Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
- Individuals aged between 18 and 69 years;
- Voluntary participation in the study;
- Provision of informed consent for the use of personal data in epidemiological research;
- Active employment with one of the companies included in the study, without being on temporary disability leave at the time of participation;
- Not a habitual alcohol drinker;
- Not suffering from known liver disease, thyroid disease, celiac disease, or drug addiction.
- Age below 18 or above 69 years.
- Lack of employment within the participating companies;
- Refusal to participate in the study;
- Refusal to grant consent for the use of personal data in epidemiological research;
- Absence of a required parameter necessary for scale calculations;
- Habitual alcohol drinker;
- Known liver disease;
- Hypothyroidism;
- Hypopituitarism;
- Celiac disease;
- Drug addiction;
- Inborn errors of metabolism;
- Patients on antiretroviral therapy.
2.2. Variable Determination
- Medical History Assessment: A comprehensive clinical history was obtained, covering sociodemographic variables such as age, sex, social class, smoking status, physical activity levels, and adherence to the Mediterranean diet;
- Anthropometric and Clinical Measurements: Parameters including height, weight, waist and hip circumference, and both systolic and diastolic blood pressure were recorded;
- Laboratory Analyses: Blood lipid profile and glucose levels were measured.
2.3. Anthropometric Measurements
2.4. Clinical Measurements
2.5. Laboratory Analyses
2.6. Risk Assessment Scales
- Adherence to the Mediterranean Diet: Assessed using the PREDIMED questionnaire, a validated 14-item instrument in which each question is assigned a score of zero or one. A total score of nine or higher indicates strong adherence to the Mediterranean diet [59].
- Physical Activity Levels: Evaluated using the International Physical Activity Questionnaire (IPAQ), a self-reported survey capturing physical activity over the previous seven days [60].
- Smoking Status: Individuals who had smoked at least one cigarette per day (or its equivalent) in the past 30 days, or who had quit smoking within the last 12 months, were classified as smokers. Non-smokers included individuals who had abstained from smoking for at least one year or had never smoked.
- Socioeconomic Classification: Defined according to the Spanish Society of Epidemiology guidelines based on the 2011 National Classification of Occupations [61].
- ○
- Class I: Senior executives, directors, and university-educated professionals.
- ○
- Class II: Intermediate professionals and self-employed individuals.
- ○
- Class III: Manual laborers.
- Metabolic Age: Determined using a TANITA MC-780 S MA bioimpedance meter (TANITA Corporation, Tokyo, Japan).Avoidable Lost Life Years (ALLY): Calculated as the difference between metabolic age and chronological age. Previous studies suggest that a metabolic age at least 12 years lower than chronological age is associated with reduced cardiovascular risk. ALLY classification [62]:
- ○
- Low: Difference of less than three years;
- ○
- Normal: Difference of three to eleven years;
- ○
- High: Difference of 12 years or more;
- ○
- A metabolic age exceeding one’s chronological age by 12 years or more was considered a high-risk threshold.
- Fatty Liver Index (FLI) [63] FLI = (e0.953 × log (triglycerides) + 0.139 × BMI + 0.718 × log (GGT) + 0.053 × waist circumference − 15.745)/(1 + e0.953 × log (triglycerides) + 0.139 × BMI + 0.718 × log (GGT) + 0.053×waist circumference − 15.745) × 100. FLI values above 60 are considered high risk;
- Hepatic Steatosis Index (HSI) [64] HSI = 8 × AST/ALT + BMI + 2 if diabetic and + 2 if female. Values above 36 are considered high risk;
- Zhejiang University Index (ZJU index) [65] ZJU = BMI + glycemia (mmol L) + triglycerides (mmol L) + 3 AST/ALT + 2 if female. Values above 38 are considered high risk;
- Fatty Liver Disease Index (FLD) [66] FLD = BMI + triglycerides + 3 × (AST/ALT) + 2 × hyperglycemia (present = 1; absent = 0). Values above 37 are considered high risk;
- Lipid Accumulation Product (LAP) [67] = Men. (waist (cm) − 65) × (triglycerides (mMol)) and Women: (waist (cm) − 58) × (triglycerides (mMol)). Values above 42.7 are considered high risk.
