Characterizing Plasma-Based Metabolomic Signatures for Metastasis in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. FIE-FTICR Experiment
2.4. MS Data Processing
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. High-Throughput and Reproducible FIE-FTICR MS-Based Platform
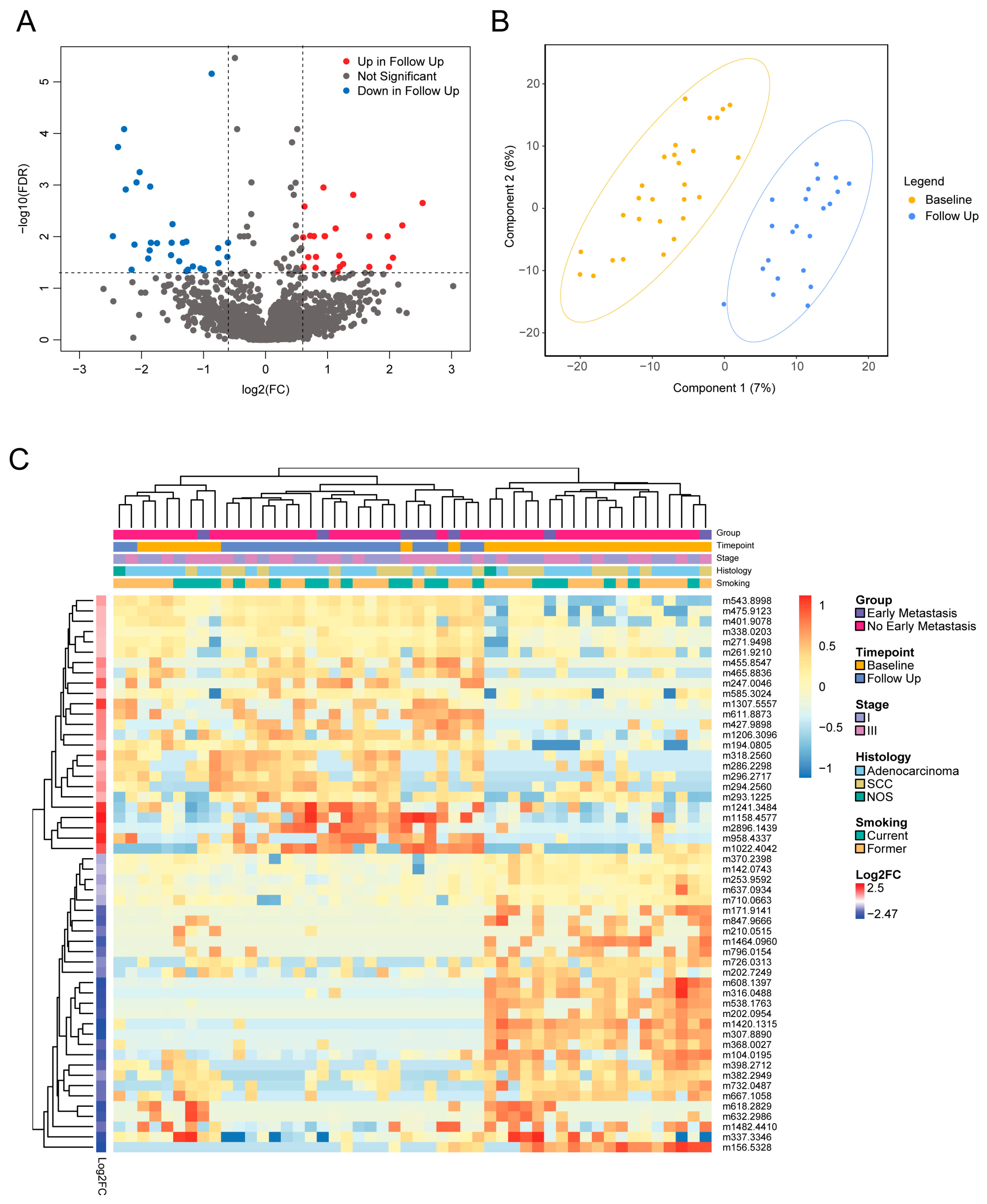
3.3. Serial Analysis of Metabolites Reveals Treatment Effects
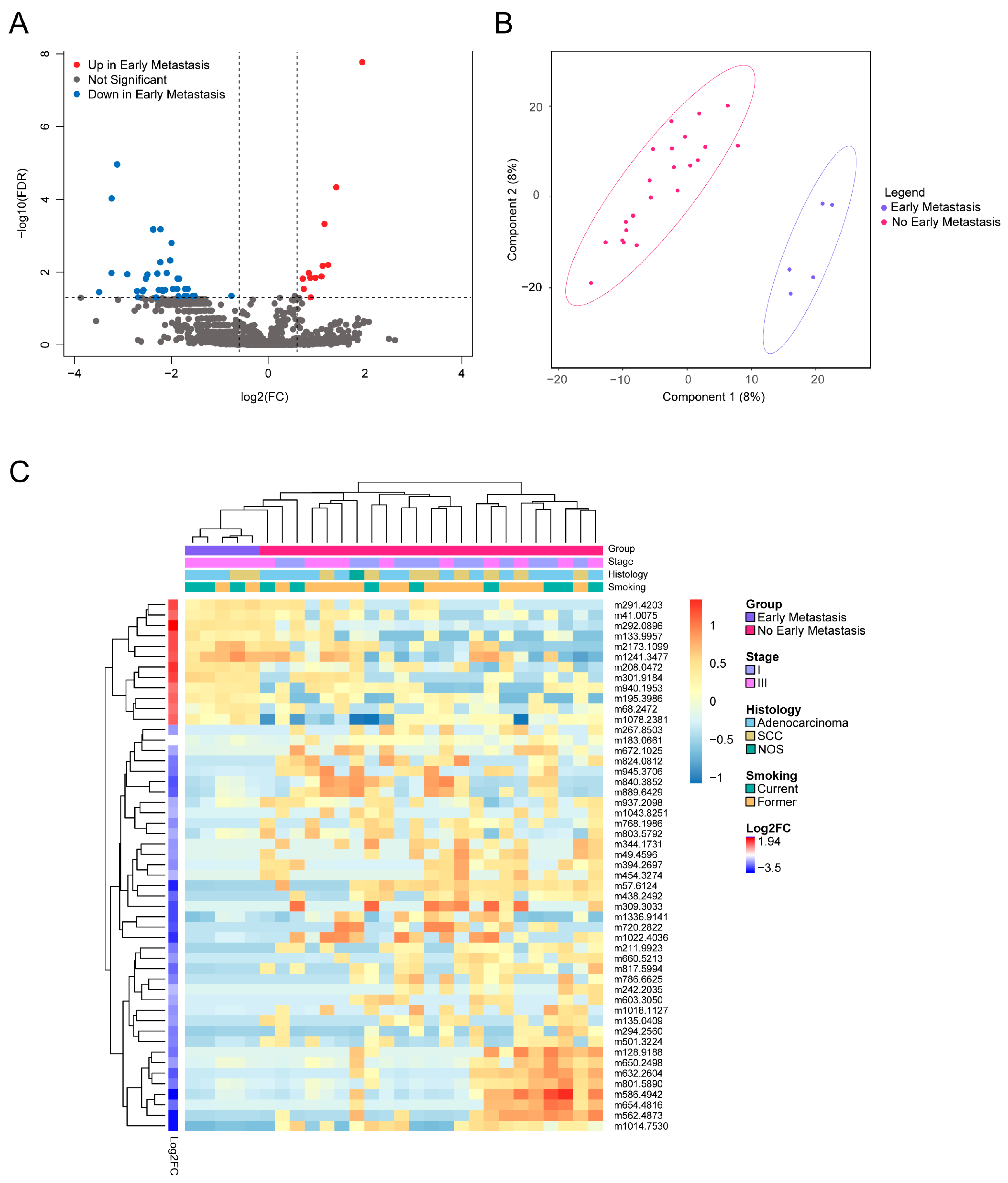
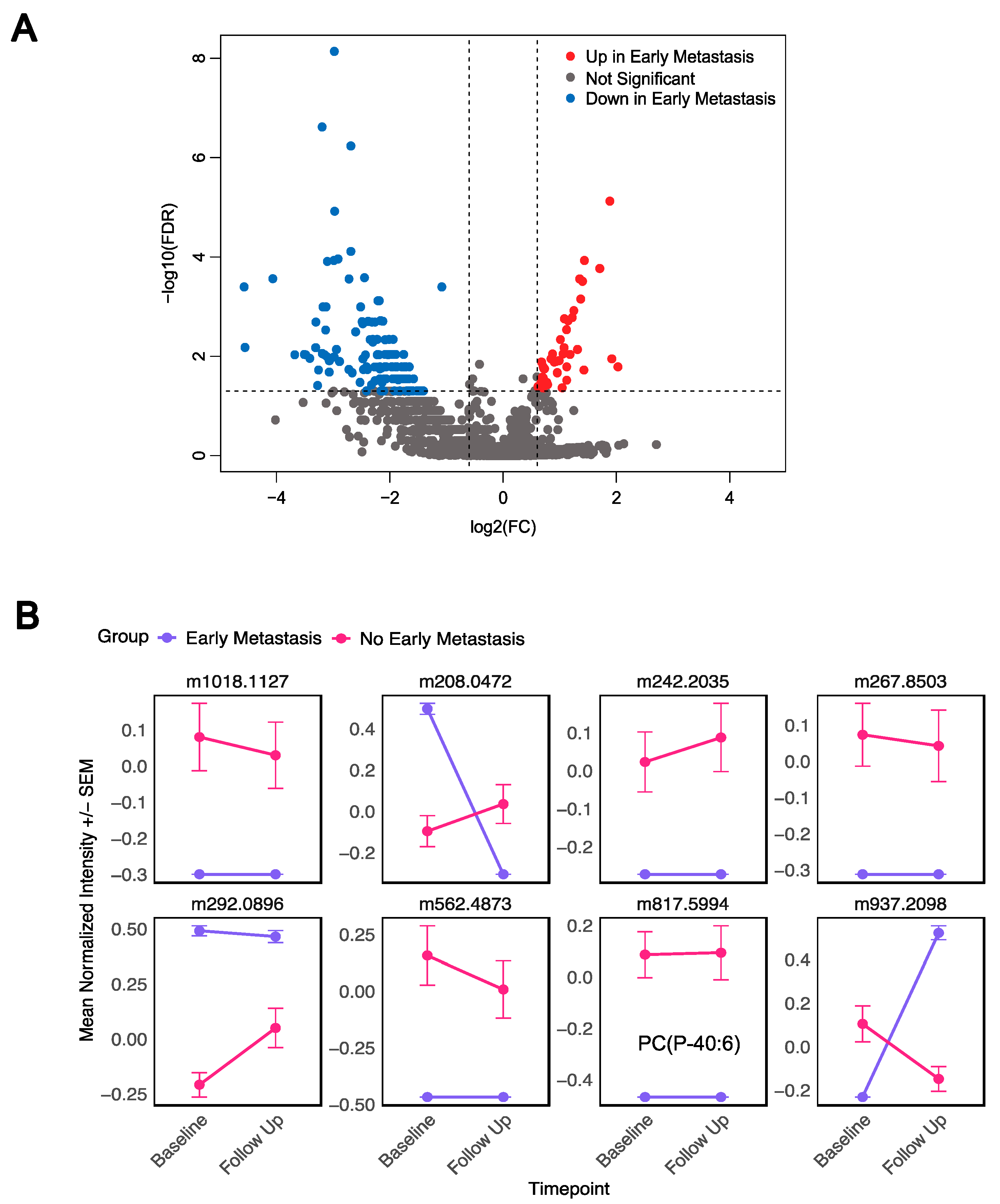
3.4. Distinct Metabolites Characterize Presence of Early Metastases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FIE FTICR MS | Flow injection electrospray–Fourier transform ion cyclotron resonance mass spectrometry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Log2FC | Log2 fold change |
| PLS-DA | Partial Least-Squares Discriminant Analysis |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Boyd, J.A.; Hubbs, J.L.; Kim, D.W.; Hollis, D.; Marks, L.B.; Kelsey, C.R. Timing of Local and Distant Failure in Resected Lung Cancer: Implications for Reported Rates of Local Failure. J. Thorac. Oncol. 2010, 5, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non–Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung Cancer Statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Krasna, M.J. Clinical Significance of Micrometastasis in Lung and Esophageal Cancer: A New Paradigm in Thoracic Oncology. Ann. Thorac. Surg. 2002, 74, 278–284. [Google Scholar] [CrossRef]
- Elia, I.; Doglioni, G.; Fendt, S.-M. Metabolic Hallmarks of Metastasis Formation. Trends Cell Biol. 2018, 28, 673–684. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic Rewiring in the Promotion of Cancer Metastasis: Mechanisms and Therapeutic Implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Morii, E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites 2021, 11, 28. [Google Scholar] [CrossRef]
