Genistein Reduces Anxiety-like Behavior During Metestrus–Diestrus Phase Without Changing Estradiol or Progesterone Levels in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Vaginal Smears
2.4. Experimental Groups and Treatments
2.5. Behavioral Tests
2.5.1. Elevated Plus Maze
2.5.2. Locomotor Activity Test
2.5.3. Light/Dark Box
2.6. Determination of Plasma Concentration of Estradiol and Progesterone
2.7. Statistical Analysis
3. Results
3.1. Grouping of Ovarian Cycle Phases According to Treatments
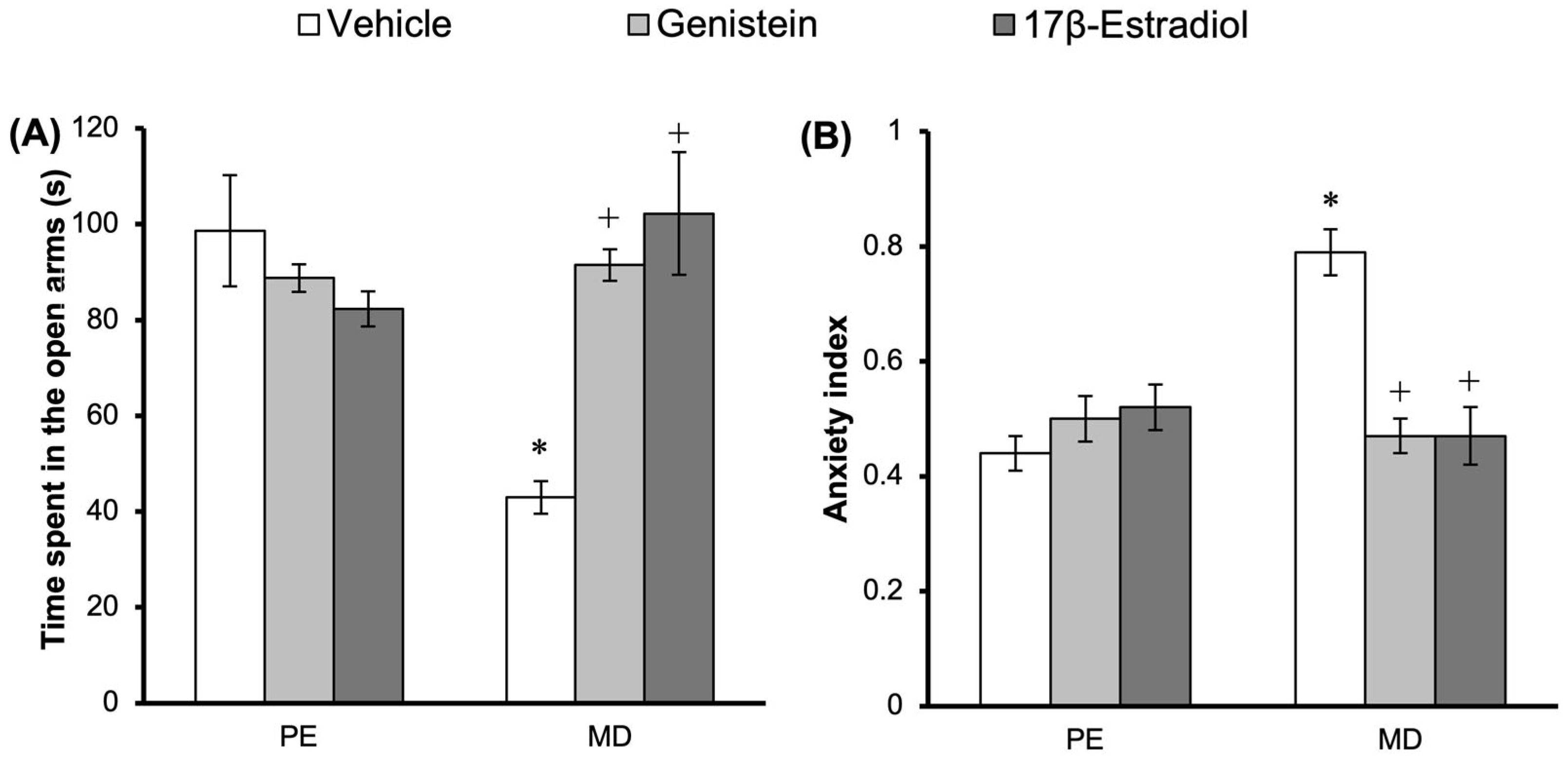
3.2. Effect of Treatments on Anxiety-like Behavior in the EPM
3.3. Effect of Treatments on Crossing, Rearing, and Grooming in the LAT
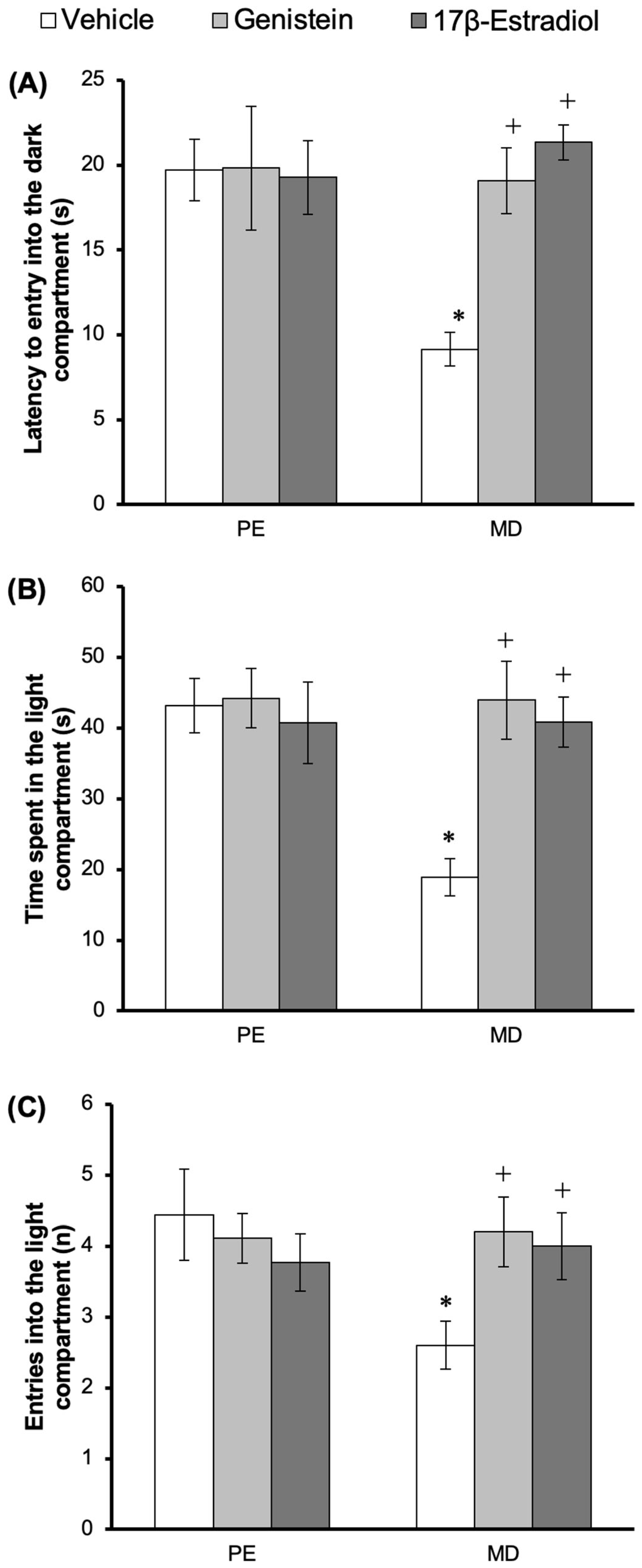
3.4. Effect of Treatments on Anxiety-like Behavior in the LDB
3.5. Effect of the Treatments on Estradiol and Progestogen Plasma Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERα | estrogen receptor α |
| ERβ | estrogen receptor β |
| EPM | elevated plus maze |
| LAT | locomotor activity test |
| LDB | light/dark box |
| AI | anxiety index |
| GPER | G-protein-coupled estrogen receptor |
| i.p. | intraperitoneal injection |
| s.c. | subcutaneous injection |
References
- Farhane-Medina, N.Z.; Luque, B.; Tabernero, C.; Castillo-Mayén, R. Factors associated with gender and sex differences in anxiety prevalence and comorbidity: A systematic review. Sci. Prog. 2022, 105, 368504221135469. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Han, L. Gender difference in anxiety and related factors among adolescents. Front. Public Health 2025, 12, 1410086. [Google Scholar] [CrossRef]
- Park, H.Y. Sex/gender differences in depression and anxiety disorders. In Sex/Gender-Specific Medicine in Clinical Areas; Kim, N., Ed.; Springer: Singapore, 2024; pp. 369–379. [Google Scholar] [CrossRef]
- Stanikova, D.; Luck, T.; Pabst, A.; Bae, Y.J.; Hinz, A.; Glaesmer, H.; Stanik, J.; Sacher, J.; Engel, C.; Enzenbach, C.; et al. Associations between anxiety, body mass index, and sex hormones in women. Front. Psychiatry 2019, 10, 479. [Google Scholar] [CrossRef]
- Perich, T.A.; Roberts, G.; Frankland, A.; Sinbandhit, C.; Meade, T.; Austin, M.P.; Mitchell, P.B. Clinical characteristics of women with reproductive cycle-associated bipolar disorder symptoms. Aust. N. Z. J. Psychiatry 2017, 51, 161–167. [Google Scholar] [CrossRef]