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koshy, A. Evolving Global Etiology of Hepatocellular Carcinoma (HCC): Insights and Trends for 2024. J. Clin. Exp. Hepatol. 2025, 15, 102406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Wong, G.; Anstee, Q.M.; Henry, L. The Global Burden of Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D’ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma: New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Martínez Jover, A.; López González, A.A.; Tomás Gil, P.; Coll Villalonga, J.L.; Martí Lliteras, P.; Ramírez Manent, J.I. Association between nonalcoholic fatty liver disease risk scales and metabolic syndrome scales in 418.343 spanish workers. Acad. J. Health Sci. 2023, 38, 130–136. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, Y.; Duan, C.; Wang, S.; He, W.; Zhang, Y.; An, X.; He, M. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res. Rev. 2023, 84, 101833. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Datz, C.; Reiberger, T.; Trauner, M. Diet and exercise in NAFLD/NASH: Beyond the obvious. Liver Int. 2021, 41, 2249–2268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022, 75, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, Z.; Zhang, Y.; Tan, J.; Chen, Z. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int. 2024, 44, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of Insulin Resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, S.M.; Hosseini, S.M. Inflammation-related miRNAs in obesity, CVD, and NAFLD. Cytokine 2024, 182, 156724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Wang, D.; Huang, Z.; Xiao, X.; Zheng, Q.; Li, S.; Long, D.; Feng, L. Mitochondrial Dysfunction in Metabolic Dysfunction Fatty Liver Disease (MAFLD). Int. J. Mol. Sci. 2023, 24, 17514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, D.-Q.; Jin, Y.; Wang, T.-Y.; Zheng, K.I.; Rios, R.S.; Zhang, H.-Y.; Targher, G.; Byrne, C.D.; Yuan, W.-J.; Zheng, M.-H. MAFLD and risk of CKD. Metabolism 2021, 115, 154433. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrault, N.A.; Francoz, C.; Berenguer, M.; Charlton, M.; Heimbach, J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin. Gastroenterol. Hepatol. 2023, 21, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, H.; Zhang, X.; Liu, W.; Ding, Y.; Huang, D.; Zhai, J.; Wei, W.; Wen, J.; Chen, D.; et al. METTL3 drives NAFLD-related hepatocellular carcinoma and is a therapeutic target for boosting immunotherapy. Cell Rep. Med. 2023, 4, 101144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2023, 23, 273–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gómez Cobo, C.; Morell García, D.; Berga Montaner, F.; Barceló Bennasar, A. Concordancia entre pruebas no invasivas para la implementación de algoritmos de cribado de fibrosis hepática en pacientes de alto riesgo. Acad. J. Health Sci. 2023, 34, 84–91. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Melania, G.; Luisella, V.; Salvina, D.P.; Francesca, G.; Silvia, T.A.; Fabrizia, B.; Maristella, M.; Filomena, N.; Kyriazoula, C.; Vassalle, C. Concordance between indirect fibrosis and steatosis indices and their predictors in subjects with overweight/obesity. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2022, 27, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, A.M.; Bojko, E.R. Fatty liver index (FLI): More than a marker of hepatic steatosis. J. Physiol. Biochem. 2024, 80, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.; Ramírez-Manent, J.I.; Riera Routon, K.; López-González, A.A. Variables that influence the values of 7 scales that determine the risk of nonalcoholic fatty liver disease and liver fibrosis in 219,477 spanish workers. Acad. J. Health Sci. 2023, 38, 9–16. [Google Scholar] [CrossRef]
