev. Orig. Health Dis. 2013, 4, 101–115. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13. [Google Scholar] [CrossRef]
- Marks, J.; Carvou, N.J.; Debnam, E.S.; Srai, S.K.; Unwin, R.J. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J. Physiol. 2003, 553, 137–145. [Google Scholar] [CrossRef]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martin, S. Placental structure in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef]
- Ehlers, E.; Talton, O.O.; Schust, D.J.; Schulz, L.C. Placental structural abnormalities in gestational diabetes and when they develop: A scoping review. Placenta 2021, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Gole, E.; Moutafi, A.; Sfikas, C.; Oehrl, W.; Samiotaki, M.; Papadimitriou, A.; Papaevagelou, V.; Nicolaidou, P. Calcium sensing receptor in pregnancies complicated by gestational diabetes mellitus. Placenta 2014, 35, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Thymara, E.; Maratou, E.; Kanellopoulos, G.; Papaevangelou, V.; Kalantaridou, S.; Kanellakis, S.; Triantafyllidou, P.; Valsamakis, G.; Mastorakos, G. Human Placental LRP5 and Sclerostin are Increased in Gestational Diabetes Mellitus Pregnancies. J. Clin. Endocrinol. Metab. 2023, 108, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Hofmann, H.H.; Weiss, P.A. Insulin binding to trophoblast plasma membranes and placental glycogen content in well-controlled gestational diabetic women treated with diet or insulin, in well-controlled overt diabetic patients and in healthy control subjects. Diabetologia 1992, 35, 45–55. [Google Scholar] [CrossRef]
- Houshmand-Oeregaard, A.; Hjort, L.; Kelstrup, L.; Hansen, N.S.; Broholm, C.; Gillberg, L.; Clausen, T.D.; Mathiesen, E.R.; Damm, P.; Vaag, A. DNA methylation and gene expression of TXNIP in adult offspring of women with diabetes in pregnancy. PLoS ONE 2017, 12, e0187038. [Google Scholar] [CrossRef]
- Mogami, H.; Yura, S.; Kondoh, E.; Masutani, H.; Yodoi, J.; Konishi, I. Differential expression of thioredoxin binding protein-2/Txnip in human placenta: Possible involvement of hypoxia in its suppression during early pregnancy. J. Obstet. Gynaecol. Res. 2017, 43, 50–56. [Google Scholar] [CrossRef]
- Sarina; Li, D.F.; Feng, Z.Q.; Du, J.; Zhao, W.H.; Huang, N.; Jia, J.C.; Wu, Z.Y.; Alamusi; Wang, Y.Y.; et al. Mechanism of Placenta Damage in Gestational Diabetes Mellitus by Investigating TXNIP of Patient Samples and Gene Functional Research in Cell Line. Diabetes Ther. 2019, 10, 2265–2288. [Google Scholar] [CrossRef]
- Triantafyllidou, P.; Papadopoulou, A.; Thymara, E.; Papaevangelou, V.; Mastorakos, G.; Papadimitriou, A.; Kalantaridou, S.; Stratakis, C.A.; Alexopoulou, E. Aortic Intima-Media Thickness is Increased in Neonates of Mothers with Gestational Diabetes Mellitus: The Role of Thioredoxin-Interacting Protein as a Marker of Oxidative Stress. Curr. Vasc. Pharmacol. 2023, 21, 234–245. [Google Scholar] [CrossRef]
- Sa, R.; Ma, J.; Yang, J.; Li, D.F.; Du, J.; Jia, J.C.; Li, Z.Y.; Huang, N.; Lamusi, A.; Sha, R.; et al. High TXNIP expression accelerates the migration and invasion of the GDM placenta trophoblast. BMC Pregnancy Childbirth 2023, 23, 235. [Google Scholar] [CrossRef]