- Zhou, D.; Duan, Z.; Li, Z.; Ge, F.; Wei, R.; Kong, L. The Significance of Glycolysis in Tumor Progression and Its Relationship with the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 1091779. [Google Scholar] [CrossRef]
- Bamji-Stocke, S.; van Berkel, V.; Miller, D.M.; Frieboes, H.B. A Review of Metabolism-Associated Biomarkers in Lung Cancer Diagnosis and Treatment. Metabolomics 2018, 14, 81. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Kim, K.; DeFelice, B.C.; Taylor, S.L.; Gandara, D.R.; Yoneda, K.Y.; Cooke, D.T.; Fiehn, O.; Kelly, K.; Miyamoto, S. Investigation of Metabolomic Blood Biomarkers for Detection of Adenocarcinoma Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Jia, Z.; Lu, Y.; Zhang, H.; Ng, C.T.; Bay, B.H.; Shen, H.M.; Ong, C.N. Metabolic Signatures of Four Major Histological Types of Lung Cancer Cells. Metabolomics 2018, 14, 118. [Google Scholar] [CrossRef]
- Hori, S.; Nishiumi, S.; Kobayashi, K.; Shinohara, M.; Hatakeyama, Y.; Kotani, Y.; Hatano, N.; Maniwa, Y.; Nishio, W.; Bamba, T.; et al. A Metabolomic Approach to Lung Cancer. Lung Cancer 2011, 74, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hu, F.; Huang, L.; Lu, J.; Yang, X.; Xu, J.; Wang, S.; Shen, Y.; Zhong, R.; Chu, T.; et al. Association of Metabolomics with PD-1 Inhibitor plus Chemotherapy Outcomes in Patients with Advanced Non-Small-Cell Lung Cancer. J. Immunother. Cancer 2024, 12, e008190. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, Z.; Li, X.; Yang, Y.; Qi, R. Metabolomics Profiling in Prediction of Chemo-Immunotherapy Efficiency in Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2023, 12, 1025046. [Google Scholar] [CrossRef]
- Brown, S.C.; Kruppa, G.; Dasseux, J.-L. Metabolomics Applications of FT-ICR Mass Spectrometry. Mass Spectrom. Rev. 2005, 24, 223–231. [Google Scholar] [CrossRef]