- Luo, S.; Weng, X.; Xu, J.; Lin, H. Correlation between ZJU index and hepatic steatosis and liver fibrosis in American adults with NAFLD. Front. Med. 2024, 11, 1443811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.; Ramírez-Manent, J.I.; Martí-Lliteras, P.; López-González, A.A. Relationship between values of 7 NAFLD scales and different RCV scales in 219,477 Spanish workers. Acad. J. Health Sci. 2023, 38, 52–59. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Luo, H.; Lin, R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front. Endocrinol. 2022, 13, 977625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez-Gallegos, I.; Marina-Arroyo, M.; López-González, Á.A.; Vallejos, D.; Martínez-Almoyna-Rifá, E.; López, P.J.T.; Ramírez-Manent, J.I. Associations Between Metabolic Age, Sociodemographic Variables, and Lifestyle Factors in Spanish Workers. Nutrients 2024, 16, 4207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chero-Sandoval, L.; Martínez-Urbistondo, M.; Cuevas-Sierra, A.; Higuera-Gómez, A.; Martin-Domenech, E.; Castejón, R.; Mellor-Pita, S.; Moreno-Torres, V.; Ramos-Lopez, O.; de Luis, D.; et al. Comparison of Metabolic Syndrome, Autoimmune and Viral Distinctive Inflammatory Related Conditions as Affected by Body Mass Index. J. Clin. Med. 2024, 13, 6298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastberg, S.E.; Lund, H.S.; De Lucia-Rolfe, E.; Kaduka, L.U.; Boit, M.K.; Corpeleijn, E.; Friis, H.; Bernard, S.; Paquette, M.; Baass, A.; et al. Hepatic steatosis is associated with anthropometry, cardio-metabolic disease risk, sex, age and urbanisation, but not with ethnicity in adult Kenyans. Trop. Med. Int. Health 2022, 27, 49–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kain, P. COVID-19 Pandemic and Metabolic Aging. Acta Sci. Neurol. 2022, 5, 30–33. [Google Scholar] [CrossRef]
- Majzoub, A.; Elbardisi, H.; Madani, S.; Leisegang, K.; Mahdi, M.; Agarwal, A.; Henkel, R.; Khalafalla, K.; ElSaid, S.; Arafa, M. Impact of body composition analysis on male sexual function: A metabolic age study. Front. Endocrinol. 2023, 13, 1050441. [Google Scholar] [CrossRef]
- Vásquez-Alvarez, S.; Bustamante-Villagomez, S.K.; Vazquez-Marroquin, G.; Porchia, L.M.; Pérez-Fuentes, R.; Torres-Rasgado, E.; Herrera-Fomperosa, O.; Montes-Arana, I.; Gonzalez-Mejia, M.E. Metabolic Age, an Index Based on Basal Metabolic Rate, Can Predict Individuals That are High Risk of Developing Metabolic Syndrome. High Blood Press. Cardiovasc. Prev. 2021, 28, 263–270. [Google Scholar] [CrossRef]
- Aging Biomarker Consortium; Suo, J.; Gan, Y.; Xie, Y.; Xu, S.; Wang, J.; Chen, D.; Chen, L.; Deng, L.; Feng, S.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar]
- Wang, J.G.; Zhang, Y.; Chen, H.E.; Li, Y.; Cheng, X.G.; Xu, L.; Guo, Z.; Zhao, X.-S.; Sato, T.; Cao, Q.-Y.; et al. Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J. Strength Cond. Res. 2013, 27, 236–243. [Google Scholar] [CrossRef]