- Pasternak, Y.; Ohana, M.; Biron-Shental, T.; Cohen-Hagai, K.; Benchetrit, S.; Zitman-Gal, T. Thioredoxin, thioredoxin interacting protein and transducer and activator of transcription 3 in gestational diabetes. Mol. Biol. Rep. 2020, 47, 1199–1206. [Google Scholar] [CrossRef]
- Eren, E.; Aykal, G.; Sayrac, S.; Erol, O.; Ellidag, H.Y.; Yilmaz, N. Relationship between thioredoxin and thioredoxin-binding protein in patients with gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2017, 30, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Sobrevia, L.; Valero, P.; Grismaldo, A.; Villalobos-Labra, R.; Pardo, F.; Subiabre, M.; Armstrong, G.; Toledo, F.; Vega, S.; Cornejo, M.; et al. Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165948. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Vanderpeet, C.L.; Bartho, L.A.; McKeating, D.R.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V. Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus. J. Physiol. 2021, 599, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fu, N.; Yang, P. MiR-17 Downregulation by High Glucose Stabilizes Thioredoxin-Interacting Protein and Removes Thioredoxin Inhibition on ASK1 Leading to Apoptosis. Toxicol. Sci. 2016, 150, 84–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, L.; Zhang, H.; Chen, Y.; Gao, P.; Zhang, J.; Zhou, X.; Zhu, S.; Du, Y.; Fang, C.; et al. miR-17-5p Promotes Glucose Uptake of HTR8/SVneo Trophoblast Cells by Inhibiting TXNIP/NLRP3 Inflammasome Pathway. Diabetes Metab. Syndr. Obes. 2022, 15, 3361–3374. [Google Scholar] [CrossRef]
- Mando, C.; Castiglioni, S.; Novielli, C.; Anelli, G.M.; Serati, A.; Parisi, F.; Lubrano, C.; Zocchi, M.; Ottria, R.; Giovarelli, M. Placental Bioenergetics and Antioxidant Homeostasis in Maternal Obesity and Gestational Diabetes. Antioxidants 2024, 13, 858. [Google Scholar] [CrossRef]
- Gong, J.S.; Kim, G.J. The role of autophagy in the placenta as a regulator of cell death. Clin. Exp. Reprod. Med. 2014, 41, 97–107. [Google Scholar] [CrossRef]
- Carvajal, L.; Gutierrez, J.; Morselli, E.; Leiva, A. Autophagy Process in Trophoblast Cells Invasion and Differentiation: Similitude and Differences with Cancer Cells. Front. Oncol. 2021, 11, 637594. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Liu, X.; Zha, X.; Elsabagh, M.; Ma, Y.; Jiang, H.; Wang, H.; Wang, M. Autophagy attenuates placental apoptosis, oxidative stress and fetal growth restriction in pregnant ewes. Environ. Int. 2023, 173, 107806. [Google Scholar] [CrossRef]
- Hung, T.H.; Huang, S.Y.; Chen, S.F.; Wu, C.P.; Hsieh, T.T. Decreased placental apoptosis and autophagy in pregnancies complicated by gestational diabetes with large-for-gestational age fetuses. Placenta 2020, 90, 27–36. [Google Scholar] [CrossRef]
- Gu, Y.; Cao, M.; Chen, Y.; Li, J.; Hu, L.; Yang, X.J. Knockdown of TXNIP alleviates gestational diabetes mellitus by activating autophagy to regulate cell proliferation and apoptosis in high glucose-treated trophoblasts. Reprod. Biol. 2024, 24, 100841. [Google Scholar] [CrossRef] [PubMed]
- Slupecka-Ziemilska, M.; Wychowanski, P.; Puzianowska-Kuznicka, M. Gestational Diabetes Mellitus Affects Offspring’s Epigenome. Is There a Way to Reduce the Negative Consequences? Nutrients 2020, 12, 2792. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Juvinao-Quintero, D.L.; Ronkainen, J.; Ott, R.; Alfano, R.; Canouil, M.; Geurtsen, M.L.; Khamis, A.; Kupers, L.K.; Lim, I.Y.; et al. Maternal Glycemic Dysregulation During Pregnancy and Neonatal Blood DNA Methylation: Meta-analyses of Epigenome-Wide Association Studies. Diabetes Care 2022, 45, 614–623. [Google Scholar] [CrossRef] [PubMed]