- Modzelewski, S.; Oracz, A.; Żukow, X.; Iłendo, K.; Śledzikowka, Z.; Waszkiewicz, N. Premenstrual syndrome: New insights into etiology and review of treatment methods. Front. Psychiatry 2024, 15, 1363875. [Google Scholar] [CrossRef]
- Hofmeister, S.; Bodden, S. Premenstrual syndrome and premenstrual dysphoric disorder. Am. Fam. Physician 2016, 94, 236–240. [Google Scholar]
- Brown, R.D.; Bondy, E.; Prim, J.; Dichter, G.; Schiller, C.E. The behavioral and physiological correlates of affective mood switching in premenstrual dysphoric disorder. Front. Psychiatry 2024, 15, 1448914. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef]
- Bishnoi, I.R.; Ossenkopp, K.P.; Kavaliers, M. Sex and age differences in locomotor and anxiety-like behaviors in rats: From adolescence to adulthood. Dev. Psychobiol. 2021, 63, 496–511. [Google Scholar] [CrossRef]
- D’Souza, D.; Sadananda, M. Estrous cycle phase-dependent changes in anxiety- and depression-like profiles in the late adolescent Wistar-Kyoto rat. Ann. Neurosci. 2017, 24, 136–145. [Google Scholar] [CrossRef]
- Pestana, J.E.; Graham, B.M. The impact of estrous cycle on anxiety-like behaviour during unlearned fear tests in female rats and mice: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 164, 105789. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Miguel, K.J.; Melo, L.L.; Spadari-Bratfisch, R.C. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 2001, 74, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lovick, T.A. GABA in the female brain: Oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharmacol. Biochem. Behav. 2008, 90, 43–50. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; López-Rubalcava, C. Can animal models resemble a premenstrual dysphoric condition? Front. Neuroendocrinol. 2022, 66, 101007. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Guillén-Ruiz, G.; Hernández-López, F.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav. Brain Res. 2021, 397, 112952. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef]
- Reilly, T.J.; Wallman, P.; Clark, I.; Knox, C.L.; Craig, M.C.; Taylor, D. Intermittent selective serotonin reuptake inhibitors for premenstrual syndromes: A systematic review and meta-analysis of randomized trials. J. Psychopharmacol. 2023, 37, 261–267. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Russo, I.H.; Russo, J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int. J. Cancer 2006, 118, 1862–1868. [Google Scholar] [CrossRef]