- Roubenoff, R. Applications of bioelectrical impedance analysis for body composition to epidemiologic studies. Am. J. Clin. Nutr. 1996, 64, 459S–462S. [Google Scholar] [CrossRef]
- Kreissl, A.; Jorda, A.; Truschner, K.; Skacel, G.; Greber-Platzer, S. Clinically relevant body composition methods for obese pediatric patients. BMC Pediatr. 2019, 19, e84. [Google Scholar] [CrossRef]
- Coufalová, K.; Komarc, M.; Cochrane, D.J. Comparison of Bioelectrical Impedance Analysis and Air Displacement Plethysmography. Int. J. Morphol. 2019, 37, 985–990. [Google Scholar] [CrossRef]
- Lührmann, P.M.; Herbert, B.M.; Neuhäuser-Berthold, M. Effects of fat mass and body fat distribution on resting metabolic rate in the elderly. Metabolism 2001, 50, 972–975. [Google Scholar]
- Tanita Corporation of America Inc. Tanita Technical Bulletin: Regression Formula for Basal Metabolic Rate (BMR); Report No:TBRFLT1013.2013; Tanita Corporation of America Inc.: Arlington Heights, IL, USA, 2013. [Google Scholar]
- Song, K.; Seol, E.G.; Yang, H.; Jeon, S.; Shin, H.J.; Chae, H.W.; Kim, E.K.; Kwon, Y.J.; Lee, J.W. Bioelectrical impedance parameters add incremental value to waist-to-hip ratio for prediction of metabolic dysfunction associated steatotic liver disease in youth with overweight and obesity. Front. Endocrinol. 2024, 15, 1385002. [Google Scholar] [CrossRef]
- Day, K.; Kwok, A.; Evans, A.; Mata, F.; Verdejo-Garcia, A.; Hart, K.; Ward, L.C.; Truby, H. Comparison of a Bioelectrical Impedance Device against the Reference Method Dual Energy X-Ray Absorptiometry and Anthropometry for the Evaluation of Body Composition in Adults. Nutrients 2018, 10, 1469. [Google Scholar] [CrossRef]
- Rudnev, S.G.; Godina, E.Z. Studies on human body composition in Russia: Past and present. J. Physiol. Anthropol. 2022, 41, 18. [Google Scholar] [CrossRef]
- Feng, Q.; Beševic, J.; Conroy, M.; Omiyale, W.; Lacey, B.; Allen, N. Comparison of body composition measures assessed by bioelectrical impedance analysis versus dual-energy X-ray absorptiometry in the United Kingdom Biobank. Clin. Nutr. ESPEN 2024, 63, 214–225. [Google Scholar] [CrossRef]
- Cretescu, I.; Horhat, R.; Mocanu, V.; Munteanu, O. Bioelectrical Impedance Versus Air-Displacement Plethysmography for Body Fat Measurements in Subjects with Abdominal Obesity: A Comparative Study. Appl. Sci. 2025, 15, 2056. [Google Scholar] [CrossRef]
- Wentz, L.M.; Webb, P.S.; Burks, K. A Comprehensive Method of Assessing Body Composition Using Kinanthropometry in Human Performance Training. J. Spec. Oper. Med. 2022, 22, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Roeschlau, P.; Bernt, E.; Gruber, W. Enzymatic determination of total cholesterol in serum. Z. Klin. Chem. Klin. Biochem. 1974, 12, 226. [Google Scholar]
- Sastre-Alzamora, T.; Tomás-Gil, P.; Paublini, H.; Pallarés, L.; Ramírez-Manent, J.I.; López-González, A.A. Relationship between heart age and insulin resistance risk scales in 139634 Spanish workers. Acad. J. Health Sci. 2024, 39, 16–22. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; I Gonzalez-Requero, A.; I Perez-Caballero, A.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Montero-Sandiego, E.; Ruiz-Robledillo, N.; Ferrer-Cascales, R.; Clement-Carbonell, V.; Alcocer-Bruno, C.; Albaladejo-Blázquez, N. Spanish validation of the simple lifestyle indicator questionnaire: Validity and reliability analysis. Front. Public Health 2024, 11, 1146010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of overweight and obesity scales in 386,924 Spanish workers. Acad. J. Health Sci. 2024, 39, 27–35. [Google Scholar] [CrossRef]