| Tissue/Cell Culture | TXNIP Expression Levels | TXNIP mRNA/Protein Expression Levels | Associated Parameters | Proposed Mechanism | Refs. |
|---|---|---|---|---|---|
| Placental tissue from GDM patients | ↑ | Protein | - | Oxidative stress, mitochondrial dysfunction, and apoptosis | [97] |
| HG-treated HTR-8/SVneo cells | ↑ | mRNA and protein | ↑mitochondrial fragmentation, ↑caspase-3 | ||
| HTR-8/SVneo cells transfected with TXNIP vector (TXNIP overexpression) | ↑ | mRNA and protein | ↓TRX, ↓cell proliferation, ↓cell migration, ↑caspase-3, ↑apoptotic cells, ↑ROS, ↑mitochondrial fragmentation, ↓mitochondrial membrane potential | ||
| Placental tissue from GDM patients | ↑ | mRNA and protein | - | Impairment of trophoblast morphology through STAT-3 and Vimentin-related mechanisms | [99] |
| HTR-8/SVneo cells overexpressing TXNIP (Tet-on system) | ↑ | mRNA and protein | ↑cell migration ability, ↑cell invasion ability, ↑densified cells, ↑Vimentin, ↑E-cadherin, ↓N-cadherin, ↑p-STAT3 | ||
| HTR-8/SVneo cells with TXNIP knockout (CRISPR-Cas9) | ↓ | mRNA and protein | ↓cell migration ability, ↓cell invasion ability, ↑shrunken cells with disintegrated cytoplasm, ↓Vimentin, ↑N-cadherin, ↓E-cadherin, ↓p-STAT3 | ||
| Maternal blood from GDMA2 patients | ↑ | mRNA | ↑TRX, ↑NFkΒ-50 | Oxidative stress and inflammation | [100] |
| Placental tissue from GDMA2 patients | ↑ | Protein | ↑TRX, ↑TRX/TXNIP ratio compared to maternal, ↑NFkΒ-50, ↑p-STAT3, ↑SOCS3 | Fetal adaptations to GDM-related oxidative stress and inflammation | |
| Umbilical cord blood from GDMA2 patients | ↓ | mRNA | ↓TRX compared to placenta, ↑TXN/TXNIP ratio- compared to maternal, ↓NFkΒ-50 compared to both maternal and placental | ||
| Maternal serum from GDM patients | ↓ | Protein | ↑TRX/TXNIP ratio | Fetal adaptations to GDM-related oxidative stress | [101] |
| E8.5 embryos from diabetic and non-diabetic mice | - | - | ↓miR-17-5p | Apoptosis through ASK1 activation | [104] |
| HG-treated C17.2 neural stem cells | ↑ | mRNA and protein | ↓miR-17-5p, ↓TRX/ASK1 complex, ↑ASK1 phosphorylation, ↑apoptotic cells, ↑cleaved caspase-3 | ||
| HG-treated C17.2 neural stem cells transfected with miR-17 mimic | ↓ | mRNA and protein | ↑TRX/ASK1 complex, ↓ASK1 phosphorylation, ↓apoptotic cells, ↓cleaved caspase-3 | ||
| HG-treated C17.2 neural stem cells transfected with miR-17 inhibitor | ↑ | mRNA and protein | ↓TRX/ASK1 complex, ↑ASK1 phosphorylation, ↑apoptotic cells, ↑cleaved caspase-3 | ||
| HG-treated C17.2 neural stem cells transfected with TXNIP siRNA (TXNIP Knockdown) | ↓ | mRNA and protein | ↑TRX/ASK1 complex, ↓ASK1 phosphorylation, ↓apoptotic cells, ↓cleaved caspase-3 | ||
| HG-treated C17.2 neural stem cells transfected with TXNIP vector (TXNIP overexpression) | ↑ | mRNA and protein | ↑TXNIP/TRX complex, ↓TXN/ASK1 complex, ↑ASK1 phosphorylation, ↑apoptotic cells, ↑cleaved caspase-3 | ||
| Placental tissue from GDM patients | ↑ | mRNA and protein | ↑NLRP3, ↓miR-17-5p | Inflammation through NLRP3 inflammasome activation | [105] |
