Urine Metabolomic Profiling and Machine Learning in Autism Spectrum Disorder Diagnosis: Toward Precision Treatment
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Urine Collection
2.3. Urine Analysis
2.4. LC-MS Analysis of Polar Metabolites
2.5. Creatinine Normalization
2.6. Data Processing and Identification
2.7. Datasets
- (1)
- Endogenous metabolites: A total of 189 metabolites in the body can be endogenously produced or obtained from endogenous metabolism, such as amino acids or participants in the Krebs cycle.
- (2)
- Exogenous metabolites: 104 metabolites originating from the exposome, meaning metabolites that exclusively originate from the environment and are subsequently processed by the body. These include food metabolites, metabolites produced by the microbiome, and metabolites present in the surroundings, such as food packaging, cosmetics, and pharmaceuticals.
- (3)
- All 293 metabolites were combined.
2.8. Machine Learning Classifiers
2.9. Questionnaires
2.10. Feature Selection
3. Results and Discussion
3.1. Classification Based on All 293 Metabolites
3.2. Classification of 189 Endogenous Metabolites
3.3. Classification Based on 104 Exogenous Metabolites
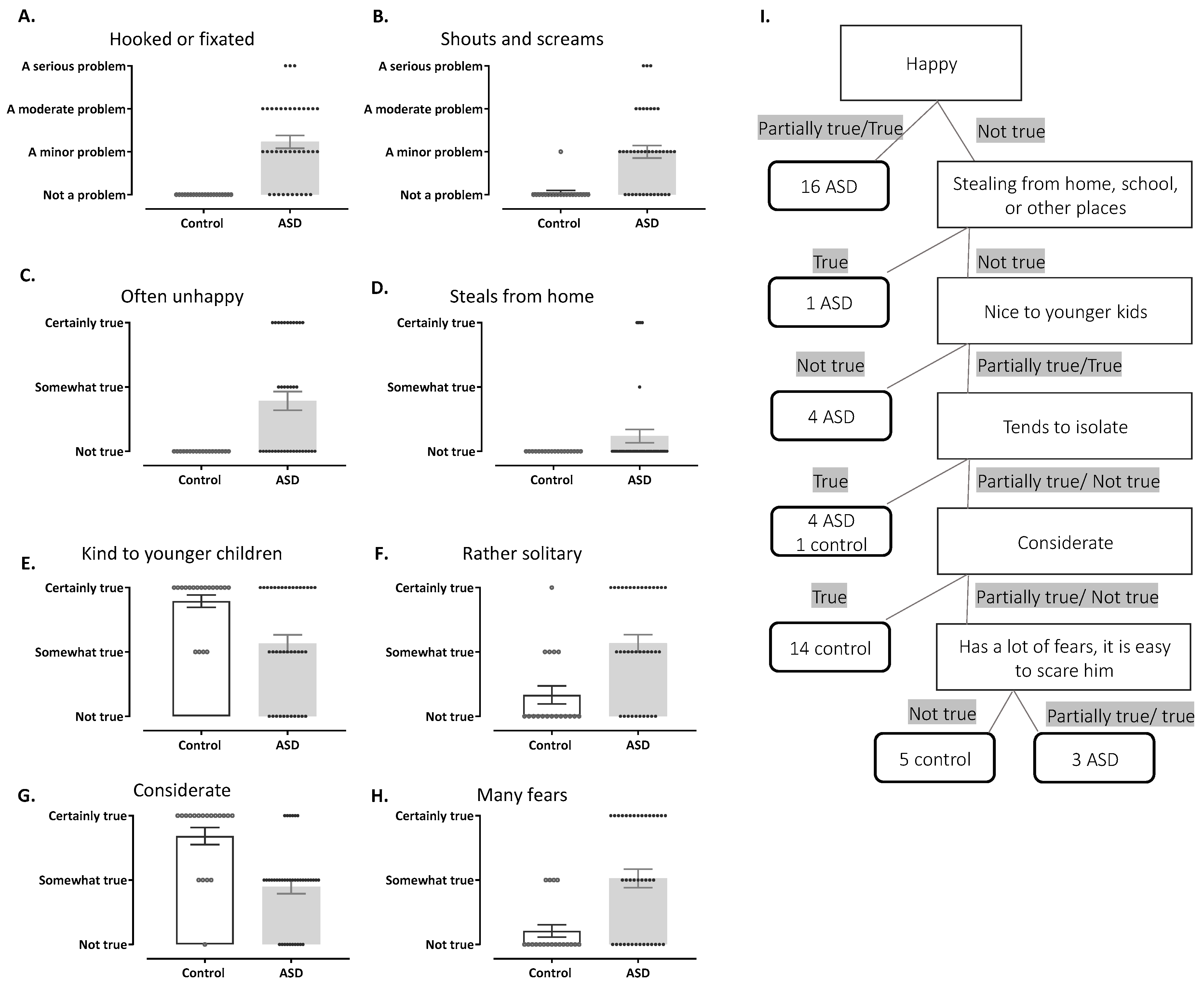
3.4. Behavioral Classifiers
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CDC. Data and Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/autism/data-research/index.html (accessed on 25 February 2025).
