Propionyl Carnitine Metabolic Profile: Optimizing the Newborn Screening Strategy Through Customized Cut-Offs
Abstract
1. Introduction
2. Materials and Methods
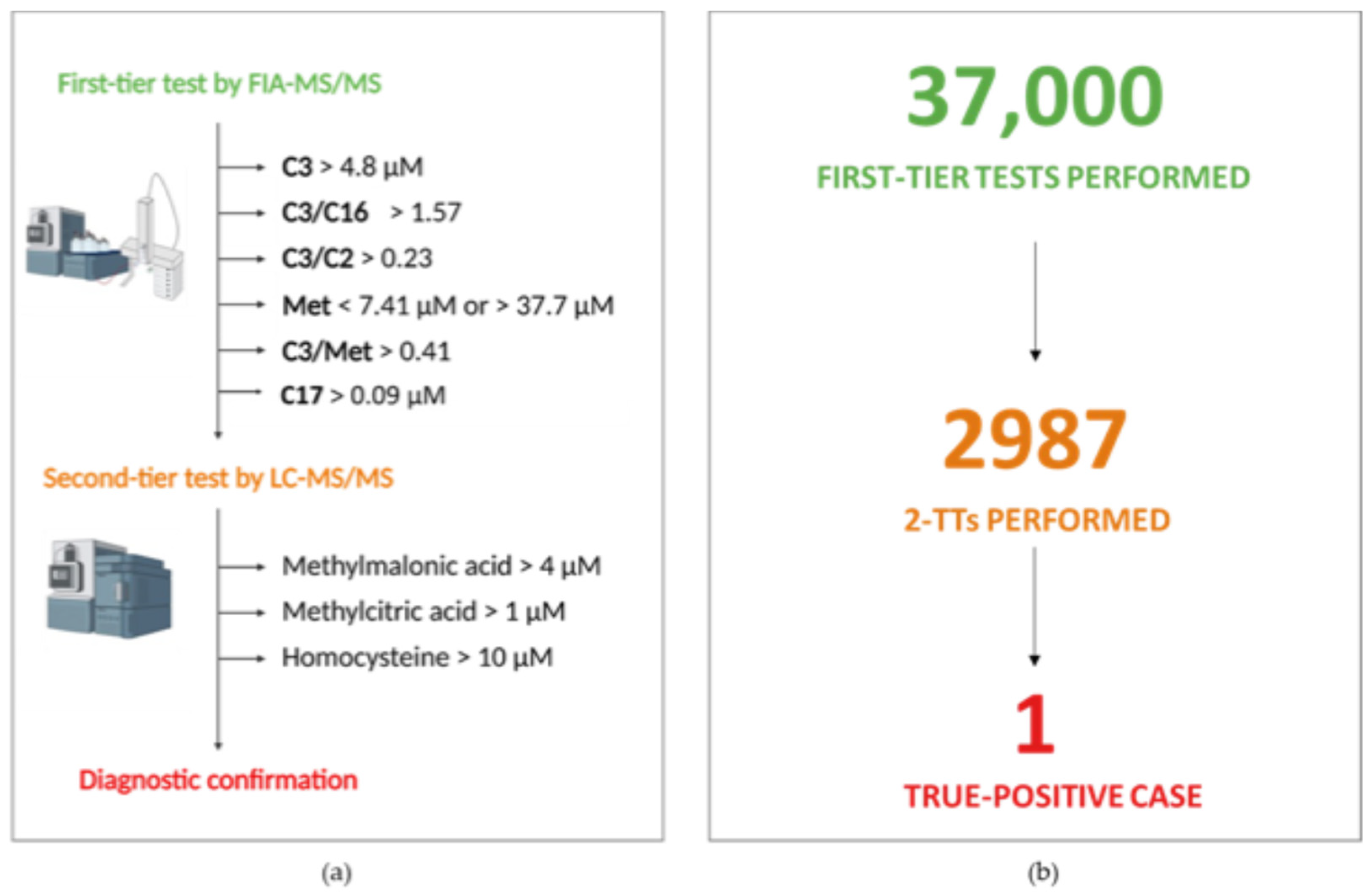
2.1. Newborn Screening Analysis and Second-Tier Testing
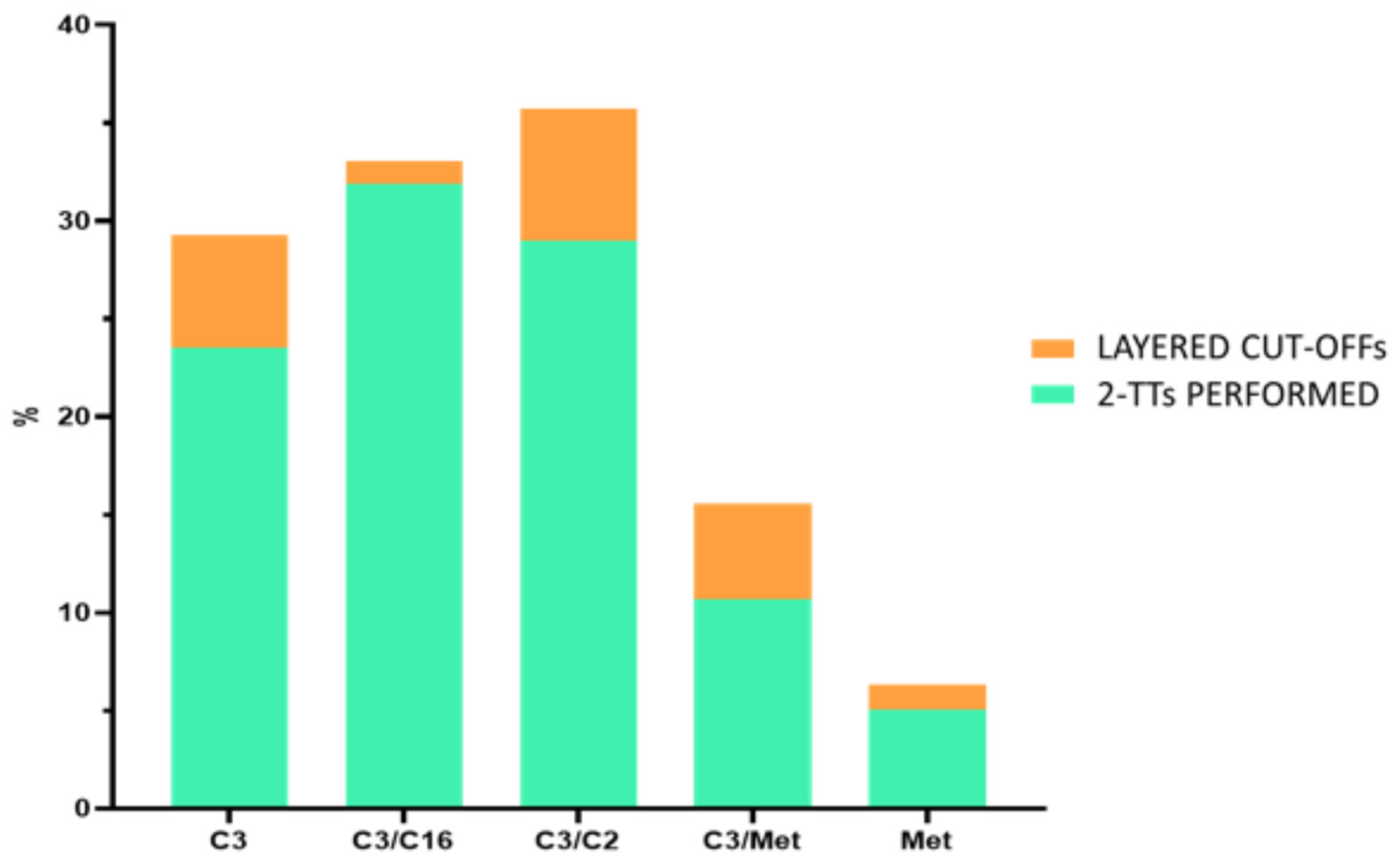
2.2. Creation of Layered Cut-Offs
2.3. Statistical Analysis
3. Results
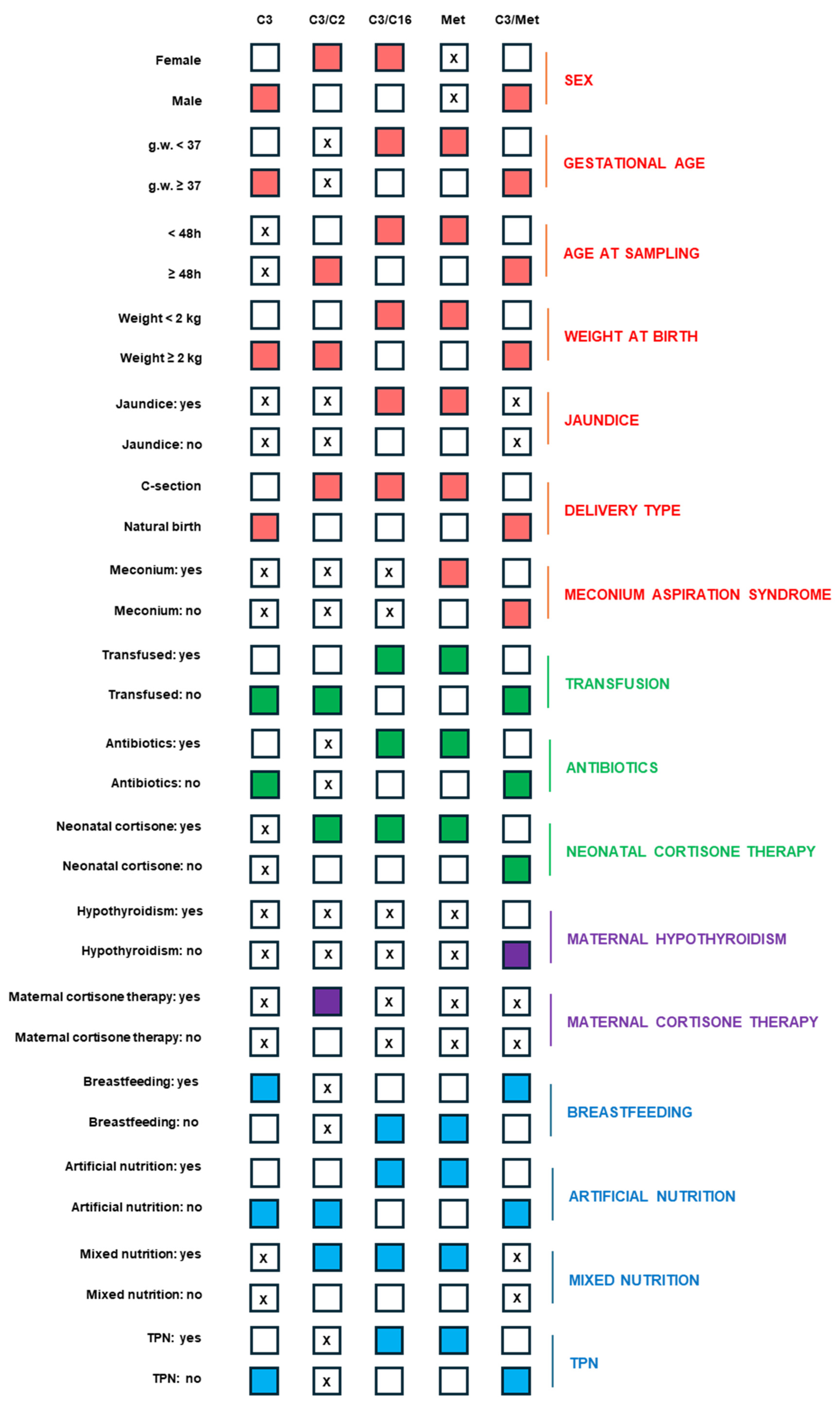
3.1. Setting a Layered Cut-Off According to Neonatal Variables
3.2. Descriptive Statistics
3.3. Insights into the Influence of Neonatal and Maternal Variables on Propionate-Related Biomarker Levels
3.4. Alarm Thresholds for Propionate-Related Biomarkers Based on Neonatal and Maternal Variables to Define the Degree of Urgency for 2-TT Execution
3.5. Post-Analytic Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gramer, G.; Hoffmann, G.F. Second-tier strategies in newborn screening—Potential and limitations. Med. Genetik. 