Intestinal Epithelial-Derived Exosomes Under Cold Stimulation Promote Adipose Thermogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation, Identification, and Injection of IEC-Exo
2.3. In Situ Injection of BAT and sWAT
2.4. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
2.5. Mice Body Weight and Surface Temperature
2.6. Western Blotting
2.7. Small RNA Sequencing
2.8. Transfection of microRNA Mimics in Primary Adipocytes
2.9. Seahorse
2.10. The Uptake of Fatty Acid and Glucose
2.11. Quantitative Real-Time PCR
2.12. Statistics
3. Results
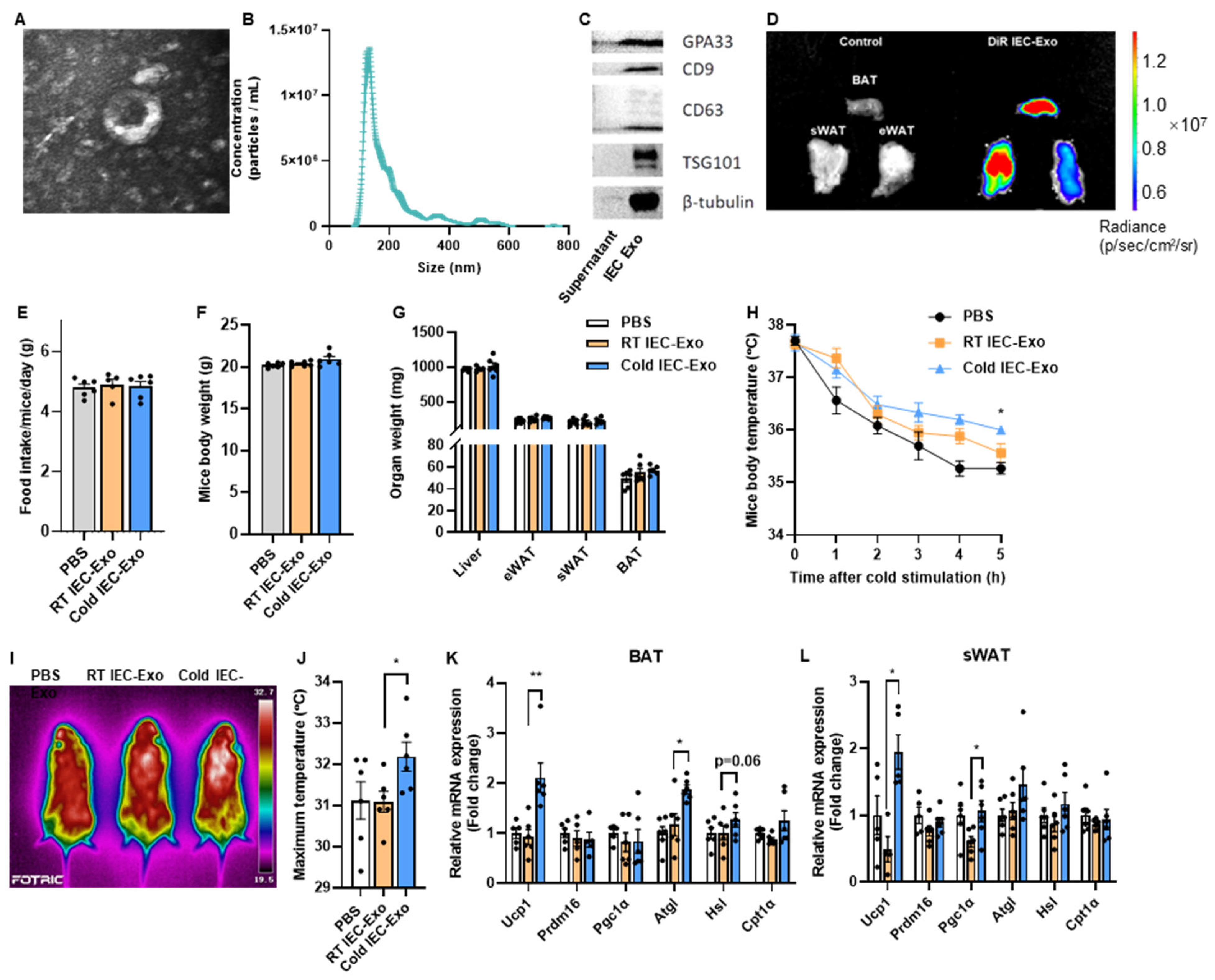
3.1. IEC-Exo Under Cold Stimulation Promote Adipose Thermogenesis in Mice
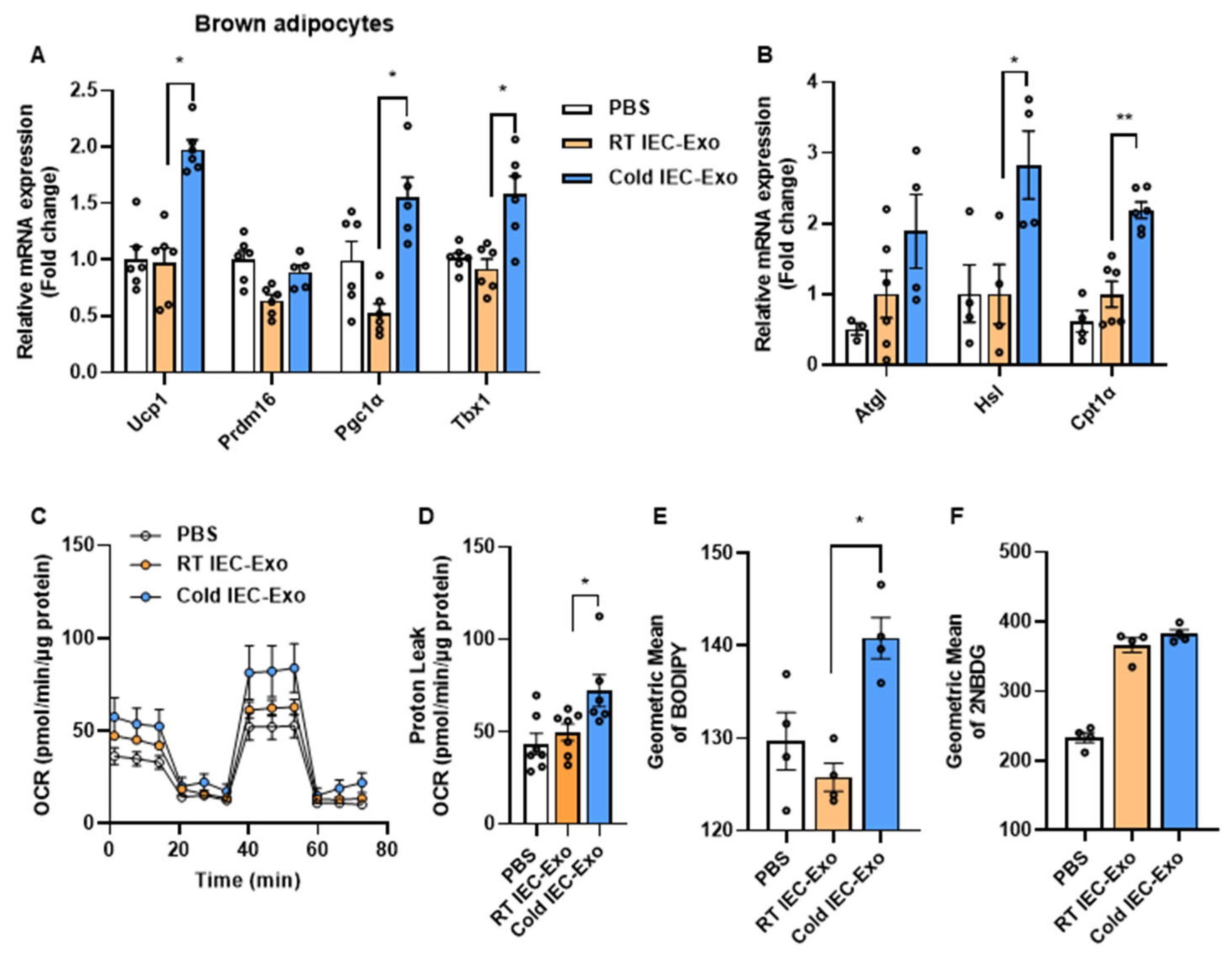
3.2. IEC-Exo Under Cold Stimulation Promote the Thermogenesis of Primary Adipocytes
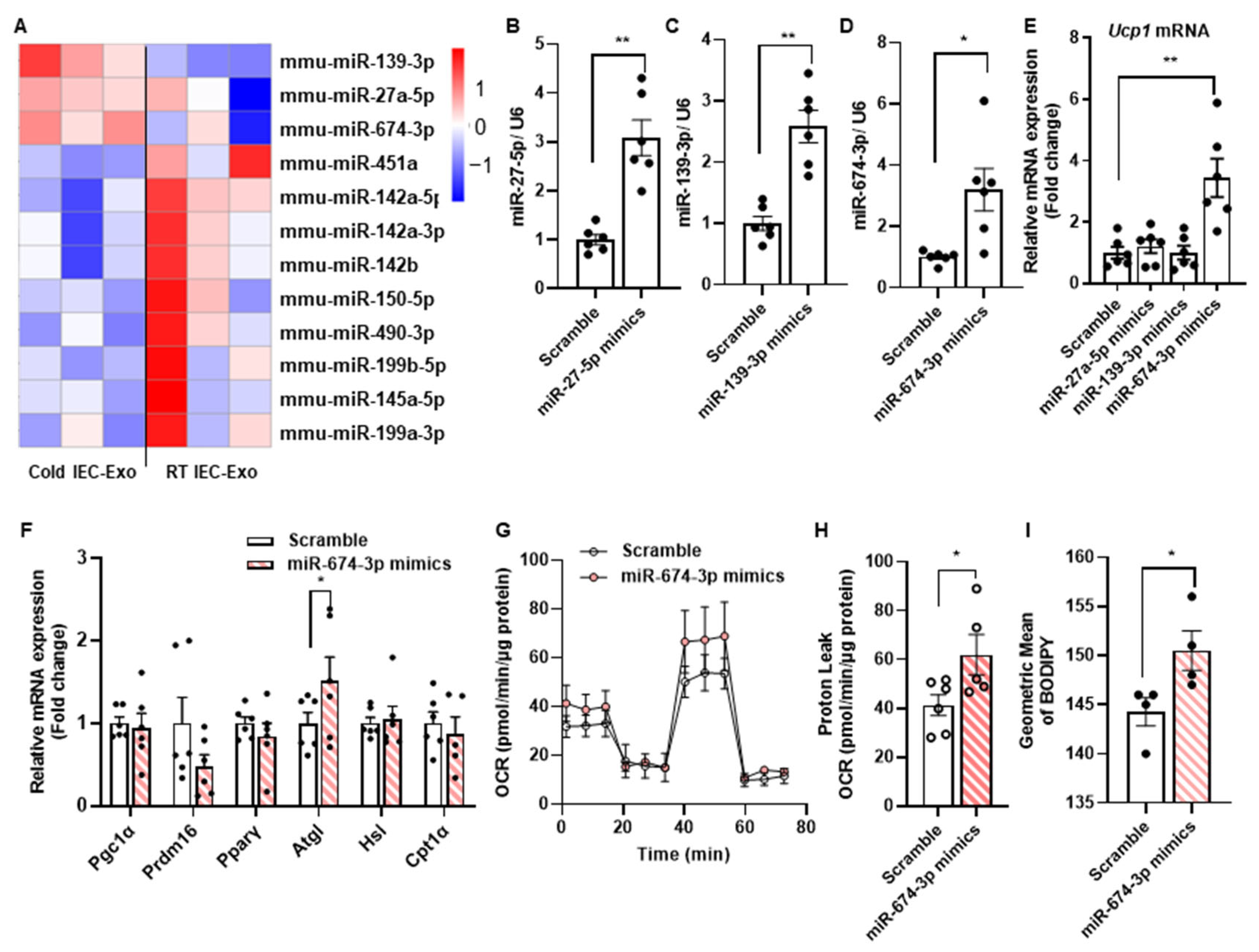
3.3. The miR-674-3p in Cold IEC-Exo Promotes the Thermogenesis of Adipocytes
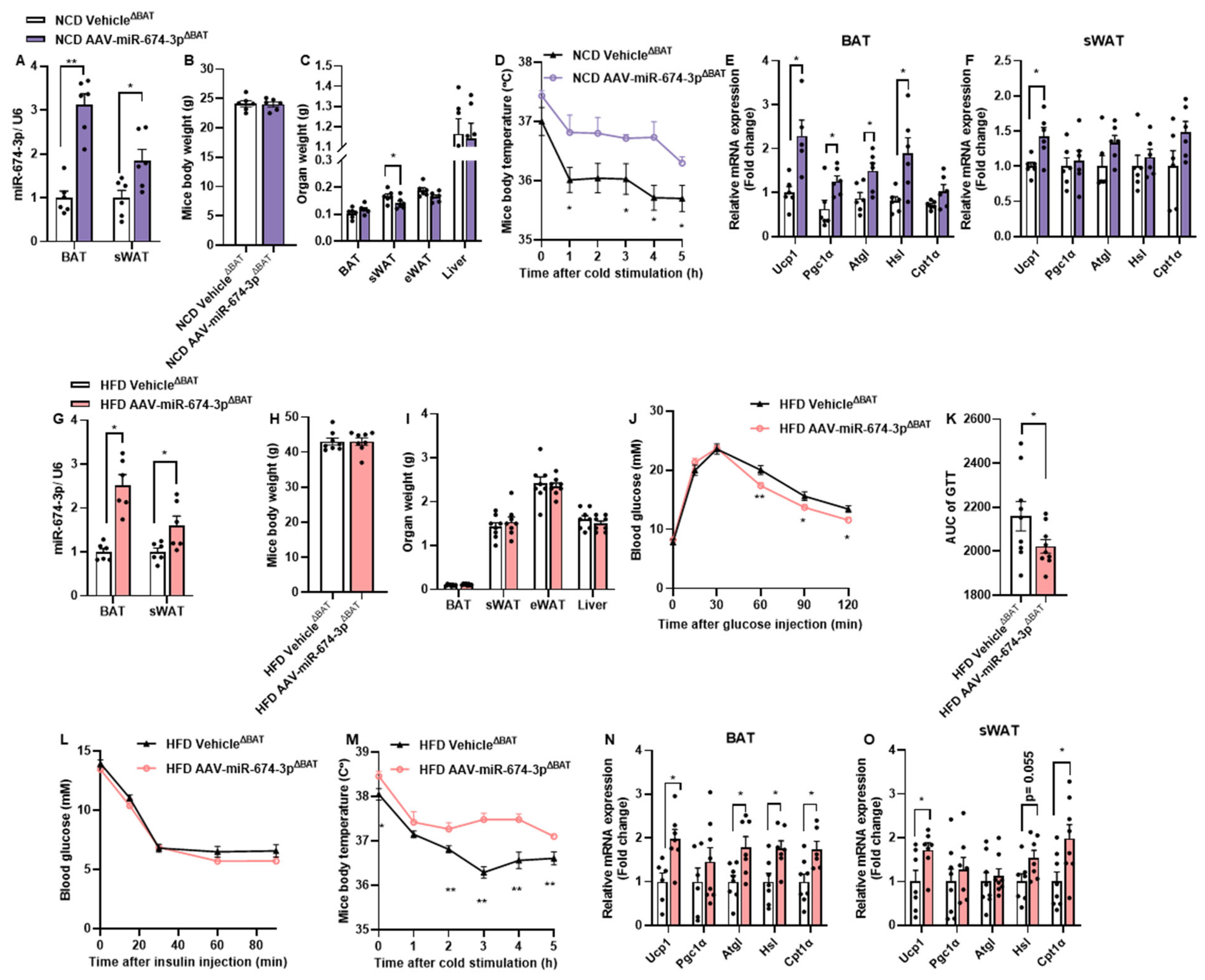