- Brantley, K.D.; Ziegler, R.G.; Craft, N.E.; Hankinson, S.E.; Eliassen, A.H. Circulating estrogen metabolites and risk of breast cancer among postmenopausal women in the nurses’ health study. Cancer Epidemiol. Biomark. Prev. 2025, 34, 375–384. [Google Scholar] [CrossRef]
- Maharaj, S.; Trevino, K. A comprehensive review of treatment options for premenstrual syndrome and premenstrual dysphoric disorder. J. Psychiatr. Pract. 2015, 21, 334–350. [Google Scholar] [CrossRef]
- Russo, R.; Corosu, R. The clinical use of a preparation based on phyto-oestrogens in the treatment of menopausal disorders. Acta Biomed. 2003, 74, 137–143. [Google Scholar] [PubMed]
- Quattrocchi, T.; Micali, E.; Gentile, A.; La Ferrera, E.G.; Barbaro, L.; Ciarcià, S.; Corrado, F.; Di Costa, M.; Fazio, R.; Licenziato, R.; et al. Effects of a phyto complex on well-being of climacteric women. J. Obstet. Gynaecol. Res. 2015, 41, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Blum, A.; Luskin, J.R.; Wilson, M.E. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav. Neurosci. 2005, 119, 587–594. [Google Scholar] [CrossRef]
- Kalandakanond-Thongsong, S.; Daendee, S.; Tongta, S.; Thongsong, B.; Srikiatkhachorn, A. Preventive and therapeutic effects of genistein and daidzein on anxiety-like behaviors in ovariectomized rats. Neurosci. Lett. 2025, 845, 138073. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Hernández-Figueroa, J.D.; Hernández-Calderón, B.C.; Saavedra, M. Anxiolytic-like effect of phytoestrogen genistein in rats with long-term absence of ovarian hormones in the black and white model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 367–372. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Cueto-Escobedo, J.; Puga-Olguín, A.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V.; Santos-Torres, A. The phytoestrogen genistein produces similar effects as 17β-estradiol on anxiety-like behavior in rats at 12 weeks after ovariectomy. Biomed. Res. Int. 2017, 2017, 9073816. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. NOM-ZOO-062-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio; México City, México. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 26 January 2025).