- Wancewicz, B.; Zhu, Y.; Fenske, R.J.; Weeks, A.M.; Wenger, K.; Pabich, S.; Daniels, M.; Punt, M.; Nall, R.; Peter, D.C.; et al. Metformin Monotherapy Alters the Human Plasma Lipidome Independent of Clinical Markers of Glycemic Control and Cardiovascular Disease Risk in a Type 2 Diabetes Clinical Cohort. J. Pharmacol. Exp. Ther. 2023, 386, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Truchan, N.A.; Fenske, R.J.; Sandhu, H.K.; Weeks, A.M.; Patibandla, C.; Wancewicz, B.; Pabich, S.; Reuter, A.; Harrington, J.M.; Brill, A.L.; et al. Human Islet Expression Levels of Prostaglandin E2 Synthetic Enzymes, But Not Prostaglandin EP3 Receptor, Are Positively Correlated with Markers of β-Cell Function and Mass in Nondiabetic Obesity. ACS Pharmacol. Transl. Sci. 2021, 4, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Schaid, M.D.; Harrington, J.M.; Kelly, G.M.; Sdao, S.M.; Merrins, M.J.; Kimple, M.E. EP3 Signaling Is Decoupled from the Regulation of Glucose-Stimulated Insulin Secretion in β-Cells Compensating for Obesity and Insulin Resistance. Islets 2023, 15, 2223327. [Google Scholar] [CrossRef]
- Zhu, Y.; Wancewicz, B.; Schaid, M.; Tiambeng, T.N.; Wenger, K.; Jin, Y.; Heyman, H.; Thompson, C.J.; Barsch, A.; Cox, E.D.; et al. Ultrahigh-Resolution Mass Spectrometry-Based Platform for Plasma Metabolomics Applied to Type 2 Diabetes Research. J. Proteome Res. 2021, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Baschnagel, A.M.; Flakus, M.J.; Wallat, E.M.; Wuschner, A.E.; Chappell, R.J.; Bayliss, R.A.; Kimple, R.J.; Christensen, G.E.; Reinhardt, J.M.; Bassetti, M.F.; et al. A Phase 2 Randomized Clinical Trial Evaluating 4-Dimensional Computed Tomography Ventilation-Based Functional Lung Avoidance Radiation Therapy for Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1393–1402. [Google Scholar] [CrossRef]
- Chen, R.; Li, Z.; Yuan, Y.; Zhu, Z.; Zhang, J.; Tian, X.; Zhang, X. A Comprehensive Analysis of Metabolomics and Transcriptomics in Non-Small Cell Lung Cancer. PLoS ONE 2020, 15, e0232272. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, K.; Peng, Z.; Ding, L.; Li, H.; Fan, Y. Evaluating for Correlations between Specific Metabolites in Patients Receiving First-Line or Second-Line Immunotherapy for Metastatic or Recurrent NSCLC: An Exploratory Study Based on Two Cohorts. Mole. Cancer Ther. 2024, 23, 733–742. [Google Scholar] [CrossRef]