3.4. In Situ Overexpression of miR-674-3p in BAT and sWAT Promotes Thermogenesis in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Xi, S.; Wang, Y.; Wu, C.; Peng, W.; Zhu, Y.; Hu, W. Intestinal Epithelial Cell Exosome Launches IL-1β-Mediated Neuron Injury in Sepsis-Associated Encephalopathy. Front. Cell. Infect. Microbiol. 2022, 11, 783049. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat. Commun. 2016, 7, 13045. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, X.; Chen, M.; Xiang, Z.; Li, X.; Zhang, J.; Dong, W. Exosomal-miR-129-2-3p derived from Fusobacterium nucleatum-infected intestinal epithelial cells promotes experimental colitis through regulating TIMELESS-mediated cellular senescence pathway. Gut Microbes 2023, 15, 2240035. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Liang, Y.; Sun, L.; Feng, L.; Min, J.; Mulholland, M.W.; Yin, Y.; Zhang, W. Regulation of hepatic lipid metabolism by intestine epithelium-derived exosomes. Life Metab. 2023, 2, load044. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Zhan, J.; Huang, W. p-Coumaric acid prevents obesity via activating thermogenesis in brown adipose tissue mediated by mTORC1-RPS6. FASEB J. 2020, 34, 7810–7824. [Google Scholar] [CrossRef]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef] [PubMed]