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academic Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Johns Hopkins Bloomberg School of Public Health: Baltimore, MD, USA, 2005; Available online: https://caat.jhsph.edu/the-principles-of-humane-experimental-technique/ (accessed on 10 March 2025).
- Hakami, T.; Mahmoud, M.I.; de Juan, E.; Cooney, M. Pharmacokinetics of genistein distribution in blood and retinas of diabetic and non-diabetic rats. Drug Metab. Pharmacokinet. 2021, 39, 100404. [Google Scholar] [CrossRef] [PubMed]
- Mamagkaki, A.; Bouris, I.; Parsonidis, P.; Vlachou, I.; Gougousi, M.; Papasotiriou, I. Genistein as a dietary supplement; formulation, analysis and pharmacokinetics study. PLoS ONE 2021, 16, e0250599. [Google Scholar] [CrossRef]
- Zuluaga, M.J.; Agrati, D.; Pereira, M.; Uriarte, N.; Fernández-Guasti, A.; Ferreira, A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol. Behav. 2005, 84, 279–286. [Google Scholar] [CrossRef]
- Guillén-Ruiz, G.; Cueto-Escobedo, J.; Hernández-López, F.; Rivera-Aburto, L.E.; Herrera-Huerta, E.V.; Rodríguez-Landa, J.F. Estrous cycle modulates the anxiogenic effects of caffeine in the elevated plus maze and light/dark box in female rats. Behav. Brain Res. 2021, 413, 113469. [Google Scholar] [CrossRef]
- Vargas-Moreno, I.; Acosta-Mesa, H.G.; Rodríguez-Landa, J.F.; Avendaño-Garido, M.L.; Fernández-Demeneghi, R.; Herrera-Meza, S. An alternative analysis of computational learning within behavioral neuropharmacology in an experimental anxiety model investigation. Math. Comput. Appl. 2024, 29, 76. [Google Scholar] [CrossRef]
- Doornweerd, A.M.; Gerritsen, L. 28 days later: A prospective daily study on psychological well-being across the menstrual cycle and the effects of hormones and oral contraceptives. Psychol. Med. 2025, 55, e19. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Nunez, C.; Susser, L.; Gershengoren, L. Understanding premenstrual exacerbation: Navigating the intersection of the menstrual cycle and psychiatric illnesses. Front. Psychiatry 2024, 15, 1410813. [Google Scholar] [CrossRef]
- Beral, V.; Bull, D.; Reeves, G. Million women study collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Jespersen, C.; Lauritsen, M.P.; Frokjaer, V.G.; Schroll, J.B. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Cochrane Database Syst. Rev. 2024, 8, CD001396. [Google Scholar] [CrossRef]
- Ho, H.P.; Olsson, M.; Westberg, L.; Melke, J.; Eriksson, E. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: An animal model of premenstrual irritability? Neuropsychopharmacology 2001, 24, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, Z.; Skuza, G. Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol. Rep. 2011, 63, 1547–1552. [Google Scholar] [CrossRef]
- Fernández-Demeneghi, R.; Rodríguez-Landa, J.F.; Guzmán-Gerónimo, R.I.; Acosta-Mesa, H.G.; Meza-Alvarado, E.; Vargas-Moreno, I.; Herrera-Meza, S. Effect of blackberry juice (Rubus fruticosus L.) on anxiety-like behaviour in Wistar rats. Int. J. Food Sci. Nutr. 2019, 70, 856–867. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhao, J.; Pang, Z.; Guo, F. Genistein inhibits Nlrp3/caspase-1 signalling to alleviate traumatic brain injury-induced anxiety-like behaviours in rats. Acta Neuropsychiatr. 