2022, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Gavrilov, D.; Rinaldo, P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: The Mayo Clinic experience (2004–2007). J. Inherit. Metab. Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Cicalini, I.; Di Michele, S.; Fusilli, P.; Cotugno, G.; Ferrante, R.; Bucci, I.; Dionisi-Vici, C.; Stuppia, L.; De Laurenzi, V.; et al. A Case of Suspected Hyperphenylalaninemia at Newborn Screening by Tandem Mass Spectrometry During Total Parenteral Nutrition. Metabolites 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Siri, B.; Olivieri, G.; Angeloni, A.; Cairoli, S.; Carducci, C.; Cotugno, G.; Di Michele, S.; Giovanniello, T.; La Marca, G.; Lepri, F.R.; et al. The diagnostic challenge of mild citrulline elevation at newborn screening. Mol. Genet. Metab. 2022, 135, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Rizzo, C.; Zucchelli, M.; Consalvo, A.; Valentinuzzi, S.; Semeraro, D.; Gasparroni, G.; Brindisino, P.; Gazzolo, D.; et al. A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 3601. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Jotta, R.; Ramos, R.; Florindo, C.; Ventura, F.V.; Vilarinho, L.; de Almeida, I.T.; Gaspar, A. Follow-up of fatty acid β-oxidation disorders in expanded newborn screening era. Eur. J. Pediatr. 2019, 178, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Cicalini, I.; Pieragostino, D.; Rizzo, C.; Verrocchio, S.; Semeraro, D.; Zucchelli, M.; Di Michele, S.; Dionisi-Vici, C.; Stuppia, L.; De Laurenzi, V.; et al. Partial Biotinidase Deficiency Revealed Imbalances in Acylcarnitines Profile at Tandem Mass Spectrometry Newborn Screening. Int. J. Environ. Res. Public Health 2021, 18, 1659. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, D.; Verrocchio, S.; Di Dalmazi, G.; Rossi, C.; Pieragostino, D.; Cicalini, I.; Ferrante, R.; Di Michele, S.; Stuppia, L.; Rizzo, C.; et al. High Incidence of Partial Biotinidase Deficiency in the First 3 Years of a Regional Newborn Screening Program in Italy. Int. J. Environ. Res. Public Health 2022, 19, 8141. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tang, Y.; Cowan, T.M.; Enns, G.M.; Zhao, H.; Scharfe, C. Reducing False-Positive Results in Newborn Screening Using Machine Learning. Int. J. Neonatal Screen. 2020, 6, 16. [Google Scholar] [CrossRef]
- Malvagia, S.; Forni, G.; Ombrone, D.; la Marca, G. Development of Strategies to Decrease False Positive Results in Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Overview of Cutoff Determinations and Risk Assessment Methods Used in Dried Blood Spot Newborn Screening-Role of Cutoffs and Other Methods of Data Analysis; Association of Public Health Laboratories: Bethesda, MD, USA, 2022.