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, J.; Feng, Y.Q. Mass Spectrometry-Based Metabolomics for Clinical Study: Recent Progresses and Applications. TrAC Trends Anal. Chem. 2023, 158, 116896. [Google Scholar] [CrossRef]
- Khamis, M.M.; Adamko, D.J.; El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass. Spectrom. Rev. 2017, 36, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Emond, P.; Mavel, S.; Aïdoud, N.; Nadal-Desbarats, L.; Montigny, F.; Bonnet-Brilhault, F.; Barthélémy, C.; Merten, M.; Sarda, P.; Laumonnier, F.; et al. GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal. Bioanal. Chem. 2013, 405, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Siracusano, M.; Noto, A.; Fattuoni, C.; Riccioni, A.; Rajula, H.S.R.; Fanos, V.; Curatolo, P.; Barberini, L.; Mazzone, L. The Urine Metabolome of Young Autistic Children Correlates with Their Clinical Profile Severity. Metabolites 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, C.; Dunstan, R.H.; Rothkirch, T.; Roberts, T.K.; Reichelt, K.L.; Cosford, R.; Deed, G.; Ellis, L.B.; Sparkes, D.L. Altered amino acid excretion in children with autism. Nutr. Neurosci. 2008, 11, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bitar, T.; Mavel, S.; Emond, P.; Nadal-Desbarats, L.; Lefèvre, A.; Mattar, H.; Soufia, M.; Blasco, H.; Vourc’h, P.; Hleihel, W.; et al. Identification of metabolic pathway disturbances using multimodal metabolomics in autistic disorders in a Middle Eastern population. J. Pharm. Biomed. Anal. 2018, 152, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Gomez-Fernández, A.; de la Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Gil-Campos, M. Metabolic profiling in children with autism spectrum disorder with and without mental regression: Preliminary results from a cross-sectional case-control study. Metabolomics 2019, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Likhitweerawong, N.; Thonusin, C.; Boonchooduang, N.; Louthrenoo, O.; Nookaew, I.; Chattipakorn, N.; Chattipakorn, S.C. Profiles of urine and blood metabolomics in autism spectrum disorders. Metab. Brain Dis. 2021, 36, 1641–1671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giulivi, C.; Zhang, Y.F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial dysfunction in autism. JAMA 2010, 304, 2389–2396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naviaux, R.K. Metabolic features of the cell danger response. Mitochondrion 2014, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Zickgraf, H. Atypical Eating Behavior in Children and Adolescents with Autism, ADHD, Other Disorders, and Typical Development. Res. Autism Spectr. Disord. 1999, 64, 76–83. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.S.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gnainsky, Y.; Itkin, M.; Mehlman, T.; Brandis, A.; Malitsky, S.; Soen, Y. Protocol for studying microbiome impact on host energy and reproduction in. STAR Protoc. 2022, 3, 101253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench. Online Appendix for “Data Mining: Practical Machine Learning Tools and Techniques”; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- Mahapatra, S.; Vyshedsky, D.; Martinez, S.; Kannel, B.; Braverman, J.; Edelson, S.M.; Vyshedskiy, A. Autism Treatment Evaluation Checklist (ATEC) Norms: A “Growth Chart” for ATEC Score Changes as a Function of Age. Children 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, C.L.; Guo, B.; Valentine, A.Z.; Groom, M.J.; Daley, D.; Sayal, K.; Hollis, C. The validity of the Strengths and Difficulties Questionnaire (SDQ) for children with ADHD symptoms. PLoS ONE 2019, 14, e0218518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zehavi, Y.; Mandel, H.; Eran, A.; Ravid, S.; Abu Rashid, M.; Jansen, E.E.W.; Wamelink, M.M.C.; Saada, A.; Shaag, A.; Elpeleg, O.; et al. Severe infantile epileptic encephalopathy associated with D-glyceric aciduria: Report of a novel case and review. Metab. Brain Dis. 2019, 34, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Aru, A.; Munk-Nielsen, L.; Federspiel, B.H. The soil fungus Chaetomium in the human paranasal sinuses. Eur. Arch. Otorhinolaryngol. 