2024, 36, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Ni, G.L.; Shao, A.M.; Cui, R. Genistein alleviates anxiety-like behaviors in post-traumatic stress disorder model through enhancing serotonergic transmission in the amygdala. Psychiatry Res. 2017, 255, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Nisha, A.; Shamim, A.; Rizvi, A.; Mahmood, T.; Goswami, B.; Ahsan, F.; Sharique, M.; Parveen, S. A comprehensive review of experimental models of stress: Pragmatic insight into psychoneuroimmunology. Health Care Sci. 2025, 4, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Silva, O.A.; de Almeida, A.A.; Cerqueira, G.S.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol, in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Azimi Sanavi, M.; Ghazvini, H.; Zargari, M.; Ghalehnoei, H.; Hosseini-Khah, Z. Effects of clozapine and risperidone antipsychotic drugs on the expression of CACNA1C and behavioral changes in rat ‘Ketamine model of schizophrenia. Neurosci. Lett. 2022, 770, 136354. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Yoriki, K.; Mori, T.; Aoyama, K.; Tarumi, Y.; Kataoka, H.; Kokabu, T.; Kitawaki, J. Genistein induces long-term expression of progesterone receptor regardless of estrogen receptor status and improves the prognosis of endometrial cancer patients. Sci. Rep. 2022, 12, 10303. [Google Scholar] [CrossRef]
- Albert, A.; Altabre, C.; Baró, F.; Buendía, E.; Cabero, A.; Cancelo, M.J.; Castelo-Branco, C.; Chantre, P.; Duran, M.; Haya, J.; et al. Efficacy and safety of a phytoestrogen preparation derived from Glycine max (L.) Merr in climacteric symptomatology: A multicentric, open, prospective and non-randomized trial. Phytomedicine 2002, 9, 85–92. [Google Scholar] [CrossRef]
- Yonkers, K.A.; O’Brien, P.M.; Eriksson, E. Premenstrual syndrome. Lancet 2008, 371, 1200–1210. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Andrade, J.E.; Helferich, W. Is soy consumption good or bad for the breast? J. Nutr. 2010, 140, 2326S–2334S. [Google Scholar] [CrossRef]
- Bloedon, L.T.; Jeffcoat, A.R.; Lopaczynski, W.; Schell, M.J.; Black, T.M.; Dix, K.J.; Thomas, B.F.; Albright, C.; Busby, M.G.; Crowell, J.A.; et al. Safety and pharmacokinetics of purified soy isoflavones: Single-dose administration to postmenopausal women. Am. J. Clin. Nutr. 2002, 76, 1126–1137. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Ma, B.; Pei, X.; Wang, W.; Gong, W. Mechanism of action of genistein on breast cancer and differential effects of different age stages. Pharm. Biol. 2025, 63, 141–155. [Google Scholar] [CrossRef] [PubMed]
- MacLusky, N.J.; Thomas, G.; Leranth, C. Low dietary soy isoflavonoids increase hippocampal spine synapse density in ovariectomized rats. Brain Res. 2017, 1657, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gend. Med. 2009, 6, 300–311. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Sollozo-Dupont, I.; Islas-Preciado, D.; González-Trujano, M.E.; Carro-Juárez, M.; López-Rubalcava, C. Anxiolytic- and anxiogenic-like effects of Montanoa tomentosa (Asteraceae): Dependence on the endocrine condition. J. Ethnopharmacol. 2019, 241, 112006. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Hernández-López, F.; Saavedra, M. Involvement of estrogen receptors in the anxiolytic-like effect of phytoestrogen genistein in rats with 12-weeks postovariectomy. Pharmacol. Pharm. 2012, 3, 439–446. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Gianquinto, E.; Rossetti, G.; Cruciani, G.; Lorenzetti, S.; Spyrakis, F. Binding of androgen- and estrogen-like flavonoids to their cognate (non)nuclear receptors: A comparison by computational prediction. Molecules 2021, 26, 1613. [Google Scholar] [CrossRef]