- Ficicioglu, C. New tools and approaches to newborn screening: Ready to open Pandora’s box? Cold Spring Harb. Mol. Case Stud. 2017, 3, a001842. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tang, Y.; Gandotra, N.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Ethnic variability in newborn metabolic screening markers associated with false-positive outcomes. J. Inherit. Metab. Dis. 2020, 43, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Malvagia, S.; Haynes, C.A.; Grisotto, L.; Ombrone, D.; Funghini, S.; Moretti, E.; McGreevy, K.S.; Biggeri, A.; Guerrini, R.; Yahyaoui, R.; et al. Heptadecanoylcarnitine (C17) a novel candidate biomarker for newborn screening of propionic and methylmalonic acidemias. Clin. Chim. Acta 2015, 450, 342–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valentinuzzi, S.; Tommolini, M.L.; Frisco, A.; Zucchelli, M.; Cicalini, I.; Cufaro, M.C. Second-tier tests do not always require chromatographic separation: The case of hydroxylated and dicarboxylic acyl-carnitine couples. J. Innate Metab. 2024, 1, e456. [Google Scholar]
- Cicalini, I.; Moffa, S.; Tommolini, M.L.; Valentinuzzi, S.; Zucchelli, M.; Bucci, I.; Chiacchiaretta, P.; Fontana, A.; Federici, L.; De Laurenzi, V.; et al. Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile. Nutrients 2023, 15, 2297. [Google Scholar] [CrossRef]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External validation of prognostic models: What, why, how, when and where? Clin. Kidney J. 2021, 14, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Hewelt-Belka, W.; Kot-Wasik, A.; Sundekilde, U.K. Nuclear Magnetic Resonance Metabolomics Reveals Qualitative and Quantitative Differences in the Composition of Human Breast Milk and Milk Formulas. Nutrients 2020, 12, 921. [Google Scholar] [CrossRef]
- Dounousi, E.; Zikou, X.; Koulouras, V.; Katopodis, K. Metabolic acidosis during parenteral nutrition: Pathophysiological mechanisms. Indian. J. Crit. Care Med. 2015, 19, 270–274. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, O.; Couce Pico, M.L.; Pérez-Muñuzuri, A.; Castiñeiras Ramos, D.E.; Fernández-Lorenzo, J.R. Hypermethioninemia in the preterm newborn. Predisposing factors. An. Pediatr. 2010, 72, 179–184. [Google Scholar] [CrossRef] [PubMed]
| Panel | C3 | C3/C2 | C3/C16 | Met | C3/Met |
|---|---|---|---|---|---|
| Weight ≥ 2 kg, GA > 37, age at sampling > 48 h (n = 29,595) | 6.10 | 0.28 | 1.83 | 7.36–30.99 | 0.47 |
| Weight ≥ 2 kg, GA > 37, age at sampling < 48 h (n = 1674) | 7.75 | 0.32 | 2.36 | 6.8–43.96 | 0.50 |
| Weight ≥ 2 kg, GA < 37, age at sampling < 48 h (n = 290) | 9.30 | 0.48 | 3.89 | 8.47–69.58 | 0.64 |
| Weight ≥ 2 kg, GA < 37, age at sampling > 48 h (n = 1453) | 7.48 | 0.41 | 2.79 | 8.47–44.73 | 0.64 |
| Weight < 2 kg, GA < 37, Age at sampling < 48 h (n = 397) | 7.30 | 0.36 | 3.32 | 7.10–128.93 | 0.65 |
| Weight < 2 kg, GA < 37, age at sampling > 48 h (n = 504) | 7.88 | 0.44 | 5.49 | 7.25–108.65 | 0.64 |
| Weight < 2 kg, GA > 37, age at sampling < 48 h (n = 15) | 3.85 | 0.18 | 1.32 | 5.24–28.14 | 0.33 |
| Weight < 2 kg, GA > 37, age at sampling > 48 h (n = 70) | 4.64 | 0.24 | 2.80 | 9.41–41.05 | 0.29 |