- Gallegos, I.R.; Arroyo, M.M.; López-González, Á.A.; Vicente-Herrero, M.T.; Vallejos, D.; Sastre-Alzamora, T.; Ramírez-Manent, J.I. The Effect of a Program to Improve Adherence to the Mediterranean Diet on Cardiometabolic Parameters in 7034 Spanish Workers. Nutrients 2024, 16, 1082. [Google Scholar] [CrossRef] [PubMed]
- Biciusca, T.; Stan, S.I.; Balteanu, M.A.; Cioboata, R.; Ghenea, A.E.; Danoiu, S.; Bumbea, A.-M.; Biciusca, V. The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics 2023, 13, 3316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, J.W.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Kim, S.U. Hepatic Steatosis Index in the Detection of Fatty Liver in Patients with Chronic Hepatitis B Receiving Antiviral Therapy. Gut Liver 2021, 15, 117–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, K.; Yin, Y.; Guo, H.; Ma, L.; Liu, R.; Zhao, T.; Wei, Y.; Zhao, Z.; Cheng, W. Association between the ZJU index and risk of new-onset non-alcoholic fatty liver disease in non-obese participants: A Chinese longitudinal prospective cohort study. Front. Endocrinol. 2024, 15, 1340644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.; Ramírez-Manent, J.I.; Martí-Lliteras, P.; López-González, A.A. Relationship between nonalcoholic fatty liver disease and liver fibrosis risk scales with overweight and obesity scales in 219,477 spanish workers. Acad. J. Health Sci. 2023, 38, 92–100. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Rabizadeh, S.; Sarzaeim, M.; Yadegar, A.; Mohammadi, F.; Bahri, R.A.; Pakravan, P.; Shafiekhani, P.; et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Lipids Health Dis. 2023, 22, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machado, M.V.; Cortez-Pinto, H. NAFLD, MAFLD and obesity: Brothers in arms? Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mavromati, M.; Jornayvaz, F.R. Hypothyroidism-Associated Dyslipidemia: Potential Molecular Mechanisms Leading to NAFLD. Int. J. Mol. Sci. 2021, 22, 12797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malandrino, N.; Bhat, S.Z.; Alfaraidhy, M.; Grewal, R.S.; Kalyani, R.R. Obesity and Aging. Endocrinol. Metab. Clin. N. Am. 2023, 52, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Gasperín-Rodríguez, E.I.; Gómez-Figueroa, J.A.; Gómez-Miranda, L.M.; Ríos-Gallardo, P.T.; Palmeros-Exsome, C.; Hernández-Lepe, M.A.; Moncada-Jiménez, J.; Bonilla, D.A. Body Composition Profiles of Applicants to a Physical Education and Sports Major in Southeastern Mexico. Int. J. Environ. Res. Public Health 2022, 19, 15685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Netto, A.M.; Kashiwagi, N.M.; Minanni, C.A.; Santos, R.D.; Cesena, F.Y. Adiposity, hepatic steatosis, and metabolic health transitions in people with obesity: Influences of age and sex. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, S.; Wu, J.; Wang, Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: New insights from pathogenic mechanisms to clinically targeted therapy. J. Transl. Med. 2023, 21, 510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Milic, J.; Menozzi, V.; Schepis, F.; Malagoli, A.; Besutti, G.; Franconi, I.; Raimondi, A.; Carli, F.; Mussini, C.; Sebastiani, G.; et al. Liver steatosis and nonalcoholic fatty liver disease with fibrosis are predictors of frailty in people living with HIV. AIDS 2020, 34, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Maeso-Díaz, R.; Gracia-Sancho, J. Aging and Chronic Liver Disease. Semin. Liver Dis. 2020, 40, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Horwich, T.B.; Calfon Press, M.; Gornbein, J.; Watson, K.E. Sex Differences in the Association of Body Composition and Cardiovascular Mortality. J. Am. Heart Assoc. 