| Group | Subgroups | ||
|---|---|---|---|
| Proestrus–Estrus | Metestrus–Diestrus | Total | |
| Vehicle | 9 | 10 | 19 |
| Genistein, 0.09 mg/kg | 9 | 9 | 18 |
| 17β-Estradiol, 0.09 mg/kg | 9 | 10 | 19 |
| Variable/Group | Subgroups | |
|---|---|---|
| Proestrus–Estrus | Metestrus–Diestrus | |
| Crossing | ||
| Vehicle | 46.00 ± 4.96 | 43.20 ± 2.52 |
| Genistein, 0.09 mg/kg | 47.44 ± 2.40 | 44.80 ± 1.80 |
| 17β-Estradiol, 0.09 mg/kg | 46.78 ± 3.25 | 49.78 ± 2.44 |
| Rearing | ||
| Vehicle | 21.92 ± 2.66 | 21.53 ± 2.85 |
| Genistein, 0.09 mg/kg | 23.52 ± 2.40 | 23.73 ± 2.80 |
| 17β-Estradiol, 0.09 mg/kg | 15.46 ± 1.83 + | 17.82 ± 2.23 + |
| Grooming | ||
| Vehicle | 24.57 ± 1.29 | 9.51 ± 0.70 * |
| Genistein, 0.09 mg/kg | 26.22 ± 2.82 | 21.45 ± 1.63 # |
| 17β-Estradiol, 0.09 mg/kg | 26.36 ± 1.69 | 17.87 ± 3.31 # |
| Hormone/Group | Subgroups | |
|---|---|---|
| Proestrus–Estrus | Metestrus–Diestrus | |
| Estradiol, pg/mL | ||
| Vehicle | 26.36 ± 1.08 | 11.06 ± 0.63 * |
| Genistein, 0.09 mg/kg | 29.46 ± 1.32 | 13.89 ± 1.81 + |
| 17β-Estradiol, 0.09 mg/kg | 75.68 ± 4.64 # | 88.64 ± 7.49 # |
| Progesterone, ng/mL | ||
| Vehicle | 31.29 ± 2.35 | 29.52 ± 2.64 |
| Genistein, 0.09 mg/kg | 33.83 ±1.72 | 31.83 ± 1.76 |
| 17β-Estradiol, 0.09 mg/kg | 35.25 ± 2.16 | 32.95 ± 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Landa, J.F.; Olmos-Vázquez, O.J.; Quiñonez-Bailón, C.F.; Guillén-Ruiz, G.; Limón-Vázquez, A.K.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B. Genistein Reduces Anxiety-like Behavior During Metestrus–Diestrus Phase Without Changing Estradiol or Progesterone Levels in Wistar Rats. Metabolites 2025, 15, 311. https://doi.org/10.3390/metabo15050311
Rodríguez-Landa JF, Olmos-Vázquez OJ, Quiñonez-Bailón CF, Guillén-Ruiz G, Limón-Vázquez AK, Cueto-Escobedo J, Rivadeneyra-Domínguez E, Bernal-Morales B. Genistein Reduces Anxiety-like Behavior During Metestrus–Diestrus Phase Without Changing Estradiol or Progesterone Levels in Wistar Rats. Metabolites. 2025; 15(5):311. https://doi.org/10.3390/metabo15050311
Chicago/Turabian StyleRodríguez-Landa, Juan Francisco, Oscar Jerónimo Olmos-Vázquez, Carlos Fabrizio Quiñonez-Bailón, Gabriel Guillén-Ruiz, Ana Karen Limón-Vázquez, Jonathan Cueto-Escobedo, Eduardo Rivadeneyra-Domínguez, and Blandina Bernal-Morales. 2025. "Genistein Reduces Anxiety-like Behavior During Metestrus–Diestrus Phase Without Changing Estradiol or Progesterone Levels in Wistar Rats" Metabolites 15, no. 5: 311. https://doi.org/10.3390/metabo15050311
APA StyleRodríguez-Landa, J. F., Olmos-Vázquez, O. J., Quiñonez-Bailón, C. F., Guillén-Ruiz, G., Limón-Vázquez, A. K., Cueto-Escobedo, J., Rivadeneyra-Domínguez, E., & Bernal-Morales, B. (2025). Genistein Reduces Anxiety-like Behavior During Metestrus–Diestrus Phase Without Changing Estradiol or Progesterone Levels in Wistar Rats. Metabolites, 15(5), 311. https://doi.org/10.3390/metabo15050311