1997, 254, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Anderson, G.M.; Kemperman, R.F.; Oosterloo-Duinkerken, A.; Minderaa, R.B.; Kema, I.P. Urinary excretion of 5-hydroxyindoleacetic acid, serotonin and 6-sulphatoxymelatonin in normoserotonemic and hyperserotonemic autistic individuals. Neuropsychobiology 2010, 61, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wink, L.K.; Adams, R.; Wang, Z.; Klaunig, J.E.; Plawecki, M.H.; Posey, D.J.; McDougle, C.J.; Erickson, C.A. A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol. Autism 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghanizadeh, A.; Moghimi-Sarani, E. A randomized double blind placebo controlled clinical trial of N-Acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry 2013, 13, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hardan, A.Y.; Fung, L.K.; Libove, R.A.; Obukhanych, T.V.; Nair, S.; Herzenberg, L.A.; Frazier, T.W.; Tirouvanziam, R. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol. Psychiatry 2012, 71, 956–961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anastasescu, C.M.; Gheorman, V.; Popescu, F.; Stepan, M.D.; Stoicănescu, E.C.; Udriștoiu, I. A Clinical Study of Urine Amino Acids in Children with Autism Spectrum Disorder. Life 2024, 14, 629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Qiao, Y.; Xu, X.J.; You, X.; Tao, Y. Urine Organic Acids as Potential Biomarkers for Autism-Spectrum Disorder in Chinese Children. Front. Cell. Neurosci. 2019, 13, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lord, R.S.; Burdette, C.K.; Bralley, J.A. Significance of urinary tartaric acid. Clin. Chem. 2005, 51, 672–673. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 2023, 13, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tye, C.; Runicles, A.K.; Whitehouse, A.J.O.; Alvares, G.A. Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychiatry 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prosperi, M.; Santocchi, E.; Guiducci, L.; Frinzi, J.; Morales, M.A.; Tancredi, R.; Muratori, F.; Calderoni, S. Interventions on Microbiota: Where Do We Stand on a Gut–Brain Link in Autism? A Systematic Review. Nutrients 2022, 14, 462. [Google Scholar] [CrossRef]
- Ruggeri, B.; Sarkans, U.; Schumann, G.; Persico, A.M. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y.; et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, S.H.; Iyer, A.; Russell, W.R. Impact of protein on the composition and metabolism of the human gut microbiota and health. Proc. Nutr. Soc. 2021, 80, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M. Autism medical comorbidities. World J. Clin. Pediatr. 2021, 10, 15–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanctuary, M.R.; Kain, J.N.; Angkustsiri, K.; German, J.B. Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Front. Nutr. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pesko, M.J.; Burbige, E.M.; Sannar, E.M.; Beresford, C.; Rogers, C.; Ariefdjohan, M.; Stutzman, D. The Use of N-acetylcysteine Supplementation to Decrease Irritability in Four Youths With Autism Spectrum Disorders. J. Pediatr. Pharmacol. Ther. 2020, 25, 149–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shazman, S.; Carmel, J.; Itkin, M.; Malitsky, S.; Shalan, M.; Soreq, E.; Elliott, E.; Lebow, M.; Kuperman, Y. Urine Metabolomic Profiling and Machine Learning in Autism Spectrum Disorder Diagnosis: Toward Precision Treatment. Metabolites 2025, 15, 332. https://doi.org/10.3390/metabo15050332
Shazman S, Carmel J, Itkin M, Malitsky S, Shalan M, Soreq E, Elliott E, Lebow M, Kuperman Y. Urine Metabolomic Profiling and Machine Learning in Autism Spectrum Disorder Diagnosis: Toward Precision Treatment. Metabolites. 2025; 15(5):332. https://doi.org/10.3390/metabo15050332
Chicago/Turabian StyleShazman, Shula, Julie Carmel, Maxim Itkin, Sergey Malitsky, Monia Shalan, Eyal Soreq, Evan Elliott, Maya Lebow, and Yael Kuperman. 2025. "Urine Metabolomic Profiling and Machine Learning in Autism Spectrum Disorder Diagnosis: Toward Precision Treatment" Metabolites 15, no. 5: 332. https://doi.org/10.3390/metabo15050332
APA StyleShazman, S., Carmel, J., Itkin, M., Malitsky, S., Shalan, M., Soreq, E., Elliott, E., Lebow, M., & Kuperman, Y. (2025). Urine Metabolomic Profiling and Machine Learning in Autism Spectrum Disorder Diagnosis: Toward Precision Treatment. Metabolites, 15(5), 332. https://doi.org/10.3390/metabo15050332