2021, 10, e017511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.; Oh, J.H.; Won, C.W. Sex-Specific Differences in Lower Body Fat Distribution and Association with Physical Performance among Healthy Community-Dwelling Older Adults: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 4201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-based differences in insulin resistance. J. Endocrinol. 2024, 261, e230245. [Google Scholar] [CrossRef] [PubMed]
- McMaughan, D.J.; Oloruntoba, O.; Smith, M.L. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front. Public Health 2020, 8, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiao, G.; Chen, M.; Mohammadpour, H.; MacDonald, C.R.; Bucsek, M.J.; Hylander, B.L.; Barbi, J.J.; Repasky, E.A. Chronic Adrenergic Stress Contributes to Metabolic Dysfunction and an Exhausted Phenotype in T Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2021, 9, 651–664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, B.; Sun, L.; Zeng, G.; Shen, Z.; Wang, K.; Yin, L.; Xu, F.; Wang, P.; Ding, Y.; Nie, Q.; et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature 2022, 610, 562–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, S.; Montalcini, T.; Pujia, A. Mediterranean Diet In Healthy Aging. J. Nutr. Health Aging. 2021, 25, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Bo’, C.; Perna, S.; Allehdan, S.; Rafique, A.; Saad, S.; AlGhareeb, F.; Rondanelli, M.; Tayyem, R.F.; Marino, M.; Martini, D.; et al. Does the Mediterranean Diet Have Any Effect on Lipid Profile, Central Obesity and Liver Enzymes in Non-Alcoholic Fatty Liver Disease (NAFLD) Subjects? A Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2023, 15, 2250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bédard, A.; Carsin, A.-E.; Fuertes, E.; Accordini, S.; Dharmage, S.C.; Garcia-Larsen, V.; Heinrich, J.; Janson, C.; Johannessen, A.; Leynaert, B.; et al. Physical activity and lung function-Cause or consequence? PLoS ONE 2020, 15, e0237769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szekeres, R.; Priksz, D.; Bombicz, M.; Pelles-Tasko, B.; Szilagyi, A.; Bernat, B.; Posa, A.; Varga, B.; Gesztelyi, R.; Somodi, S.; et al. Exercise Types: Physical Activity Mitigates Cardiac Aging and Enhances Mitochondrial Function via PKG-STAT3-Opa1 Axis. Aging Dis. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Aguirre, L.; Gurney, B.; Sinacore, D.R.; Fowler, K.; Gregori, G.; Armamento-Villareal, R.; Qualls, C.; Villareal, D.T. Effect of Aerobic or Resistance Exercise, or Both, on Intermuscular and Visceral Fat and Physical and Metabolic Function in Older Adults With Obesity While Dieting. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 131–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mascaró, C.M.; Bouzas, C.; Montemayor, S.; Casares, M.; Llompart, I.; Ugarriza, L.; Borràs, P.-A.; Martínez, J.A.; Tur, J.A. Effect of a Six-Month Lifestyle Intervention on the Physical Activity and Fitness Status of Adults with NAFLD and Metabolic Syndrome. Nutrients 2022, 14, 1813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.W.; Yoo, J.J.; Kim, S.G.; Kim, Y.S. Bioelectrical Impedance Analysis Can Be an Effective Tool for Screening Fatty Liver in Patients with Suspected Liver Disease. Healthcare 2022, 10, 2268. [Google Scholar] [CrossRef] [PubMed]