- Rocha, C.M.; Barros, A.S.; Goodfellow, B.J.; Carreira, I.M.; Gomes, A.; Sousa, V.; Bernardo, J.; Carvalho, L.; Gil, A.M.; Duarte, I.F. NMR Metabolomics of Human Lung Tumours Reveals Distinct Metabolic Signatures for Adenocarcinoma and Squamous Cell Carcinoma. Carcinogenesis 2015, 36, 68–75. [Google Scholar] [CrossRef]
- Merino Salvador, M.; Gómez de Cedrón, M.; Moreno Rubio, J.; Falagán Martínez, S.; Sánchez Martínez, R.; Casado, E.; Ramírez de Molina, A.; Sereno, M. Lipid Metabolism and Lung Cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The Role of Lipids in Cancer Progression and Metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Breslow, D.K.; Weissman, J.S. Membranes in Balance: Mechanisms of Sphingolipid Homeostasis. Mol. Cell 2010, 40, 267. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Sengupta, A.; Ding, K.; Ubeydullah, E.; Krishnaiah, S.; Leighl, N.B.; Shepherd, F.A.; Seymour, L.; Weljie, A. Metabolites as Prognostic Markers for Metastatic Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Platinum-Doublet Chemotherapy. Cancers 2020, 12, 1926. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty Acid Synthase and the Lipogenic Phenotype in Cancer Pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.; Schulze, A. Lipid Metabolism at the Nexus of Diet and Tumor Microenvironment. Trends Cancer 2019, 5, 693–703. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Kisluk, J.; Pietrowska, K.; Godzien, J.; Kozlowski, M.; Reszeć, J.; Sierko, E.; Naumnik, W.; Mróz, R.; Moniuszko, M.; et al. The Ability of Metabolomics to Discriminate Non-Small-Cell Lung Cancer Subtypes Depends on the Stage of the Disease and the Type of Material Studied. Cancers 2021, 13, 3314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, J.; Li, T.; Hu, H.; Li, X.; Xing, H.; Wang, J.; Yang, F.; Ma, Q.; Liu, B.; et al. Accurate Classification of Non-Small Cell Lung Cancer (NSCLC) Pathology and Mapping of EGFR Mutation Spatial Distribution by Ambient Mass Spectrometry Imaging. Front. Oncol. 2019, 9, 804. [Google Scholar] [CrossRef]