| Sample Code | C3 (Cut-Off < 6.10 μM) | C3/C2 (Cut-Off < 0.28) | C3/C16 (Cut-Off < 1.83) | Met (n.v. 7.36–30.99 μM) | C3/Met (Cut-Off < 0.47) | Confirmed Diagnosis |
|---|---|---|---|---|---|---|
| Sample 001 | 7.75 | 0.71 | 5.88 | 3.37 | 2.3 | MMA (CbIC) |
| C3 | C3/C2 | C3/C16 | Met | C3/Met | ||
|---|---|---|---|---|---|---|
| Female | 8.97 | 0.50 | 5.11 | 9.71–64.06 | 0.77 | NEONATAL CONDITIONS |
| Male | 10.13 | 0.44 | 5.33 | 8.05–65.14 | 0.83 | |
| GA < 37 | 9.25 | 0.52 | 5.47 | 10.8–93.77 | 0.68 | |
| Age at sampling < 48 h | 8.67 | 0.45 | 5.50 | 8.84–88.42 | 0.63 | |
| Weight < 2 kg | 8.20 | 0.71 | 7.94 | 9.74–161.68 | 0.61 | |
| Weight ≥ 2 kg | 9.77 | 0.45 | 4.19 | 8.87–51.39 | 0.79 | |
| Natural birth | 9.63 | 0.44 | 5.05 | 8.34–62.39 | 0.79 | |
| C-section | 10.36 | 0.46 | 5.78 | 10.2–67.63 | 0.76 | |
| Jaundice | 11.26 | 0.39 | 15.25 | 10.3–83.8 | 0.92 | |
| Meconium | 7.78 | 0.29 | 1.98 | 9.23–59.81 | 0.76 | |
| Transfusion | 6.35 | 0.48 | 6.48 | 9.10–91.28 | 0.41 | NEONATAL TREATMENTS |
| Antibiotics | 10.41 | 0.44 | 7.71 | 11.0–94.33 | 0.77 | |
| Neonatal cortisone | 8.58 | 0.86 | 5.94 | 10.32–90.47 | 0.52 | |
| Breastfeeding | 9.16 | 0.45 | 3.78 | 6.92–48.23 | 0.78 | NUTRITION |
| Artificial nutrition | 8.34 | 0.84 | 8.10 | 11.38–119.66 | 0.79 | |
| Mixed nutrition | 10.40 | 0.45 | 7.73 | 9.85–50.94 | 0.77 | |
| TPN | 9.54 | 0.44 | 5.43 | 12.9–93.76 | 0.53 | |
| Maternal cortisone | 5.95 | 0.32 | 2.44 | 11.13–38.63 | 0.45 | MATERNAL VARIABLES |
| Hypothyroidism | 9.79 | 0.40 | 4.19 | 8.77–58.7 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommolini, M.L.; Cufaro, M.C.; Valentinuzzi, S.; Cicalini, I.; Zucchelli, M.; Frisco, A.; Simonetti, S.; Perrone Donnorso, M.; Moccia, S.; Bucci, I.; et al. Propionyl Carnitine Metabolic Profile: Optimizing the Newborn Screening Strategy Through Customized Cut-Offs. Metabolites 2025, 15, 308. https://doi.org/10.3390/metabo15050308
Tommolini ML, Cufaro MC, Valentinuzzi S, Cicalini I, Zucchelli M, Frisco A, Simonetti S, Perrone Donnorso M, Moccia S, Bucci I, et al. Propionyl Carnitine Metabolic Profile: Optimizing the Newborn Screening Strategy Through Customized Cut-Offs. Metabolites. 2025; 15(5):308. https://doi.org/10.3390/metabo15050308
Chicago/Turabian StyleTommolini, Maria Lucia, Maria Concetta Cufaro, Silvia Valentinuzzi, Ilaria Cicalini, Mirco Zucchelli, Alberto Frisco, Simonetta Simonetti, Michela Perrone Donnorso, Sara Moccia, Ines Bucci, and et al. 2025. "Propionyl Carnitine Metabolic Profile: Optimizing the Newborn Screening Strategy Through Customized Cut-Offs" Metabolites 15, no. 5: 308. https://doi.org/10.3390/metabo15050308
APA StyleTommolini, M. L., Cufaro, M. C., Valentinuzzi, S., Cicalini, I., Zucchelli, M., Frisco, A., Simonetti, S., Perrone Donnorso, M., Moccia, S., Bucci, I., Aricò, M., De Laurenzi, V., Federici, L., Pieragostino, D., & Rossi, C. (2025). Propionyl Carnitine Metabolic Profile: Optimizing the Newborn Screening Strategy Through Customized Cut-Offs. Metabolites, 15(5), 308. https://doi.org/10.3390/metabo15050308