| HTR-8/SVneo cells transfected with TXNIP vector (TXNIP overexpression) | ↑ | mRNA and protein | ↑NLRP3, ↓glucose consumption | ||
| HTR-8/SVneo cells transfected with miR-17-5p mimic | ↓ | mRNA and protein | ↓NLRP3, ↑glucose consumption | ||
| HTR-8/SVneo cells co-transfected with miR-17-5p mimic and TXNIP vector | ↑ | mRNA and protein | ↑NLRP3, ↓glucose consumption | ||
| HG-treated HTR-8/SVneo cells | ↑ | mRNA and protein | ↑NLRP3, ↓glucose consumption | ||
| HG-treated HTR-8/SVneo cells transfected with miR-17-5p mimic | ↓ | mRNA and protein | ↓NLRP3, ↑glucose consumption | ||
| HG-treated HTR-8/SVneo cells | ↑ | mRNA | ↑LDH, ↓cell viability, ↑apoptosis, ↓autophagosomes, ↑P62, ↓LC3-II/LC3-I ratio | Inhibition of autophagy | [111] |
| HG-treated HTR-8/SVneo cells transfected with siRNA TXNIP (TXNIP knockdown) | ↓ | mRNA | ↓LDH, ↑cell viability, ↓apoptosis, ↑autophagosomes, ↓P62, ↑LC3-II/LC3-I ratio | ||
| HTR-8/SVneo cells transfected with siRNA TXNIP (TXNIP knockdown) | ↓ | mRNA | ↑autophagosomes, ↓P62, ↑LC3-II/LC3-I ratio | ||
| HTR-8/SVneo cells transfected with siRNA TXNIP (TXNIP knockdown) and treated with autophagy inhibitor 3-MA | ↓ | mRNA | ↓autophagosomes, ↑P62, ↓LC3-II/LC3-I ratio | ||
| HG-treated HTR-8/SVneo cells transfected with siRNA TXNIP (TXNIP knockdown) and treated with 3-MA | ↓ | mRNA | ↓cell viability, ↑apoptosis |
| Tissue/ Cell Culture | TXNIP Expression Levels | TXNIP mRNA/protein Expression Levels | Correlations | Implications | Refs. |
|---|---|---|---|---|---|
| Subcutaneous adipose tissue from offspring of GDM patients | ↓ | mRNA | TXNIP expression levels were negatively associated with TXNIP methylation levels | Offspring compensatory mechanisms against GDM-related complications | [95] |
| Placental tissue from GDM patients | ↑trophoblasts and syncytiotrophoblasts ↓endothelial cells | Protein | TXNIP expression levels in trophoblasts were positively correlated with aIMT | Increased risk of cardiovascular disease in offsprings | [98] |
| Placental tissue from non-obese and obese patients, including a subgroup from obese patients with GDM | No difference | Protein | TXNIP expression levels were positively correlated with the placental thickness and negatively correlated with the placental surface | Decreased placental efficiency | [106] |
| Umbilical cord blood from pregnant women (metanalysis) | - | - | TXNIP methylation levels were negatively correlated with AUCgluc exclusively in non-GMD groups | Impact of maternal glycemic control on the epigenetic regulation of TXNIP | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinopoulou, I.; Papadopoulou, A. Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus. Metabolites 2025, 15, 351. https://doi.org/10.3390/metabo15060351
Kokkinopoulou I, Papadopoulou A. Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus. Metabolites. 2025; 15(6):351. https://doi.org/10.3390/metabo15060351
Chicago/Turabian StyleKokkinopoulou, Ioanna, and Anna Papadopoulou. 2025. "Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus" Metabolites 15, no. 6: 351. https://doi.org/10.3390/metabo15060351
APA StyleKokkinopoulou, I., & Papadopoulou, A. (2025). Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus. Metabolites, 15(6), 351. https://doi.org/10.3390/metabo15060351