- Hao, D.; Sarfaraz, M.O.; Farshidfar, F.; Bebb, D.G.; Lee, C.Y.; Card, C.M.; David, M.; Weljie, A.M. Temporal Characterization of Serum Metabolite Signatures in Lung Cancer Patients Undergoing Treatment. Metabolomics 2016, 12, 58. [Google Scholar] [CrossRef]
- Overy, D.P.; Enot, D.P.; Tailliart, K.; Jenkins, H.; Parker, D.; Beckmann, M.; Draper, J. Explanatory Signal Interpretation and Metabolite Identification Strategies for Nominal Mass FIE-MS Metabolite Fingerprints. Nat. Protoc. 2008, 3, 471–485. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]

| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 17 (61) |
| Female | 11 (39) |
| Median age (range) in years | 69 (52–84) |
| Stage | |
| I | 13 (46) |
| III | 15 (54) |
| Histology | |
| Squamous cell carcinoma | 10 (36) |
| Adenocarcinoma | 17 (61) |
| Not otherwise specified | 1 (3) |
| Metastasis | |
| Early metastasis | 5 (18) |
| No early metastasis | 23 (82) |
| Smoking Status | |
| Current | 11 (39) |
| Former | 17 (61) |
| Non-smoker | 0 (0) |
| Treatment | |
| Radiation only | 12 (43) |
| Radiation + Chemo | 6 (21) |
| Radiation + Chemo/ICI | 8 (29) |
| Radiation + ICI | 2 (7) |
| Mass | Name | Molecular Formula | Class | Subclass | Parent Level 1 | Log2FC | Adj p |
|---|---|---|---|---|---|---|---|
| 294.256 | FA(19:2) | C19H34O2 | Fatty acyls | Fatty acids and derivatives | Long-chain fatty acids | −2.174 | 0.034 |
| 344.1731 | 4-ETHOXYCARBONYL-6-ETHYL-5-METHYL-2-(PARA-TOLYL)PERHYDROPYRROLO(3,4-C)PYRROLE-1,3-DIONE (3A,4-CIS-6,6A-TRANS) | C19H24N2O4 | Carboxylic acids and derivatives | Amino acids, peptides, and analogs | Amino acids and derivatives | −1.8881 | 0.03 |
| 720.2822 | As-PL(16:0) | C29H58AsO13P | Diarylheptanoids | Linear diarylheptanoids | Linear diarylheptanoids | −2.593 | 0.034 |