- Balakathiresan, N.S.; Chandran, R.; Bhomia, M.; Jia, M.; Li, H.; Maheshwari, R.K. Serum and amygdala microRNA signatures of posttraumatic stress: Fear correlation and biomarker potential. J. Psychiatr. Res. 2014, 57, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.-Y.; Han, S.B.; Kim, J.; Blundon, J.A.; Wang, Y.-D.; Yu, J.; Anderson, K.; Kaminski, D.B.; Sakurada, S.M.; Pruett-Miller, S.M.; et al. Schizophrenia-related microdeletion causes defective ciliary motility and brain ventricle enlargement via microRNA-dependent mechanisms in mice. Nat. Commun. 2020, 11, 912. [Google Scholar] [CrossRef]
- Zurawek, D.; Kusmider, M.; Faron-Gorecka, A.; Gruca, P.; Pabian, P.; Solich, J.; Kolasa, M.; Papp, M.; Dziedzicka-Wasylewska, M. Reciprocal MicroRNA Expression in Mesocortical Circuit and Its Interplay with Serotonin Transporter Define Resilient Rats in the Chronic Mild Stress. Mol. Neurobiol. 2016, 54, 5741–5751. [Google Scholar] [CrossRef]
- Fu, W.; Hong, Z.; You, X.; Din, J.; Chen, B.; Zhao, B.; Yuan, G.; Li, Q. Enhancement of anticancer activity of docetaxel by combination with Fuzheng Yiliu decoction in a mouse model of castration-resistant prostate cancer. Biomed. Pharmacother. 2019, 118, 109374. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, M.; Chen, G.; Tong, L.; Zhang, H.; Du, D. Up-regulation of miR-335 and miR-674-3p in the rostral ventrolateral medulla contributes to stress-induced hypertension. J. Neurochem. 2022, 161, 387–404. [Google Scholar] [CrossRef]
- Zeng, M.; He, Y.; Yang, Y.; Wang, M.; Chen, Y.; Wei, X. Mesenchymal stem cell-derived extracellular vesicles relieve endothelial cell senescence via recovering CTRP9 upon repressing miR-674-5p in atherosclerosis. Regen. Ther. 2024, 27, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, Q.; Qi, L.; Hua, D.; Tao, J.; Mangan, C.J.; Lou, Y.; Li, L. MicroRNA-674-5p/5-LO axis involved in autoimmune reaction of Concanavalin A-induced acute mouse liver injury. Toxicol. Lett. 