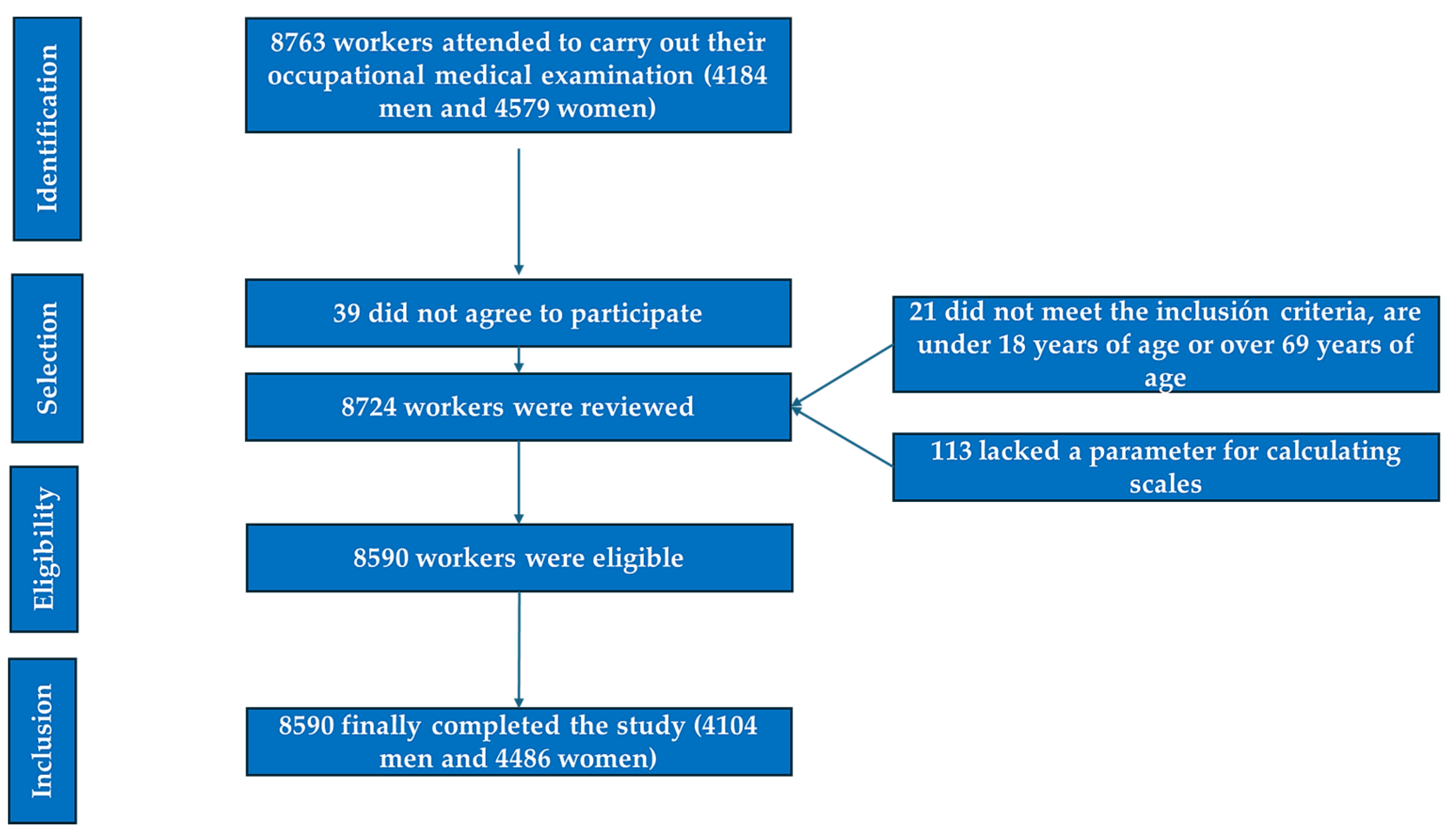

| Men n = 4104 | Women n = 4486 | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p-Value | |
| Age (years) | 41.6 (10.6) | 41.5 (10.5) | 0.492 |
| Height (cm) | 175.8 (7.2) | 162.5 (6.1) | <0.001 |
| Weight (kg) | 81.2 (14.8) | 63.9 (13.6) | <0.001 |
| Waist circumference (cm) | 89.8 (12.5) | 77.0 (12.0) | <0.001 |
| Hip circumference (cm) | 101.8 (8.7) | 99.6 (10.9) | <0.001 |
| Systolic blood pressure (mmHg) | 128.6 (13.3) | 117.2 (14.1) | <0.001 |
| Diastolic blood pressure (mmHg) | 79.9 (10.2) | 74.9 (9.9) | <0.001 |
| Glycemia (mg/dL) | 93.4 (17.8) | 88.9 (12.6) | <0.001 |
| Total cholesterol (mg/dL) | 191.8 (36.0) | 189.0 (34.8) | <0.001 |
| HDL-cholesterol (mg/dL) | 49.2 (11.3) | 59.5 (12.8) | <0.001 |
| LDL-cholesterol (mg/dL) | 124.0 (54.6) | 113.8 (30.7) | <0.001 |
| Triglycerides (mg/dL) | 107.8 (69.4) | 81.5 (46.3) | <0.001 |
| GGT (UI) | 31.5 (30.0) | 18.5 (15.9) | <0.001 |
| AST (UI) | 24.4 (17.3) | 18.2 (7.7) | <0.001 |
| ALT (UI) | 29.3 (34.9) | 17.3 (13.4) | <0.001 |
| % | % | p-value | |
| 18–29 years | 15.5 | 16.8 | 0.005 |
| 30–39 years | 27.8 | 25.1 | |
| 40–49 years | 32.7 | 34.4 | |
| 50–59 years | 19.0 | 19.7 | |
| 60–69 years | 5.0 | 4.0 | |
| Social class I | 57.1 | 50.8 | <0.001 |
| Social class II | 20.2 | 23.8 | |
| Social class III | 22.7 | 25.4 | |
| Non-smokers | 84.5 | 84.2 | 0.348 |
| Smokers | 15.5 | 15.8 | |
| No physical activity | 25.9 | 35.1 | <0.001 |
| Physical activity 1–3 days/week | 27.0 | 26.5 | |
| Physical activity more 3 days/week | 47.1 | 38.4 | |
| Mediterranean diet not followed | 44.5 | 41.6 | <0.001 |
| Mediterranean diet followed | 55.5 | 58.4 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Metabolic Age | n | Mean (SD) | p-Value | n | Mean (SD) | p-Value |
| FLI low | 2206 | −10.3 (6.1) | <0.001 | 3645 | −8.7 (8.5) | <0.001 |
| FLI moderate | 971 | −3.0 (9.7) | 478 | 6.3 (9.4) | ||
| FLI high | 1107 | 7.1 (9.6) | 361 | 11.7 (6.7) | ||
| HSI normal | 2518 | −10.2 (6.9) | <0.001 | 3268 | −10.5 (6.6) | <0.001 |
| HSI high | 1766 | 3.6 (10.6) | 1216 | 6.5 (9.6) | ||
| ZJU normal | 3102 | −9.5 (7.5) | <0.001 | 3443 | −10.2 (7.0) | <0.001 |
| ZJU high | 1182 | 6.1 (9.5) | 1041 | 8.1 (8.5) | ||
| FLD normal | 3700 | −7.2 (9.0) | <0.001 | 4175 | −7.3 (9.6) | <0.001 |
| FLD high | 584 | 12.2 (6.8) | 309 | 13.0 (5.2) | ||
| LAP normal | 3276 | −7.1 (9.4) | <0.001 | 3994 | −7.4 (9.7) | <0.001 |
| LAP high | 1008 | 5.2 (10.4) | 490 | 8.5 (9.0) |
| MA High | MA High | MA High | MA High | MA High | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Women | 1 | 1 | 1 | 1 | 1 |
| Men | 1.27 (1.20–1.34) | 1.18 (1.14–1.23) | 1.15 (1.10–1.21) | 1.10 (1.07–1.14) | 1.23 (1.18–1.29) |
| 18–29 years | 1 | 1 | 1 | 1 | 1 |
| 30–39 years | 1.13 (1.10–1.17) | 1.20 (1.16–1.25) | 1.15 (1.11–1.20) | 1.11 (1.08–1.15) | 1.16 (1.10–1.23) |
| 40–49 years | 1.29 (1.24–1.35) | 1.38 (1.31–1.45) | 1.28 (1.21–1.35) | 1.22 (1.16–1.28) | 1.30 (1.20–1.41) |