| 801.589 | PC 36:02 HETE | C44H84NO9P | Glycerophospholipids | Glycerophosphoserines | 1-alkyl,2-acyl-glycerol-3-phosphoserines | −2.19 | 0.032 |
| 817.5994 | PC(P-40:6) | C48H84NO7P | Glycerophospholipids | Glycerophosphocholines | 1-(1Z-alkenyl),2-acyl-glycerophosphocholines | −2.375 | 0.001 |
| 183.0661 | Phosphocholine | C5H14NO4P | Organonitrogen compounds | Quaternary ammonium salts | Cholines | −0.759 | 0.045 |
| 632.2604 | PI(20:5) | C29H45O13P | Glycerophospholipids | Glycerophosphoinositols | PIs (Phosphatidylinositols) | −2.528 | 0.015 |
| 786.6625 | SM(40:1) | C45H91N2O6P | Sphingolipids | Phosphosphingolipids | SMs (Sphingomyelins) | −2.13 | 0.031 |
| 660.5213 | SM(d31:1) | C36H73N2O6P | Sphingolipids | Phosphosphingolipids | SMs (Sphingomyelins) | −1.872 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhu, Y.; McIlwain, S.J.; Deng, H.; Brasier, A.R.; Ge, Y.; Kimple, M.E.; Baschnagel, A.M. Characterizing Plasma-Based Metabolomic Signatures for Metastasis in Non-Small Cell Lung Cancer. Metabolites 2025, 15, 340. https://doi.org/10.3390/metabo15050340
Liu M, Zhu Y, McIlwain SJ, Deng H, Brasier AR, Ge Y, Kimple ME, Baschnagel AM. Characterizing Plasma-Based Metabolomic Signatures for Metastasis in Non-Small Cell Lung Cancer. Metabolites. 2025; 15(5):340. https://doi.org/10.3390/metabo15050340
Chicago/Turabian StyleLiu, Manlu, Yanlong Zhu, Sean J. McIlwain, Haotian Deng, Allan R. Brasier, Ying Ge, Michelle E. Kimple, and Andrew M. Baschnagel. 2025. "Characterizing Plasma-Based Metabolomic Signatures for Metastasis in Non-Small Cell Lung Cancer" Metabolites 15, no. 5: 340. https://doi.org/10.3390/metabo15050340
APA StyleLiu, M., Zhu, Y., McIlwain, S. J., Deng, H., Brasier, A. R., Ge, Y., Kimple, M. E., & Baschnagel, A. M. (2025). Characterizing Plasma-Based Metabolomic Signatures for Metastasis in Non-Small Cell Lung Cancer. Metabolites, 15(5), 340. https://doi.org/10.3390/metabo15050340