2016, 258, 101–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Feng, T.; Yang, Y.; Zhu, Z.; Shao, F.; Sun, L.; Yin, Y.; Zhang, W. Intestinal Epithelial-Derived Exosomes Under Cold Stimulation Promote Adipose Thermogenesis. Metabolites 2025, 15, 324. https://doi.org/10.3390/metabo15050324
Han X, Feng T, Yang Y, Zhu Z, Shao F, Sun L, Yin Y, Zhang W. Intestinal Epithelial-Derived Exosomes Under Cold Stimulation Promote Adipose Thermogenesis. Metabolites. 2025; 15(5):324. https://doi.org/10.3390/metabo15050324
Chicago/Turabian StyleHan, Xue, Tiange Feng, Yaxu Yang, Ziming Zhu, Fangyu Shao, Lijun Sun, Yue Yin, and Weizhen Zhang. 2025. "Intestinal Epithelial-Derived Exosomes Under Cold Stimulation Promote Adipose Thermogenesis" Metabolites 15, no. 5: 324. https://doi.org/10.3390/metabo15050324
APA StyleHan, X., Feng, T., Yang, Y., Zhu, Z., Shao, F., Sun, L., Yin, Y., & Zhang, W. (2025). Intestinal Epithelial-Derived Exosomes Under Cold Stimulation Promote Adipose Thermogenesis. Metabolites, 15(5), 324. https://doi.org/10.3390/metabo15050324