| 50–59 years | 1.48 (1.38–1.57) | 1.56 (1.50–1.63) | 1.46 (1.38–1.54) | 1.40 (1.31–1.50) | 1.48 (1.35–1.61) |
| 60–69 years | 1.79 (1.64–1.94) | 1.75 (1.66–1.85) | 1.69 (1.58–1.80) | 1.66 (1.51–1.81) | 1.63 (1.49–1.78) |
| Social class I | 1 | 1 | 1 | 1 | 1 |
| Social class II | 1.79 (1.46–2.12) | 1.67 (1.50–1.84) | 1.84 (1.57–2.11) | 1.49 (1.35–1.632) | 1.63 (1.48–1.79) |
| Social class III | 2.33 (1.95–2.71) | 1.89 (1.70–2.09) | 2.22 (1.82–2.62) | 1.99 (1.64–2.35) | 2.43 (2.10–2.77) |
| Non-smokers | 1 | 1 | 1 | 1 | 1 |
| Smokers | 1.12 (1.10–1.15) | 1.24 (1.18–1.30) | 1.29 (1.20–1.39) | 1.09 (1.06–1.11) | 1.17 (1.10–1.24) |
| Physical activity more 3 days/week | 1 | 1 | 1 | 1 | 1 |
| Physical activity 1–3 days/week | 1.96 (1.64–2.28) | 1.88 (1.56–2.20) | 1.79 (1.64–1.94) | 1.81 (1.60–2.02) | 2.14 (1.85–2.44) |
| No physical activity | 3.19 (2.68–3.70) | 3.19 (2.66–3.72) | 3.20 (2.64–3.77) | 4.12 (3.38–4.85) | 4.20 (3.64–4.77) |
| Mediterranean diet followed | 1 | 1 | 1 | 1 | 1 |
| Mediterranean diet not followed | 2.26 (1.95–2.58) | 2.65 (2.27–3.04) | 2.35 (2.00–2.71) | 2.39 (2.03–2.76) | 2.42 (2.15–2.70) |
| FLI low | 1 | ||||
| FLI moderate | 5.47 (4.45–6.50) | ||||
| FLI high | 10.13 (8.90–11.37) | ||||
| HSI normal | 1 | ||||
| HSI high | 11.13 (9.93–12.34) | ||||
| ZJU normal | 1 | ||||
| ZJU high | 9.88 (8.60–11.17) | ||||
| FLD normal | 1 | ||||
| FLD high | 12.10 (10.80–13.51) | ||||
| LAP normal | 1 | ||||
| LAP high | 8.75 (7.56–9.95) |
| Women | Men | |
|---|---|---|
| AUC (95% CI) | AUC (95% CI) | |
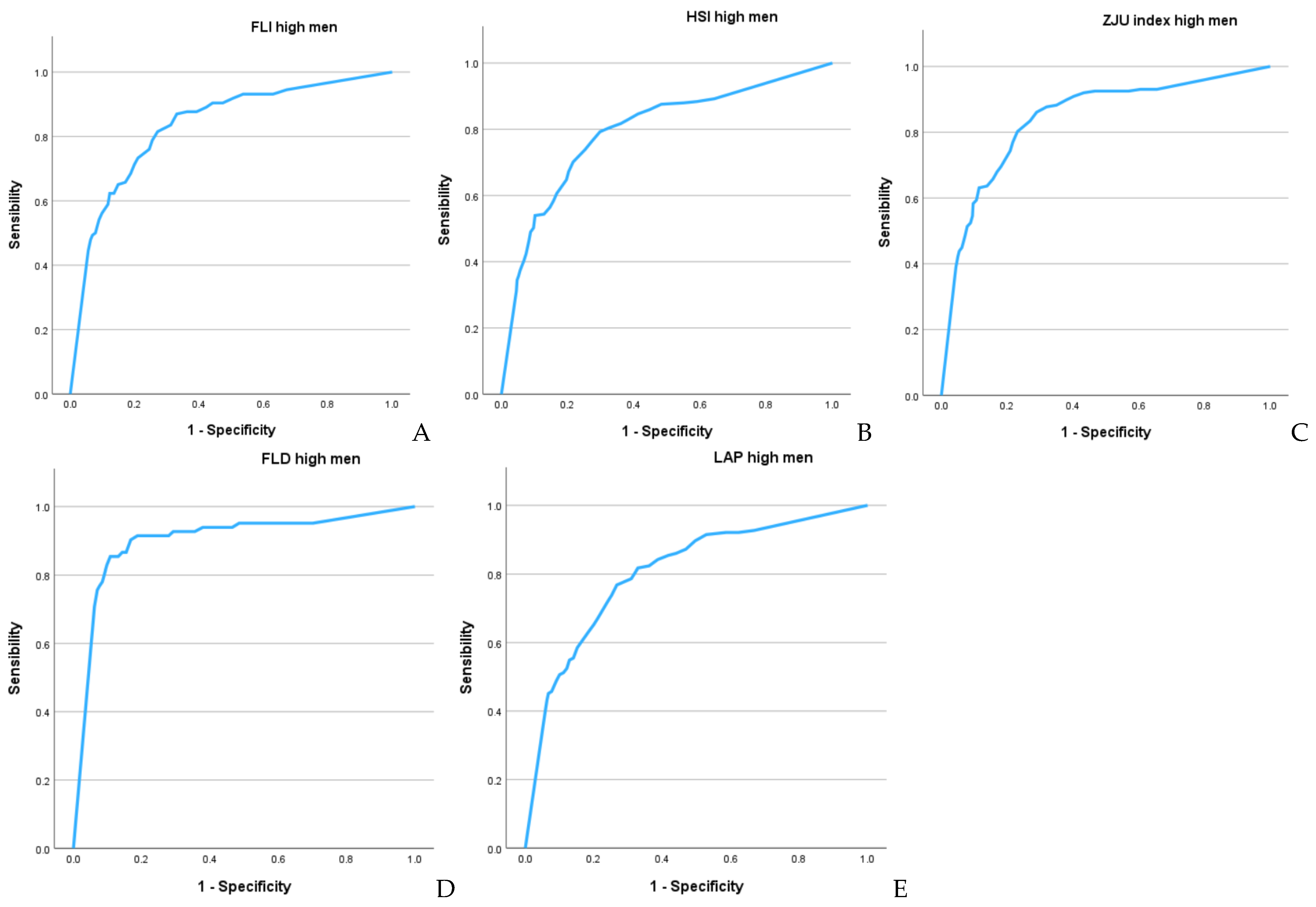
| FLI high | 0.900 (0.884–0.916) | 0.833 (0.817–0.848) |
| HSI high | 0.878 (0.866–0.890 | 0.799 (0.785–0.814) |
| ZJU high | 0.898 (0.888–0.909) | 0.852 (0.838–0.865) |
| FLD high | 0.935 (0.925–0.945) | 0.917 (0.903–0.932) |
| LAP high | 0.864 (0.848–0.881) | 0.802 (0.786–0.818) |
| cut-off-sens-specif-Youden | cut-off-sens-specif-Youden | |
| FLI high | -2–83.5–83.4–0.669 | -3–80.1–80.1–0.602 |
| HSI high | -3–81.0–80.9–0.619 | -5–77.0–74.1–0.511 |
| ZJU high | -1–83.3–83.3–0.666 | -3–81.0–77.5–0.585 |
| FLD high | 9–88.2–87.7–0.759 | 6–87.5–86.1–0.736 |
| LAP high | 1–80.4–80.3–0.607 | -3–75.6–72.7–0.483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Gallegos, I.; Busquets-Cortes, C.; Paublini, H.; López-González, Á.A.; Martínez-Almoyna-Rifá, E.; Tárraga López, P.J.; Ramírez-Manent, J.I. Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers. Metabolites 2025, 15, 343. https://doi.org/10.3390/metabo15050343
Ramírez-Gallegos I, Busquets-Cortes C, Paublini H, López-González ÁA, Martínez-Almoyna-Rifá E, Tárraga López PJ, Ramírez-Manent JI. Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers. Metabolites. 2025; 15(5):343. https://doi.org/10.3390/metabo15050343
Chicago/Turabian StyleRamírez-Gallegos, Ignacio, Carla Busquets-Cortes, Hernán Paublini, Ángel Arturo López-González, Emilio Martínez-Almoyna-Rifá, Pedro Juan Tárraga López, and José Ignacio Ramírez-Manent. 2025. "Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers" Metabolites 15, no. 5: 343. https://doi.org/10.3390/metabo15050343
APA StyleRamírez-Gallegos, I., Busquets-Cortes, C., Paublini, H., López-González, Á. A., Martínez-Almoyna-Rifá, E., Tárraga López, P. J., & Ramírez-Manent, J. I. (2025). Association Between Bioimpedance-Determined Metabolic Age and MASLD Risk Scores in Spanish Workers. Metabolites, 15(5), 343. https://doi.org/10.3390/metabo15050343